Abstract
The authors report a rare case of a carotid-cavernous fistula (CCF) secondary to Ehlers–Danlos syndrome (EDS) type IV which showed an aggressive angiographical change.
A 59-year-old woman presented with headache, right pulsatile tinnitus, and diplopia on the right side. The diagnostic angiography demonstrated a right CCF. Accordingly transarterial embolization of the fistula was attempted 5 days later. The initial right internal carotid angiography showed an aneurysm on the petrous portion of the internal carotid artery (ICA) which was not recognized in the diagnostic angiography. Spontaneous reduction of the shunt flow and long dissection of the ICA were also revealed. The aneurysm was successfully occluded with coils, and only minor shunt flow was shown on the final angiogram. EDS type IV was diagnosed with a skin biopsy for a collagen abnormality. After the operation, the stenosis of the right ICA gradually progressed, although there was no recurrence of the CCF.
Interventional treatment for patients with EDS can cause devastating vascular complication. We should be aware of the possibility of EDS type IV when a spontaneous CCF shows unusual angiographical change because early diagnosis of EDS type IV is crucial for determination of the optimum treatment option.
Keywords: Aneurysm, carotid-cavernous fistula, Ehlers–Danlos syndrome type IV, transarterial embolization
Introduction
Ehlers–Danlos syndrome (EDS) type IV, an autosomal dominant disorder, is uncommon, and the precise incidence and prevalence are still unknown.1 In patients with EDS type IV, collagen type III deficiency plays a role in the pathogenesis of intracranial saccular aneurysms, carotid-cavernous fistulas (CCF), and dissections of the cervical arteries.2 However, the optimal treatment for the intracranial vascular disorders is still controversial. We describe herein a patient with a CCF secondary to EDS type IV which showed unusual angiographical change.
Case report
A 59-year-old woman presented to our hospital with sudden onset of headache, right pulsatile tinnitus, and diplopia due to right abducens palsy. Her parents were cousins. She had suffered from intestinal perforation 3 years previously. Magnetic resonance (MR) imaging demonstrated a right CCF and a left middle cerebral artery aneurysm (Figure 1). On day 9, digital subtraction angiography was performed via the right brachial artery. Right internal carotid angiography revealed a CCF (Figure 2). Immediately after the angiography, a dissecting aneurysm developed at the puncture site in the right brachial artery which was treated conservatively. On day 14, endovascular treatment was attempted under general anesthesia. A 5-French guiding sheath (Axelguide, Medikit, Tokyo, Japan) was introduced into the right femoral artery. Intravenous anticoagulation was induced with a 4000-U heparin bolus, and the activated clotting time was maintained at approximately 250 s. The guiding sheath was navigated to the right internal carotid artery (ICA). Angiography revealed the development of an aneurysm (6.6 × 6.2 × 5.8 mm) and dissection of the ICA from the aneurysm towards the proximal portion, which were not presented in the diagnostic angiography (Figure 3(a, b)). Because the shunt flow was supplied from the newly developed aneurysm, we decided to occlude the aneurysm with platinum coils. A balloon microcatheter (7 × 7 mm HyperForm, Covidien, Irvine, CA, USA) was navigated into the right ICA and positioned across the neck of the aneurysm. A microcatheter (Excelsior SL-10, Stryker, Kalamazoo, MI, USA) was navigated into the aneurysm sac. The aneurysm was completely occluded with a total of 17 coils (143 cm) using a balloon remodeling technique, although minor residual shunt flow was still recognized on the final angiogram (Figure 3(c)).
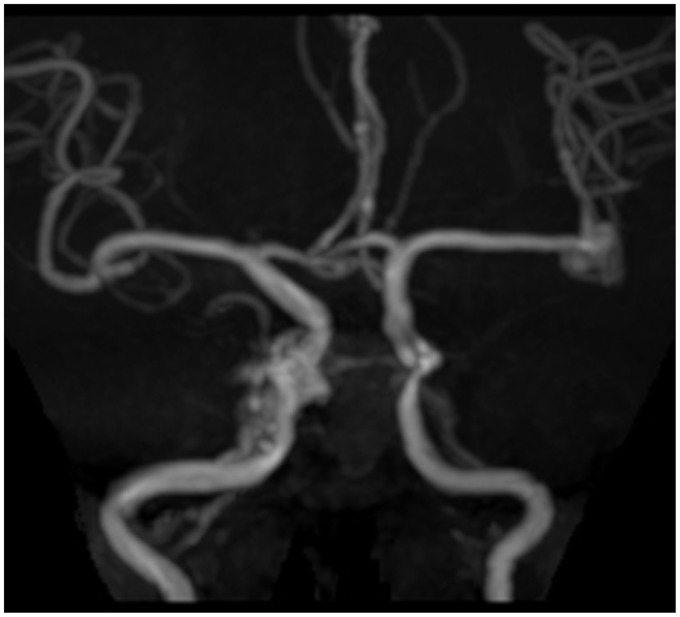
Figure 1.
TOF MRA reveals a right carotid-cavernous fistula and a left middle cerebral artery aneurysm.
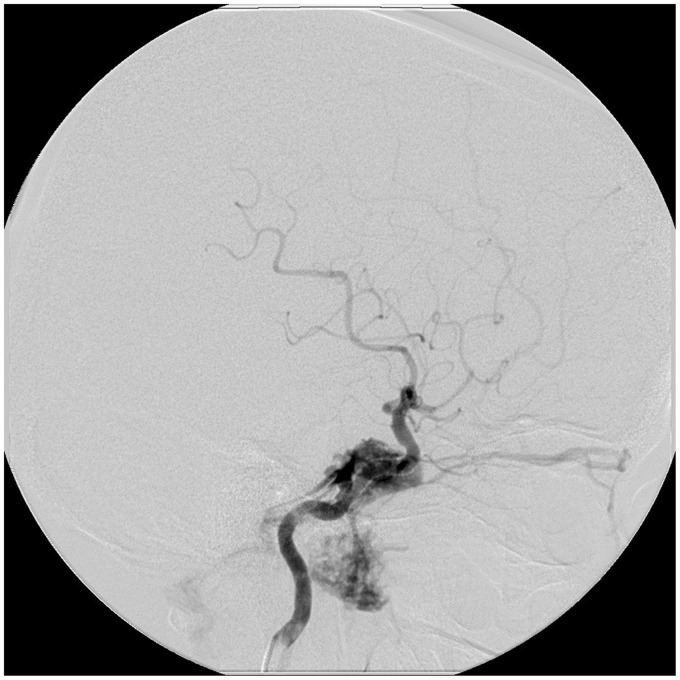
Figure 2.
The right ICA angiogram shows the carotid-cavernous fistula at C3–4. Outflow is seen via the right cavernous sinus into the right inferior petrosal sinus, right superior ophthalmic vein, the right inferior ophthalmic vein, right pterygoid plexus, and the left cavernous sinus.
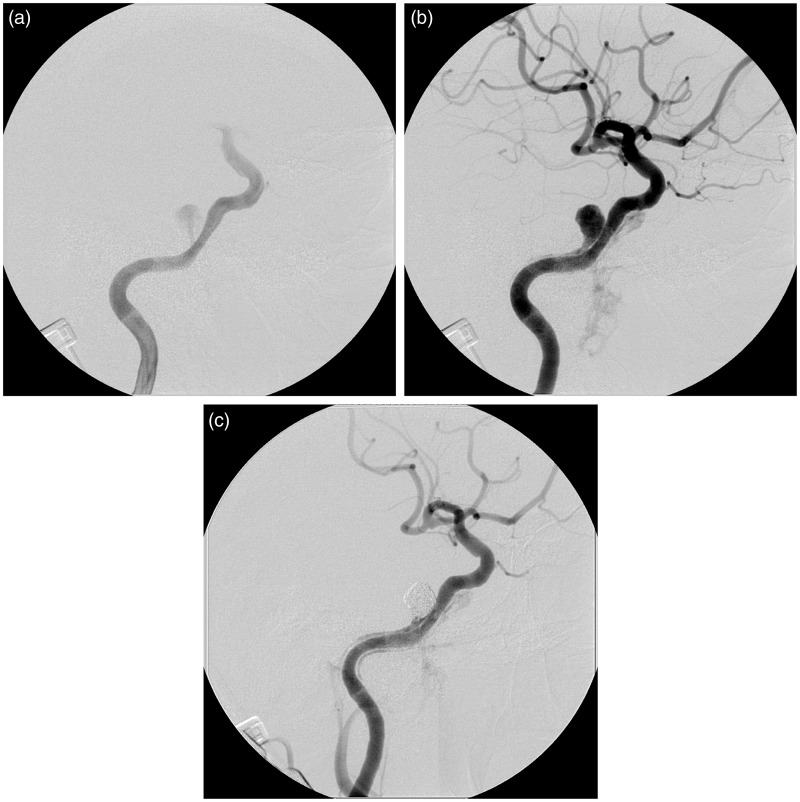
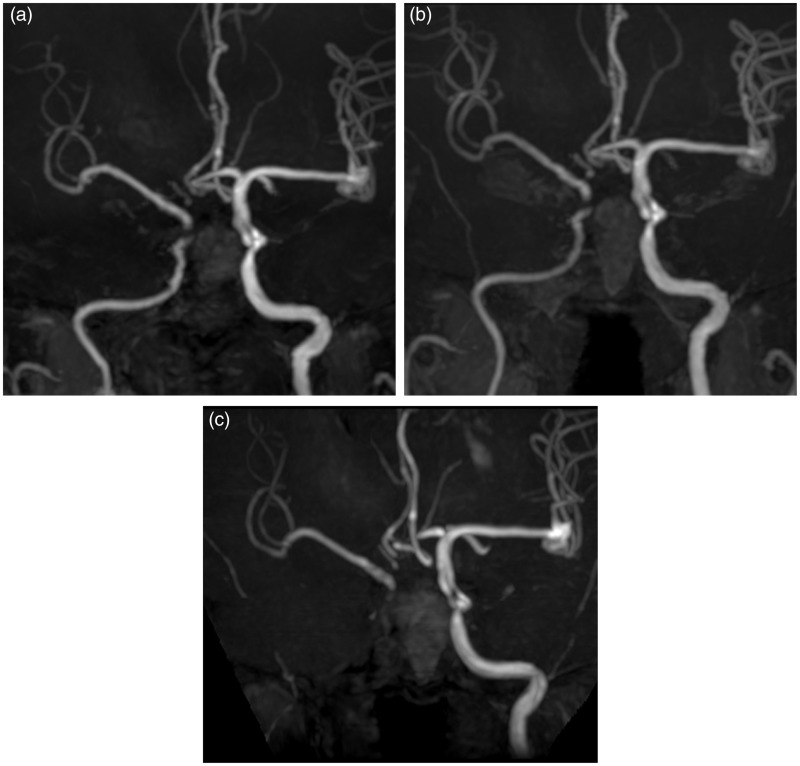
Figure 3.
Right internal carotid angiography. (a) Early arterial image demonstrating the shunt from the ICA into the newly developed aneurysm. (b) Late arterial phase demonstrating the retrograde blood flow in the pseudolumen along the internal carotid artery, suggestive of the long dissection of the internal carotid artery. The amount of the shunt flow into the cavernous sinus and the pterygoid plexus is much smaller compared with that recognized in the diagnostic angiography. (c) The final image revealing complete occlusion of the aneurysm with minimal shunt flow.
Postoperatively, the patient presented with left hemiparesis. MR images on the following day revealed the multiple cerebral infarctions and the dissection of the right ICA, although the shunt flow was not visible (Figure 4). The patient was treated with intravenous edaravone for 7 days to prevent the deterioration of the symptoms. Her right brachial artery dissecting aneurysm gradually grew, and vascular reconstruction of her right brachial artery was undertaken using a saphenous vein graft on the 28th day after the initial angiography. The unusual clinical course, past history, and family history was suggestive of EDS type IV, which was diagnosed as a collagen type III deficiency as indicated by a skin biopsy and fibroblast culture. Oral administration of celiprolol was started to prevent further vascular complications. All the neurological symptoms except for the mild right abducens nerve palsy finally resolved. The stenosis of the right ICA gradually progressed, and its occlusion was confirmed on the MR images 21 months after the operation (Figure 5). Enlargement of the unruptured aneurysm on the left middle cerebral artery and recurrence of the right CCF were not shown on the MR images.
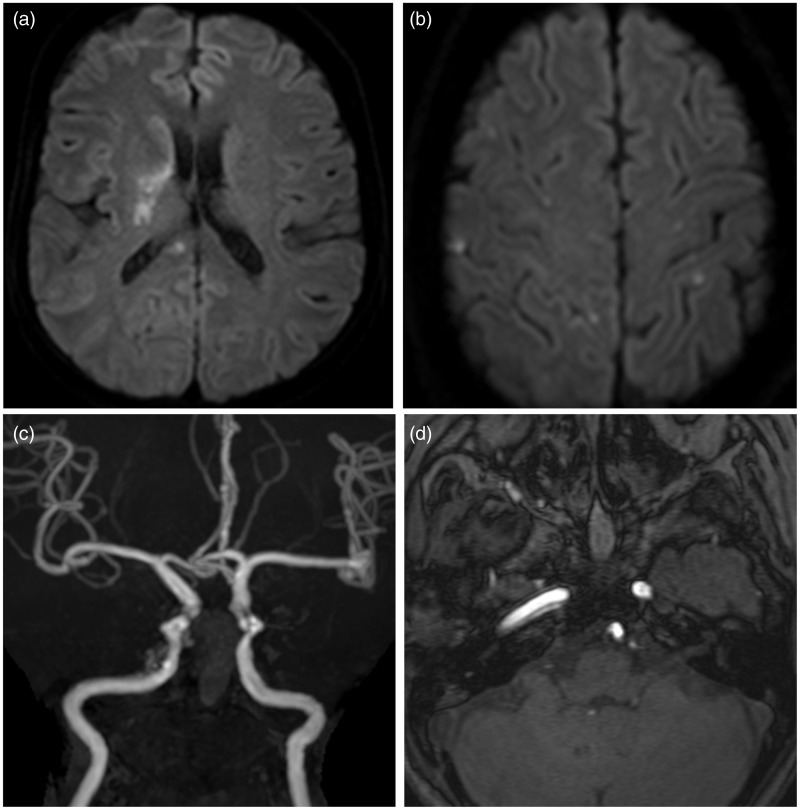
Figure 4.
MRI scans taken one day after the operation. (a, b) Diffusion-weighted MRI demonstrates acute ischemic lesions in the territories of the right middle cerebral artery, the right posterior cerebral artery, and bilateral anterior cerebral arteries. (c, d) TOF MRA reveals mild stenosis and the pseudolumen of the right internal carotid artery.
Figure 5.
TOF MRA taken 4 months (a), 15 months (b), and 21 months (c) after the operation shows the progression of the stenosis of the right internal carotid artery. There was no recurrence of the CCF.
Discussion
The estimated prevalence for all types of EDS varies between 1/10,000 and 1/25,000, and EDS type IV represents approximately 5–10% of EDS cases.3 The frequency of EDS type IV in patients with CCF is estimated at about 2–3%.4–6 Taking the low prevalence of EDS type IV into account, it is obvious that patients with EDS type IV present with CCFs much more frequently than patients without EDS type IV.
The etiology of CCFs related to EDS type IV has been well explored. An abnormally low intima-media thickness of the carotid artery generates a higher wall stress than in control subjects at the site of an elastic artery, which may increase the risk of arterial dissection and rupture.7 Weakness of the wall of the cavernous sinus associated with aneurysm formation of the ICA and its subsequent rupture are also likely to lead to the formation of a CCF.8
A previous review showed that the CCF in two patients with EDS type IV spontaneously resolved, although the CCF recurred in one of them.9 In cases of EDS type IV, however, the natural course of the CCF is still unclear because long-term conservative observation has been performed in only a small number of CCFs secondary to EDS type IV.9 There are also reported cases of EDS type IV in which the CCF developed after surgical or endovascular occlusion of the contralateral ICA as the treatment for the CCF.9,10 These cases indicate that the angioarchitecture in cases of EDS type IV can change due to hemodynamic stress.
In the present case, the reduction of the shunt flow, the formation of the aneurysm, and dissection of the ICA progressed in only 5 days. These findings were confirmed before the microcatheter was navigated to the petrous portion of the ICA. Therefore, the rapid angiographical changes were highly likely induced by the abnormal fragility of the arterial wall rather than the mechanical damage by the microcatheter. It might also be plausible that the injection of the contrast medium from the diagnostic catheter or the guiding sheath delivered pressure directly on the arterial wall which could cause the laceration and dissection in the petrous portion of the ipsilateral ICA.
The intracranial ischemic complications related to endovascular therapy in EDS type IV patients have not been reported. In the present case, however, the postoperative MR images showed multiple ischemic lesions in the territories of the right ICA, the right posterior cerebral artery, and the left anterior cerebral artery, although the endovascular therapy was performed under systemic heparinization. The dissection of the arterial wall might lead to the abnormal generation of microemboli which could cause cerebral infarction. In the present case, we administered a neuroprotective agent for the treatment of acute ischemic stroke. However, intravenous anticoagulants might have also been effective to prevent the deterioration of the cerebral infarction caused by the microemboli.
As transbrachial catheter diagnostics has been proven to be minimally invasive, and an efficient and low-risk method,11 our institute preferably performs diagnostic angiography via the brachial artery. In the present case, the unusual dissecting aneurysm had developed in the right brachial artery after the diagnostic angiography. We should be aware of the risk of the development of a dissecting aneurysm following transbrachial catheter diagnostics in patients with EDS type IV.
To the best of our knowledge, there have been no previous reports showing such an aggressive change of the CCF even in cases of EDS type IV. We should remember the possibility of EDS type IV as the etiology when we encounter a spontaneous CCF showing an unusual clinical course because endovascular treatment frequently causes catastrophic complications.9,12 In addition, we should cautiously observe the clinical course and evaluate the risk and benefit of the interventional treatment before the treatment option is decided because the CCFs are not a fatal condition.
Conclusion
We have reported herein on a rare case of CCF in a patient with EDS type IV which showed rapid angiographical change. The early diagnosis of EDS type IV is critical when deciding on the treatment option. If a spontaneous CCF shows the unusual clinical course, we should be aware of the possibility of EDS type IV.
Acknowledgments
Author contributions to the study and manuscript preparation include the following. Conception and design: Kojima, Saga, and Tomio. Acquisition of data: Kojima and Tomio. Analysis and interpretation of data: Kojima, Kosho, and Hatamochi. Drafting the article: Kojima.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Pepin M, Schwarze U, Superti-Furga A, et al. Clinical and genetic features of Ehlers-Danlos syndrome type IV, the vascular type. N Engl J Med 2000; 9: 673–680. [DOI] [PubMed] [Google Scholar]
- 2.Schievink WI, Limburg M, Oorthuys JW, et al. Cerebrovascular disease in Ehlers-Danlos syndrome type IV. Stroke 1990; 21: 626–632. [DOI] [PubMed] [Google Scholar]
- 3.Germain DP. Ehlers-Danlos syndrome type IV. Orphanet J Rare Dis 2007; 2: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Debrun GM, Aletich VA, Miller NR, et al. Three cases of spontaneous direct carotid cavernous fistulas associated with Ehlers-Danlos syndrome type IV. Surg Neurol 1996; 46: 247–252. [DOI] [PubMed] [Google Scholar]
- 5.Halbach VV, Higashida RT, Dowd CF, et al. Treatment of carotid-cavernous fistulas associated with Ehlers-Danlos syndrome. Neurosurgery 1990; 26: 1021–1027. [DOI] [PubMed] [Google Scholar]
- 6.North KN, Whiteman DA, Pepin MG, et al. Cerebrovascular complications in Ehlers-Danlos syndrome type IV. Ann Neurol 1995; 38: 960–964. [DOI] [PubMed] [Google Scholar]
- 7.Boutouyrie P, Germain DP, Fiessinger JN, et al. Increased carotid wall stress in vascular Ehlers-Danlos syndrome. Circulation 2004; 109: 1530–1535. [DOI] [PubMed] [Google Scholar]
- 8.Bashir Q, Thornton J, Alp S, et al. Carotid-cavernous fistula associated with Ehlers-Danlos syndrome type IV. A case report and review of literature. Interv Neuroradiol 1999; 20: 313–320. [DOI] [PubMed] [Google Scholar]
- 9.Schievink WI, Piepgras DG, Earnest F, 4th, et al. Spontaneous carotid-cavernous fistulae in Ehlers-Danlos syndrome Type IV. Case report. J Neurosurg 1991; 74: 991–998. [DOI] [PubMed] [Google Scholar]
- 10.Schoolman A, Kepes JJ. Bilateral spontaneous carotid-cavernous fistulae in Ehlers-Danlos syndrome. Case report. J Neurosurg 1967; 26: 82–86. [DOI] [PubMed] [Google Scholar]
- 11.Basche S, Eger C, Aschenbach R. Transbrachial angiography: An effective and safe approach. Vasa 2004; 33: 231–234. [DOI] [PubMed] [Google Scholar]
- 12.Horowitz MB, Purdy PD, Valentine RJ, et al. Remote vascular catastrophes after neurovascular interventional therapy for type 4 Ehlers-Danlos Syndrome. Am J Neuroradiol 2000; 21: 974–976. [PMC free article] [PubMed] [Google Scholar]