Abstract
Background and purpose
We aimed to determine the safety of intra-arterial Abciximab injection in the management of thromboembolic complications during endovascular treatment of ruptured cerebral aneurysms.
Methods
In a monocentric consecutive series of endovascular treatment of 783 ruptured aneurysms, 42 (5.3%) patients received Abciximab after the aneurysm was secured. Bleeding complications were registered and dichotomized as follows: new intracranial hemorrhage and peripheral bleeding. For each patient, World Federation of Neurosurgery (WFNS) subarachnoid hemorrhage (SAH) grade, shunting, and clinical outcomes in the post-operative period and at 3–6 months were recorded.
Results
SAH WFNS grades were as follows: grade I n = 14, grade II n = 10, grade III n = 11, grade IV n = 4, grade V n = 3. Ten patients had intracranial hematoma additionally to the SAH prior to embolization. Four patients (9.5%) presented more blood on the post-embolization CT but only one suffered a new clinically relevant intracranial hemorrhage. Two patients (4.8%) experienced significant peripheral bleeding but none were associated with long-term disabilities. Fourteen patients had a shunt installed less than 24 h prior to Abciximab injection and one less than 48 h later. At 3–6-month follow-up, 31 patients (74%) achieved a modified Rankin Scale score (mRS) of 2 or less, six patients (14%) had a mRS of 3–5, three were dead (7%), and two were lost at follow-up.
Conclusion
When the aneurysm is secured, intra-arterial Abciximab injection is a low complication rate treatment modality for thromboembolic events during embolization of cerebral ruptured aneurysm.
Keywords: Safety, Abciximab, cerebral aneurysm, subarachnoid hemorrhage, aneurysm coiling
Introduction
Reported rates of angiographic thromboembolic complications occurring during cerebral aneurysm embolization vary, ranging from 9–28%.1,2 Recent magnetic resonance (MR) studies indicate that these events probably occur at a much higher rate.3 Symptomatic complications are less frequent, probably around 5%.1 In endovascular treatment of cerebral aneurysms, the two mainly used molecules with high affinity for the platelet glycoprotein IIb/IIIa receptor are Abciximab and Tirofiban. With Abciximab, the effect occurs within 10 min, and though it may be reduced, it persists for as long as 48 h after the cessation of treatment.4 Data have been published5–10 but only a small series focused on the safety of this use in the case of ruptured aneurysm.11 The injection of Abciximab is not free of risk, and in the cardiologic literature the rate of major bleeding in a meta-analysis is 2.5%.12 In the neurologic literature, in case of acute ischemic stroke, symptomatic intracranial hemorrhage occurs in 5.5% of patients.13 The purpose of this study is to assess the safety of the use of Abciximab in the context of aneurismal subarachnoid hemorrhage (SAH) by recording the rates of intracranial and peripheral bleeding complications and the safety of ventricular shunting.
Material and methods
Procedures
Procedures with Abciximab (Reopro; Eli Lilly, Indianapolis, IN) injections were prospectively and consecutively recorded from November 2004 (start of Abciximab use) to November 2012. During this period, 783 patients with ruptured aneurysms were treated endovascularly in our institution. All procedures were performed under general anesthesia and patients were given an initial loading dose of 5000 IU of heparin followed by continuous infusion to maintain an activated clotting time between 250 and 300 seconds. In 42 (5.3%) procedures, a thromboembolic event occurred and Abciximab was injected (Figure 1).
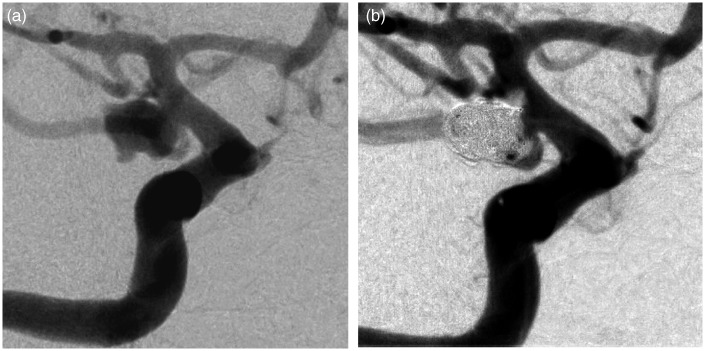
Figure 1.
Example of the use of Abciximab in a grade III SAH for non-occlusive and growing clot at the coil/parent artery interface of a right Pcom ruptured aneurysm (1(a) and 1(b)).
Thromboembolism event
Thromboembolism was defined as presence and/or progression of a thrombus, an antegrade flow disturbance and/or a missing branch. The thrombus could be located at the coil/parent artery interface or in the more distal circulation. We use the term “proximal thrombus” when it arises at the coil/parent artery interface and “distal thrombus” when its location is distal to the aneurysm but within the same vascular territory. Considering that thrombi in these procedures often occurred at the coil/artery interface and did not lead to downstream flow perturbation in all cases, the TIMI/TICI flow grades scale has not been used.
Abciximab treatment
The treatment of angiographic thromboembolic events was performed via a bolus of IA Abciximab (0.25 mg/kg) through coil delivery microcatheter or guiding catheter followed by a 12-h IV infusion (0.125 µg/kg/min) when judged necessary (one case) and/or when neurological deficits were present immediately after the procedure and not related to hemorrhage by emergency computed tomography (CT) (three cases).
Ventricular shunting
Patients were dichotomized in two groups: cerebral spinal fluid (CSF) shunts placed prior to Abciximab injection and after.
Demographic data
The study comprised 42 patients (age range 35–78 years, mean age +/− standard deviation, 54 years ± 11). There were 13 men (age range 43–72 years, mean age, 55 years ± 9.8) and 29 women (age range 35–78 years; mean age, 53 years ± 10.9).
Data analysis
Data were prospectively recorded and retrospectively analyzed. Non-enhanced CT was performed the day after the procedure in all cases, and has been retrospectively analyzed and compared with the CT before treatment by two readers to research new hemorrhage and new ischemic infarction related to the thrombus. New intracranial hemorrhage was defined as any new blood on CT or MR in the 48 h following Abciximab administration. Any hypodensity in the concerned vessel territory was defined as an ischemic infarction. Peripheral bleeding was defined as “requiring any specific imaging test or treatment.”
Post-procedural neurological morbidity was defined as any worsening of clinical status noticed in the 48 h post-embolization compared with pre-embolization clinical neurological status. Initial SAH World Federation of Neurosurgery (WFNS) scores were collected in all patients, as well as the modified Rankin Scale (mRS) score at 3 months.
Results
Clinical and radiological SAH presentation
The WFNS at hospital admission was 1 in 14 of the 42 patients (33%), 2 in 10 patients (24%), 3 in 11 patients (26%), 4 in four patients (10%), and 5 in three patients (7%). Ten of the 42 patients presented an intra-parenchymatous hematoma on the pre-treatment CT scan.
Angiographic indications for Abciximab use
Of the thromboembolic complications, 34 (81%) were at or near the coil/parent artery junction and eight cases were distal emboli (19%).
Post-operative imaging
New intracranial hemorrhage
Four patients (9.5%) suffered a new intracranial hemorrhage as defined above; however, only one appeared to be clinically relevant and greater than 1.5 cm in long axis. In the 10 patients with intra-parenchymatous hematoma additionally to SAH, one experienced an enlargement of the hematoma (Figure 2).
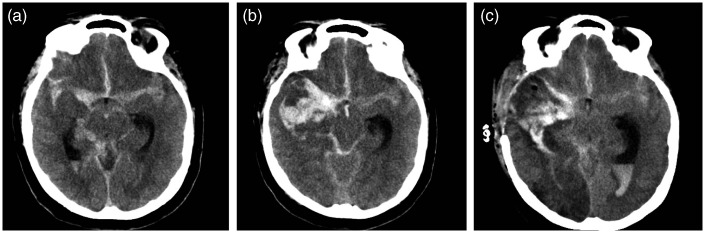
Figure 2.
Pre-treatment CT with diffuse SAH and a small internal temporal hematoma (same patient as in Figure 1) (a). Facing decline of level of consciousness and right mydriasis, CT shows the increase of the temporal hematoma and intraventricular hemorrhage (b). Re-bleeding has been ruled out by a cerebral angiography (not shown), surgical evacuation and ventricular shunting has been performed. CT shows large ischemic changes in the MCA and PCA territories (c). Patient mRS 4, 4 months later.
In the 15 patients who needed ventricular shunting, 14 were placed less than 24 h prior to the embolization and Abciximab injection and one in the 48 h following. Two patients experienced small new intracranial hemorrhage.
“Thrombus-related” infarction
Ischemic changes were observed on CT in 14 patients (33%), including eight post-procedure new deficit patients (19%).
Post-Abciximab injection peripheral bleeding
Two patients (4.8%) experienced peripheral bleeding as defined above, but none were associated with long-term disabilities.
Neurological outcome
Post-procedural outcome
Immediate post-procedural clinical examination showed six (14%) cases of worsening of neurological clinical exam compared with initial clinical status.
Three to six-month follow-up
In total, 31 patients (74%) achieved a mRS score of 2 or less, six patients (14%) had a mRS of 3–5, three were dead (7%), and two were lost at follow-up.
Discussion
In the literature, the rate of thrombus formation during the coiling of cerebral aneurysm varies from 9–20%.1,2,10 In ruptured aneurysm, Pierot et al. reported a higher rate of thromboembolic events in patients with aneurysms larger than 10 mm, in smokers and in patients with aneurysms with a neck larger than 4 mm.2
The sequence of events leading to the clot starts with the adhesion of platelets. Platelets become “activated” and glycoprotein lIb/IIIa receptors on the platelet surface assume an active conformation that binds circulating adhesive proteins and ultimately leads to thrombosis.
Facing an acute intra-arterial clot, no consensus on optimal management exists. Neuro-interventionists have several options: abstention and observation of the evolution, arterial or IV injection of antiplatelet agents, and/or mechanical intervention.9
Abciximab is not the only drug available in order to attempt lysis of an acute intra-arterial clot during neuro-interventional procedures.9 Cronqvist et al.14 described the use of Urokinase to manage thromboembolic events encountered during coil embolization. In this small case series of six patients with acutely ruptured aneurysms, three experienced symptomatic hemorrhagic complications after thrombolysis leading to a poor outcome (GOS score of 5).14 Hähnel et al.15 described the use of recombinant tPA in a case series of nine patients. The authors observed a 22% recanalization rate and reported significant morbidity and mortality, (two deaths and three morbidities).15 Following those publications and the appearance of antiplatelet agents as inhibitors of the glycoprotein IIb/IIIa receptor, lytic agents as Urokinase or tPA are no longer used as first-line therapy by most teams facing an acute thromboembolic complication.
In the past 10 years, several series have shown that Abciximab injection for thromboembolic complication during coiling was associated with a low complication rate. However, a large number of the aneurysms treated in these papers were unruptured, and most of the papers included ruptured and unruptured aneurysms.8,10,11,16,17 Tirofiban is a shorter-acting and reversible glycoprotein IIb/IIIa receptor inhibitor. According to Jeon et al. and Bruening et al., intra-arterial Tirofiban and Abciximab exhibited similar safety and recanalization rates.18,19
In our series, we used Abciximab injection in 5.3% of our ruptured aneurysm treatment cases; 81% of the clots appeared at the coil/artery interface. We generally injected the Abciximab via the microcatheter already in place in order to be as close as possible to the clot, as suggested by Duncan et al.20 Some authors have advocated that local IA delivery of Abciximab by a microcatheter, placed in the parent vessel, may require smaller doses of the agent and therefore may decrease the risk of hemorrhagic complications.6 However, this is not proven and was not our strategy; likewise, the benefit of injecting Abciximab via the microcatheter rather than through the guiding catheter or intravenously is also unproven, despite some data from the cardiologic literature.12
On the early post-operative imaging, we observed new onset of bleeding in four patients (9.5%). These numbers may seem high, but three-quarters of the new instances of intracranial bleeding were minimal and not clinically relevant. The only concerning bleeding we observed was an important extension of a pre-existing hematoma versus a re-bleeding of the aneurysm (Figure 2). Regarding ischemic changes, we observed new lesions in 14 patients (33%). However, 74% of our population still achieved a mRS score of 2 or less at 3–6 months, which is comparable with the 3–6 months follow-up mRS of ISAT patients.21 In any case this is a retrospective study not designed and not powerful enough to discuss the efficacy of Abciximab.
Conclusion
The use of Abciximab as a first-line treatment in thrombotic complications during coil embolization of ruptured cerebral aneurysm seems safe. In this series we did not observe concerning bleeding around the ventricular shunt trajectory even though shunts were placed less than 24 h prior to Abciximab injection 14 times.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Layton KF, Cloft HJ, Gray L, et al. Balloon-assisted coiling of intracranial aneurysms: Evaluation of local thrombus formation and symptomatic thromboembolic complications. Am J Neuroradiol 2007; 28: 1172–1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pierot L, Cognard C, Anxionnat R, et al. Ruptured intracranial aneurysms: Factors affecting the rate and outcome of endovascular treatment complications in a series of 782 patients (CLARITY Study). Radiology 2010; 256: 916–923. [DOI] [PubMed] [Google Scholar]
- 3.Brooks NP, Turk AS, Niemann DB, et al. Frequency of thromboembolic events associated with endovascular aneurysm treatment: Retrospective case series. J Neurosurg 2008; 108: 1095–100. [DOI] [PubMed] [Google Scholar]
- 4.Tcheng JE, Ellis SG, George BS, et al. Pharmacodynamics of chimeric glycoprotein IIb/IIIa integrin antiplatelet antibody Fab 7E3 in high-risk coronary angioplasty. Circulation 1994; 90: 1757–1764. [DOI] [PubMed] [Google Scholar]
- 5.Mounayer C, Piotin M, Baldi S, et al. Intraarterial administration of Abciximab for thromboembolic events occurring during aneurysm coil placement. Am J Neuroradiol 2003; 24: 2039–2043. [PMC free article] [PubMed] [Google Scholar]
- 6.Linfante I, Etezadi V, Andreone V, et al. Intra-arterial Abciximab for the treatment of thrombus formation during coil embolization of intracranial aneurysms. J Neurointerv Surg 2010; 2: 135–138. [DOI] [PubMed] [Google Scholar]
- 7.Gralla J, Rennie ATM, Corkill RA, et al. Abciximab for thrombolysis during intracranial aneurysm coiling. Neuroradiology 2008; 50: 1041–1047. [DOI] [PubMed] [Google Scholar]
- 8.Ries T, Siemonsen S, Grzyska U, et al. Abciximab is a safe rescue therapy in thromboembolic events complicating cerebral aneurysm coil embolization: Single center experience in 42 cases and review of the literature. Stroke 2009; 40: 1750–1757. [DOI] [PubMed] [Google Scholar]
- 9.Fiorella D, Albuquerque FC, Han P, et al. Strategies for the management of intraprocedural thromboembolic complications with Abciximab (ReoPro). Neurosurgery 2004; 54: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 10.Aggour M, Pierot L, Kadziolka K, et al. Abciximab treatment modalities for thromboembolic events related to aneurysm coiling. Neurosurgery 2010; 67: 503–508. [DOI] [PubMed] [Google Scholar]
- 11.Aviv RI, O'Neill R, Patel MC, et al. Abciximab in patients with ruptured intracranial aneurysms. Am J Neuroradiol 2005; 26: 1744–1750. [PMC free article] [PubMed] [Google Scholar]
- 12.De Luca G, Verdoia M, Suryapranata H. Benefits from intracoronary as compared to intravenous Abciximab administration for STEMI patients undergoing primary angioplasty: A meta-analysis of 8 randomized trials. Atherosclerosis 2012; 222: 426–433. [DOI] [PubMed] [Google Scholar]
- 13.Adams HP, Effron MB, Torner J, et al. Emergency administration of Abciximab for treatment of patients with acute ischemic stroke: Results of an international phase III trial: Abciximab in Emergency Treatment of Stroke Trial (AbESTT-II). Stroke 2008; 39: 87–99. [DOI] [PubMed] [Google Scholar]
- 14.Cronqvist M, Pierot L, Boulin A, et al. Local intraarterial fibrinolysis of thromboemboli occurring during endovascular treatment of intracerebral aneurysm: A comparison of anatomic results and clinical outcome. Am J Neuroradiol 1998; 19: 157–165. [PMC free article] [PubMed] [Google Scholar]
- 15.Hähnel S, Schellinger PD, Gutschalk A, et al. Local intra-arterial fibrinolysis of thromboemboli occurring during neuroendovascular procedures with recombinant tissue plasminogen activator. Stroke A J Cereb Circ 2003; 34: 1723–1728. [DOI] [PubMed] [Google Scholar]
- 16.Park JH, Kim JE, Sheen SH, et al. Intraarterial Abciximab for treatment of thromboembolism during coil embolization of intracranial aneurysms: Outcome and fatal hemorrhagic complications. J Neurosurg 2008; 108: 450–457. [DOI] [PubMed] [Google Scholar]
- 17.Jones RG, Davagnanam I, Colley S, et al. Abciximab for treatment of thromboembolic complications during endovascular coiling of intracranial aneurysms. Am J Neuroradiol 2008; 29: 1925–1929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Jeon JS, Sheen SH, Hwang G, et al. Intraarterial tirofiban thrombolysis for thromboembolisms during coil embolization for ruptured intracranial aneurysms. J Cerebrovasc Endovasc Neurosurg 2012; 14: 5–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bruening R, Mueller-Schunk S, Morhard D, et al. Intraprocedural thrombus formation during coil placement in ruptured intracranial aneurysms: Treatment with systemic application of the glycoprotein IIb/IIIa antagonist tirofiban. Am J Neuroradiol 2006; 27: 1326–1331. [PMC free article] [PubMed] [Google Scholar]
- 20.Duncan IC, Fourie PA. Catheter-directed intra-arterial abciximab administration for acute thrombotic occlusions during neurointerventional procedures. Interv Neuroradiol 2002; 8: 159–168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Molyneux A, Kerr R, Stratton I, et al. International Subarachnoid Aneurysm Trial (ISAT) of neurosurgical clipping versus endovascular coiling in 2143 patients with ruptured intracranial aneurysms: A randomised trial. Lancet 2002; 360: 1267–1274. [DOI] [PubMed] [Google Scholar]