Abstract
Background and purpose
The purpose of this article is to report on the long-term success rates of Silk flow-diverter (FD) treatment in a multicenter prospective study for the treatment of complex aneurysms.
Methods
Between May 2008 and January 2011, all consecutive patients featuring complex intracranial aneurysms eligible for FD treatment with the Silk in three neurovascular centers were included. Clinical and imaging data were assessed during hospitalization and follow-up.
Results
Five patients were initially asymptomatic, 20 patients showed various neurological symptoms. Twenty-eight FDs were implanted in 25 patients treating 28 aneurysms. The immediate procedure-related morbidity was 8% (two of 25), mortality 0%. One procedure-related death was observed during follow-up (in-stent thrombosis). Compared to the immediate result nearly two of three aneurysms improved during follow-up; all angiographically confirmed inflow changes took place within six months after treatment. Final anatomic outcome in 24 aneurysms of 22 patients comprised 14 (59%) with complete occlusion, seven (29%) with a neck remnant, two (8%) with residual filling <50%, none with residual filling >50% and one (4%) unchanged in comparison to its pretreatment status. Postinterventional recanalizations were seen in three of 13 (23%) aneurysms treated with FD alone; none were observed in 15 aneurysms treated with adjunctive coiling.
Conclusion
Anatomic presentation and location are key for successful FD treatment. The rate of successful occlusion increases during follow-up. Postinterventional monitoring for at least six months is paramount, as anatomic outcome is not reliably predictable and recanalizations may occur in initially completely occluded aneurysms.
Keywords: Flow diverter, Silk, intracranial aneurysm
Introduction
The advent of flow diverters (FDs)
Since the introduction of Guglielmi detachable coils (GDCs) in the early 1990s, significant improvements of the materials have helped neurointerventionalists in lowering complication rates and improving occlusion degrees.1 So-called complex aneurysms, being either fusiform or wide-necked ones or (partially thrombosed) giant aneurysms, however, had recurrence rates of 20–30% when treated with GDC coils.2 For certain subgroups of aneurysms, like sidewall aneurysms in the internal carotid artery’s (ICA) ophthalmic segment, alternative strategies were tried, e.g. occlusion with Onyx HD 500 (eV3, Irvine, CA, USA).3 This was abandoned though after the advent of highly flexible stents like Neuroform (Boston Scientific, Natick, MA, USA), Enterprise (Cordis, Bridgewater, NJ, USA) and LEO (Balt Extrusion, Montmorency, France), which serve as bridging-neck devices when combined with coiling. They support the coils in the aneurysm sac, which is especially important in wide-necked aneurysms with unfavorable neck/sac ratios of >0.7; furthermore they permit higher coil-packing densities.4 Intra-aneurysmal flow alterations and complete thrombosis of the sac have been reported with these stents.5–10 The evolving principle of flow diversion resulted in the introduction of two new devices, termed “flow diverters” (FDs): the Silk stent (Balt Extrusion, Montmorency, France), which has been approved for use in Europe since 2007, and the Pipeline Embolization device (PED) (eV3). Compared to conventional stents they provide a higher mesh density, resulting in a higher coverage of the target vessel’s inner surface, in order to alter the pathological hemodynamic flow and to promote laminar flow in the parent artery.11
Aims of the study
We report our clinical and angiographic experiences with the Silk in 25 cases with a total of 28 aneurysms with and without adjunctive coiling during hospitalization and mid-term follow-up (FU) of up to 36 months (mean 14.9 months).
Materials and methods
Patients
The retrospective analysis was conducted by reviewing all cases from a prospectively kept neurointerventional database starting in May 2008 in three neurovascular centers with predefined indications for the treatment of complex intracranial aneurysms in the anterior and posterior circulation and a predefined antiplatelet therapeutic regimen. Patients gave informed consent. According to the institutional regulations no ethics committee approval was necessary for conducting the retrospective analysis in an anonymized approach. The cohort comprises 25 patients with 28 aneurysms, which were treated by Silk stent placement between May 2008 and January 2011. All interventions were performed or supervised by the senior neurointerventionalists (WW, MS and BE). Interdisciplinary therapeutic alternatives were discussed in the decision-making process. With a single exception only patients with previously unruptured aneurysms were treated; one patient with a history of subarachnoid hemorrhage (SAH) (Hunt and Hess (H & H) I) was treated six months later with an FD. Indications for treatment with the Silk were defined as follows: (1) wide-necked saccular aneurysms (neck > 4 mm or neck/sac ratio > 0.7); (2) giant aneurysms (diameter > 25 mm) or aneurysms presenting with a mass effect; (3) fusiform aneurysms. In a first step the anatomy of potential perforators and the configuration of the aneurysm (sidewall or fusiform) were angiographically assessed, as this information was crucial for the indication for treatment with the Silk stent in our case series. In case of prior coil treatment and/or partially thrombosed aneurysms, we assessed the complete aneurysm size.
Silk stent morphology
Introduced to the European market in 2007, the Silk is a flexible and self-expanding device made of 48 braided strands derived from a nickel-titanium alloy (nitinol). It features flared ends and a sinusoidal radiopaque wire. Owing to its dense mesh with pore sizes of 110 to 250 µm, it provides a metal coverage of 35–55% of the target vessel’s inner surface at its nominal diameter. The available diameters range from 2 to 5 mm and the lengths vary between 15 and 40 mm.12–14
Target vessel morphology: selection criteria for the Silk
It is of critical importance to calculate the correct size of the FD. For this purpose Balt offers recommendations concerning the Silk’s appropriate length and diameter in relation to the target vessel. The potential shortening of the deployed Silk stent can be significant and has to be taken into account. One should calculate in such a way that the distal and proximal portions of the stent do at least overlap for 5 mm with the vessel wall in relation to the aneurysm neck; a bit more whenever possible to ensure the stent’s positional stability (apposition) might be useful.15
Endovascular procedure and Silk deployment
All treatments were performed via the femoral approach under general anesthesia. All neurovascular centers performed the endovascular treatment (EVT) on biplane flat panel digital subtraction units (Axiom Artis, Siemens, Erlangen, Germany). In a standardized procedure a 6F Neuron (Penumbra Inc, Alameda, CA, USA) or Fargo (Balt Extrusion) guiding catheter was coaxially introduced in a compatible shuttle sheath and positioned in the parent vessel (cavernous ICA or V3/V4 segment of the vertebral artery (VA), respectively). Then the aneurysm or its neck was passed with a Vasco (Balt Extrusion) microcatheter with the aid of a standard microwire (Silverspeed (eV3), Transend or Synchro-2 (Boston Scientific)) and positioned as far as reasonably possible in a straight portion of a distal branch. This is important, as the distal tip of the stent delivery wire may be traumatic. Then the Silk stent was inserted into the microcatheter, pushed beyond the aneurysm and positioned at the end of the microcatheter’s tip. In cases where the delivery of the Silk through the Vasco was prolonged because of difficult anatomic conditions, the microcatheter was flushed repeatedly with heparinized saline solution in order to avoid clogging of the stent meshes with fibrin. The distal tip of the delivery wire had to protrude from the microcatheter’s tip in a straight vessel portion. The microcatheter was withdrawn and one-third of the Silk unsheathed to allow for the funnel-like stent contacting the vessel wall. Then the whole system was carefully pulled back to the appropriate target-position and deployed completely by pulling the microcatheter while slightly pushing the delivery wire. The Silk resembled a crawling caterpillar during the maneuver, which was performed in a very slow manner compared to conventional intracranial stenting, because the Silk unfolds with an inherent mechanical inertia. If the Silk did not completely unfold after its release it was carefully passed with the Vasco microcatheter to complete the deployment. In cases where this maneuver was not sufficient, a remodeling balloon was used to ensure complete apposition of its meshes with the vessel wall.
Adjunctive coiling
On March 9, 2010, Balt Extrusion published an Urgent Field Safety Notice because of reports of fatal hemorrhages in eight patients treated with the Silk without adjunctive coil embolization. These aneurysms ranged between 18 and 35 mm. Balt stated that the Silk is intended for endovascular occlusion with the use of coils in the aneurysmal sac and that they did not promote the use of the Silk alone at that point of time. The company stressed that the Silk has to be associated with coiling in the treatment of large and giant aneurysms.
In the present study, adjunctive coiling was performed in part of the patient cohort. This was performed either as a “jailing-technique” whereby the coil-delivering microcatheter was secured between the parent vessel’s intima and the outer confines of the deployed FD, keeping the coils within the aneurysm and outside the reconstructed vessel lumen; or in a conventional manner by coiling the aneurysm-dome while sparing the neck and then applying the Silk stent. In two cases where there had been a previous treatment effort with an intracranial LEO stent (Balt Extrusion, Montmorency, France), an auxiliary coiling through the LEO’s stent meshes supplemented the preexisting coils.
Periprocedural antiplatelet regimen
Premedication was administered with clopidogrel and acetylsalicylic acid (ASA) as recommended by Balt, starting with a loading dose of 300 mg clopidogrel and 500 mg ASA at least five days prior to the EVT. Clopidogrel 75 mg/d and ASA 100 mg/d were continued for at least three months until the first control angiography. The further recommendations for antiplatelet medication depended on the result of the control angiography. In 2010 all study centers started with pre-interventional routine laboratory tests (Multiplate) for clopidogrel resistance, using prasugrel as an alternative in non-responders.
Assessment of clinical and angiographic outcome
A senior neurologist recorded the clinical course, including worsening of neurological symptoms and death. Clinical outcome was graded on admission and discharge and during FU according to the modified Rankin scale (mRS).
At the end of the Silk implantation as well as in the FU angiographic controls, the degree of flow modification in the aneurysm was assessed by the senior neurointerventionalists in relation to the pretreatment aneurysm volume according to the following grading: no change (if the contrast agent entered the aneurysm like prior to the FD placement); residual filling >50% (if the contrast agent filled >50% of the aneurysm sac); residual filling <50% (if the contrast agent filled <50% of the aneurysm sac); neck remnant and complete occlusion.
The basic routine FU scheme was set up as follows:
Three or six months (center’s decision): Clinical control (mRS). First angiographic control by digital subtraction angiography (DSA); computed tomography (CT) angiography or magnetic resonance (MR) angiography facultative.
Twelve months: Clinical control (mRS). Second angiographic control by DSA or alternatively CT or MR angiography in cases where a control exam had been obtained at three or six months.
Twenty-four months: Clinical control (mRS). DSA or CT or MR angiography.
Thirty-six months: Clinical control (mRS). DSA or CT or MR angiography.
In the routine FU intervals ≥12 months we tolerated a deviation of ± 3 months at each scheduled time-point for organizational reasons. Patients were encouraged to present to the corresponding center in case of any neurological symptoms. Thus, for some patients the (maximum) FU intervals differ from this scheme (see Table 1).
Table 1.
Immediate and late clinical and angiographical outcome.
| Patient no. | Clinical outcome |
Max. FU (months) | Angiographical outcome |
|||
|---|---|---|---|---|---|---|
| Admission | Discharge | Max. FU | Flow modification: immediate | Flow modification: max. FU | ||
| 1 | 3 | 3 | 0 (+) | 30 | Residual filling > 50% | Complete occlusion since eighth month, stable through FU |
| 2 | 3 | 3 | 6 (−) | 6 | Residual filling > 50% | Residual filling < 50% |
| 3 | 1 | 0 | 0 (+) | 9 | Residual filling < 50% | Complete occlusion |
| 4 | 0 | 0 | 0 (=) | 6 | Residual filling > 50% | Neck remnant |
| 5 | 1 | 1 | 1 (=) | 24 | Residual filling > 50% | Complete occlusion since ninth month, stable through FU |
| 6 | 1 | 2 | 1 (=) | 12 | Residual filling < 50% | Neck remnant |
| 7 | 2 | 2 | 2 (=) | 6 | Residual filling > 50% | Complication: complete ICA occlusion (asymptomatic), aneurysm obliterated through FU |
| 8 | 0 | 1 | 1 (−) | 12 | Complete occlusion | Complete occlusion (after recanalization on third month) |
| 9 | 1 | 1 | 1 (=) | 0 | Residual filling > 50% | n.a. |
| 10 | 1 | 1 | 1 (=) | 12 | Residual filling > 50% | Neck remnant (recanalized after complete occlusion at third month) |
| 11 | 3 | 3 | 3 (=) | 16 | Complete occlusion | Small, constant endoleak since third month fed by the VA; elective VA occlusion 10th month; stable since then |
| 12 | 0 | 0 | 0 (=) | 7 | Neck remnant | Complete occlusion since third month, stable through FU |
| 13 | 1 | 1 | 0 (+) | 36 | Residual filling < 50% | Residual filling < 50% since intervention, stable through FU |
| 14 | 0 | 0 | 0 (=) | 36 | No change | Complete occlusion since third month, stable through FU |
| 15 | 2 | 2 | 2 (=) | 12 | Complete occlusion | Complete occlusion |
| 16 | 0 | 0 | 0 (=) | 15 | Residual filling > 50% | Complete occlusion |
| 17 | 0 | 0 | 0 (=) | 10 | Residual filling > 50% | Neck remnant |
| 18 | 1 | 1 | 1 (=) | 29 | Residual filling > 50% | Complete occlusion since third month (stable through FU) |
| 19 | 0 | 0 | 0 (=) | 13 | No change | No change (stable through FU) |
| 20 | 0 | 0 | 0 (=) | 11 | Both residual filling > 50% | Both neck remnant |
| 21 | 0 | 0 | 0 (=) | 12 | Both no change | Both complete occlusion |
| 22 | 0 | 0 | 0 (=) | 3 | Residual filling < 50% | Neck remnant |
| 23 | 1 | 1 | 1 (=) | 15 | Complete occlusion | Complete occlusion |
| 24 | 2 | 2 | 6 (−) | 4 | Both residual filling > 50% | No data, patient deceased (myocardial infarction) |
| 25 | 1 | 1 | 0 (+) | 13 | Residual filling < 50% | Complete occlusion |
VA: vertebral artery; FU: follow-up; +: improved; =: stable; −: worsened..
Illustrative case (patient no. 8)
A 47-year-old female patient underwent MRI of the head for recurrent headaches; she had no other medical history. The exam depicted a paraophthalmic ICA sidewall aneurysm and she had a DSA (Figures 1 and 2). After careful evaluation and interdisciplinary discussion with the patient she opted for endovascular treatment of the lesion. Due to aneurysm morphology (complex shape, outer vessel curve location) we decided to loosely coil it before Silk placement; the final run after FD placement showed complete occlusion (Figure 3). A control DSA after three months (Figure 4) depicted a partial recanalization; the distal part of the FD had also intraluminal narrowing due to intimal hyperplasia. We kept the patient on double antiplatelets for 12 months; the final control angiography after 32 months revealed an occlusion of the formerly recanalized part of the aneurysm and regression of the intimal hyperplasia, leaving no relevant residual stenosis (Figure 5).
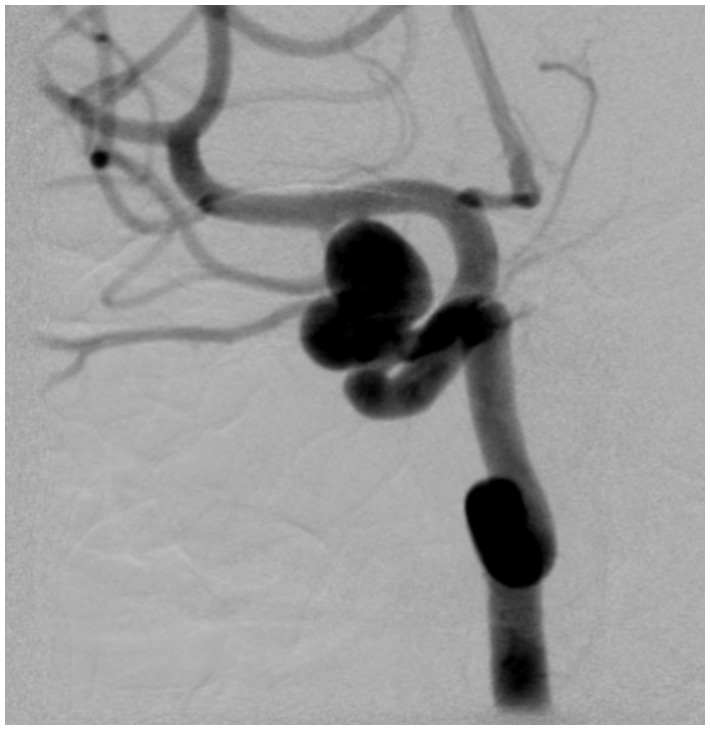
Figure 1.
Patient no. 8: LAO projection 45 degrees; pre-interventional status of the paraophthalmic ICA sidewall aneurysm. LAO: left anterior oblique; ICA: internal carotid artery.
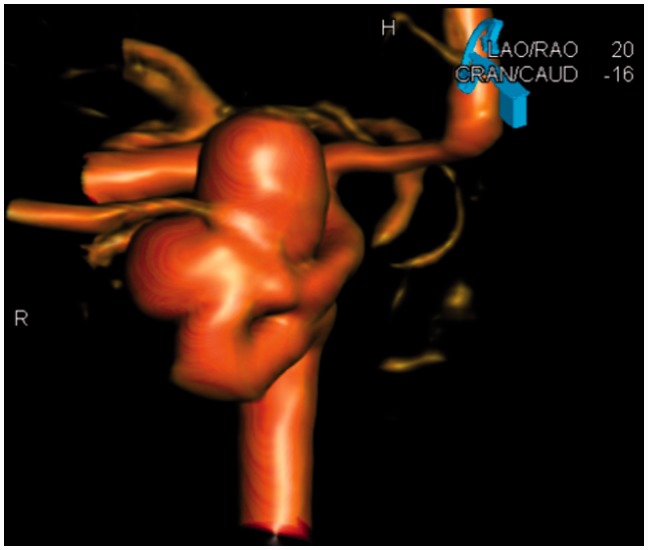
Figure 2.
Patient no. 8: 3D imaging, LAO projection 20 degrees; working projection for coiling prior to subsequent FD implantation. LAO: left anterior oblique; 3D: three-dimensional; FD: flow diverter.
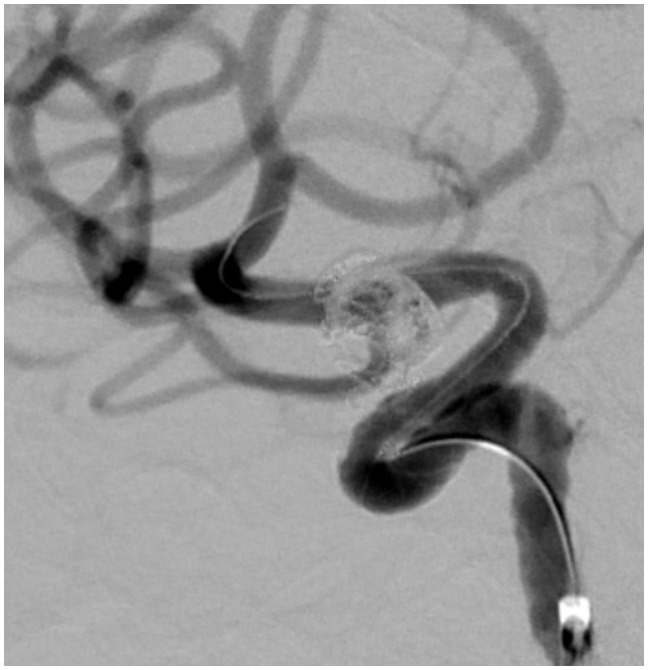
Figure 3.
Patient no. 8: LAO projection 50 degrees; control injection after coiling and FD placement showing complete occlusion of the aneurysm. LAO: left anterior oblique; FD: flow diverter.
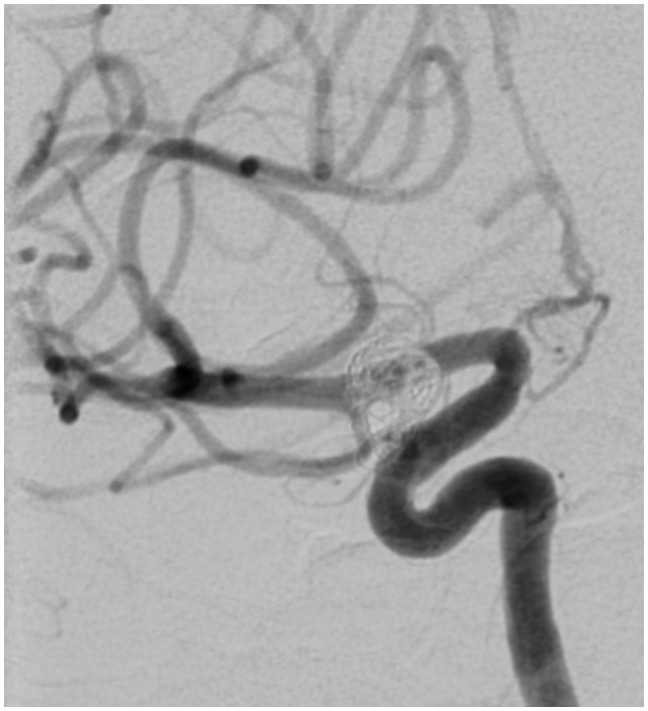
Figure 4.
Patient no. 8: FU DSA control after three months: LAO projection 50 degrees; aneurysm shows partial recanalization (bold open arrow). The distal part of the stent shows intraluminal narrowing (bold closed arrow) due to intimal hyperplasia (small arrow). FU: follow-up; DSA: digital subtraction angiography; LAO: left anterior oblique.
Figure 5.
Patient no. 8: FU DSA control after 32 months: LAO projection 50 degrees; complete occlusion of the formerly recanalized part of the aneurysm; regression of the intimal hyperplasia, leaving no relevant residual stenosis. FU: follow-up; DSA: digital subtraction angiography; LAO: left anterior oblique.
Results
Patient cohort
Overall 28 FDs were implanted in 25 patients treating 28 aneurysms. There were 19 women and six men with a mean age of 63 years, ranging from 28 to 88 years. The clinical and angiographic data of the patients and aneurysms are summarized in Table 2.
Table 2.
Aneurysm characteristics and clinical presentation.
| Patient no. | Age | Clinical presentation | Aneurysm morphology |
Silk stent diameter/ length | Coiling | ||
|---|---|---|---|---|---|---|---|
| Size neck/ sac (mm) | Location | Type | |||||
| 1 | 56 | Extensive loss of sight (mass effect on chiasma, CN. II) | 10/25 | Cavernous ICA (C4) | Sidewall, saccular | 4 × 40 | j |
| 2 | 49 | Fluctuating vision impairment, previous LEO and coiling recanalized | –/30 | VA; V3–V4 intersection | Fusiform | 4.5 × 50 4.5 × 40 4.5 × 35 | p and a |
| 3 | 49 | Cranial nerve palsy (CN. III) | 20/30 | Cavernous ICA (C4) | Sidewall, saccular | 3.5 × 35 | j |
| 4 | 69 | Incidental, previous coiling recanalized | 14/15 | Cavernous ICA (C4) | Sidewall, saccular | 3.5 × 35 | p |
| 5 | 53 | Cranial nerve palsy (CN. VI) | 15/25 | Cavernous ICA (C4) | Sidewall, saccular | 3.5 × 30 | No |
| 6 | 70 | Progressive vision impairment and diplopia (CN. II and III) | 7/25 | Supraophthalmic ICA (C6) | Sidewall, saccular | 3.5 × 30 | No |
| 7 | 69 | Cranial nerve palsy (CN. III and VI) | 12/29 | Cavernous ICA (C4) | Sidewall, saccular | 4.5 × 35 | No |
| 8 | 47 | Intermittent headaches | 7/18 | Paraophthalmic ICA (C6) | Sidewall, saccular | 4 × 20 | p |
| 9 | 88 | Cranial nerve palsy (CN. III) | –/17 | Cavernous ICA (C4) | Fusiform | 5.5 × 40 5.5 × 30 | No |
| 10 | 76 | Cranial nerve palsy (CN. VI) | 10/18 | Cavernous ICA (C4) | Sidewall, saccular | 4.5 × 40 | No |
| 11 | 39 | Psychosis | –/18 | BA | Fusiform | 5.5 × 60 | No |
| 12 | 28 | Intermittent headaches | 3/55 | Communicating ICA (C7) | Sidewall, saccular | 4 × 25 | No |
| 13 | 71 | Stroke (aneurysm-related embolic shower) | 8/25 | Cavernous ICA (C4) | Sidewall, saccular | 4.5 × 40 | j |
| 14 | 60 | V4-dissection with previous SAH | –/5 | VA; V4 | Fusiform | 3.5 × 25 | No |
| 15 | 70 | Stroke (mass effect on M2) | 7/22 | MCA; M1–M2 intersection | Sidewall, saccular | 2.5 × 25 | j |
| 16 | 54 | Intermittent headaches | 6/4 | Paraophthalmic ICA (C6) | Sidewall, saccular | 4 × 25 | no |
| 17 | 60 | Incidental | 10/25 | Paraophthalmic ICA (C6) | Sidewall, saccular | 3.5 × 25 | p |
| 18 | 61 | Incidental, previous contra-lateral stroke | 8/13 | Supraophthalmic ICA (C6) | Sidewall, saccular | 4 × 35 | j |
| 19 | 77 | Incidental | 7/12 | Communicating ICA (C7) | Sidewall, saccular | 3.5 × 30 | p |
| 20 | 69 | Intermittent headaches | First 6/4 Second 3/4 | Both paraophthalmic (C6) | Both sidewall, saccular | 4 × 30 | No |
| 21 | 59 | Transient hemi-anesthesia | First 6/11 Second 4/3 | First ICA/PcomA junction Second communicating ICA (C7) | Both sidewall, saccular | 5.5 × 60 covering both aneurysms | First p Second no |
| 22 | 72 | Incidental, diabetic cranial nerve palsy (CN. VI) | 4/26 | Paraophthalmic ICA (C6) | Sidewall, saccular | 4.5 × 25 | a |
| 23 | 71 | Intermittent headaches, previous LEO and coiling recanalized | 10/20 | Supraophthalmic ICA (C6) | Sidewall, saccular | 3.5 × 25 | p and a |
| 24 | 76 | First-time seizure (mass effect) | First 5/10 Second 7/32 | First cavernous ICA (C4) Second paraophthalmic ICA (C6) | Sidewall, saccular | 4.5 × 40 covering both aneurysms | First no Second a |
| 25 | 61 | Cranial nerve palsy (CN. III) | 6/12 | Cavernous ICA (C4) | Sidewall, saccular | 4.5 × 25 | j |
j: jailing; p: previous coiling; a: auxiliary coiling in same session before Silk deployment; no: no adjunctive coiling; ICA: internal carotid artery; MCA: middle cerebral artery; PcomA: posterior communicating artery; BA: basilar artery; CN: cranial nerve; VA: vertebral artery; SAH: subarachnoid hemorrhage; LEO: LEO stent (Balt Extrusion, Montmorency, France).
Aneurysm distribution and morphology
Pertaining to the vascular territories, 22 patients with 25 aneurysms were treated in the anterior circulation and three patients with three aneurysms in the posterior circulation. A total of 24/25 anterior circulation aneurysms were located in the ICA (10 in the cavernous (C4), 11 in the ophthalmic (C6) and three in the communicating (C7) segment); there was one middle cerebral artery (MCA) aneurysm in the M1/M2 intersection. Two of three posterior circulation aneurysms were VA aneurysms (V3/V4 intersection and V4 segment); one was a basilar artery (BA) aneurysm.
Twenty-three of 24 ICA aneurysms were saccular sidewall aneurysms; only one of 24 was of the fusiform type. All posterior circulation aneurysms (n = 3) were fusiform ones. There was no bifurcation aneurysm in the present cohort.
Except for the fusiform aneurysms, the mean aneurysm neck size was 7.3 mm (range of 4 to 15 mm). The mean aneurysm diameter was 19 mm (range of 3 to 55 mm).
Technical success and complications of the EVT
The Silk deployment was successfully performed in all 25 patients. Of the 28 aneurysms treated, four were recurrences after previous treatment with coiling alone in two and in conjunction with a LEO stent in another two cases. Among these 25 patients we observed seven (28%) adverse events in the acute phase during hospitalization; four of seven resulted in new clinical deficits. Overall, in 10/25 (40%) patients the initial apposition of the FD was not satisfactory. In nine of 10 cases we decided for an adjunctive PTA, which led in all cases to a correct apposition of the FD. However, we noticed in one case that PTA led to a significant shortening of the Silk, leading almost to an uncovering of one of the treated aneurysms.
FD alone vs. adjunctive coiling
Thirteen of 28 aneurysms were treated by FD placement alone (11 ICA, one VA and one BA). The remainder were treated by a combination of FD placement and coiling, featuring different approaches in terms of timing and applied coiling technique (Tables 2 and 3).
Table 3.
Peri-interventional and late complications.
| Patient no. | Stent thrombosis | PA stenosis | Sidebranch occlusion | Distal embolism | Mass effect/ hemorrhage |
|---|---|---|---|---|---|
| 2 | 6 months, deceased | Acute, increased mass effect with hydrocephalus | |||
| 6 | Acute, ischemia in caput of caudate nucleus | ||||
| 7 | 24 hours, ICA occlusion, asymptomatic | ||||
| 8 | 3 months, mid-grade in-stent stenosis, asymptomatic; after 12 months no residual stenosis | Acute, loss of 3/4 of visual field (ophthalmic artery) | |||
| 12 | 3 months, distal intimal hyperplasia, asymptomatic; stable after 7 months | ||||
| 15 | Acute, MCA occlusion, PTA restoring flow, asymptomatic | 12 months, mild MCA stroke without permanent deficit, new fusiform aneurysm in PTA location | |||
| 22 | Acute, embolic shower, asymptomatic | ||||
| 23 | Acute, embolic shower, transient facial hemi-anesthesia | ||||
| 25 | 3 months, proximal mid-grade in-stent-stenosis, asymptomatic; 13 months only residual stenosis; 20 months no stenosis | ||||
| Total | 3 | 3 | 1 | 4 | 1 |
PA: pulmonary artery; ICA: internal carotid artery; MCA: middle cerebral artery; PTA: percutaneous transluminal angioplasty.
Immediate outcome
In terms of mRS, the immediate clinical outcome at discharge was unchanged in 23/25 and worse in two of 25 patients due to procedural complications (patients no. 6 and no. 8). The overall immediate angiographic results at discharge comprised four aneurysms (14%) with complete occlusions, one (4%) with a neck remnant, five (18%) with residual filling <50%, 14 (50%) with residual filling >50%, and four (14%) where the inflow remained unchanged compared to the pre-interventional status. Regarding the subgroups with and without adjunctive coiling and patients with a mass effect (n = 10), the angiographic results are summarized in Table 4.
Table 4.
Subgroups: Aneurysms treated by FD placement alone vs. FD and adjunctive coiling.
| FD alone |
FD with coiling |
|||
|---|---|---|---|---|
| Angiographic result | Immediate (n = 13) | Max. FU (n = 10)a | Immediate (n = 15) | Max. FU (n = 14)b |
| Unchanged | 2 | – | 2 | 1 |
| Residual filling > 50% | 8 | – | 6 | - |
| Residual filling < 50% | 1 | – | 4 | 2 |
| Neck remnant | 1 | 4 | – | 3 |
| Complete | 1 | 6 | 3 | 8 |
The numbers given in brackets display the respective results for the 10 patients suffering from a mass effect.
n: number of aneurysms; FU: follow-up; ICA: internal carotid artery; FD: flow diverter; BA: basilar artery; PAO: parent artery occlusion; VA: vertebral artery.
For two patients no FU was available. One patient was excluded (case 7) because of early asymptomatic ICA occlusion based on in-stent thrombosis of the FD. Case no. 11, a fusiform BA aneurysm, was included as the VA-fed endoleak detected during FU was successfully treated by PAO of the VA.
For one patient no FU was available.
Complications after hospitalization
Complications after hospitalization occurred in five patients: one death due to in-stent thrombosis under continuous ASA treatment, clopidogrel had been stopped after three months as advised, and one new fusiform aneurysm formation due to intra-interventional parent vessel injury, leading to an embolic shower with mild hemiparesis and three asymptomatic parent artery stenoses that resolved after 12 months without sequalae. We observed no SAH and no displacement of the FD in the peri- and postinterventional course.
FU
All patients except for one agreed to participate in the FU. The mean FU interval was 14.9 months (total: 357 patient-months). Sixteen patients were followed ≥12 months and five patients ≥24 months.
Initial angiographic control
Twenty-three of 24 patients (96%) had the initial control DSA after three or six months, respectively. These initial postinterventional angiographies showed improvement of the anatomic outcome in 14/23 (61%), no change in eight of 23 (35%) and an increased inflow in one of 23 (4%) aneurysms; one patient (no. 7) with an ICA occlusion due to in-stent thrombosis remained stable and in case no. 11 the control DSA uncovered an endoleak.
Anatomic outcome during FU
Final anatomic outcome in 24 aneurysms of 22 patients available for FU (Table 4) included 14 (59%) with complete occlusion, seven (29%) with a neck remnant, two (8%) with residual filling <50%, none with residual filling >50% and one (4%) unchanged in comparison to its pretreatment status. Of the four of 28 aneurysms with immediate complete occlusion, only two (nos. 15 and 23) remained stable throughout the FU; case no. 8 presented with a recanalization after three months combined with an in-stent stenosis, whereby both pathological findings resolved in nine months, as shown by DSA. The other case (no. 11) developed an endoleak after three months. A parent artery occlusion (PAO) of the feeder resolved this complication and the fusiform BA aneurysm stabilized to a complete occlusion during FU. There was one more case (no. 10) with a recanalization at six months, after showing a complete occlusion in the first DSA control after three months. In the present cohort all observed changes in anatomic outcome took place within six months; none of the 21 aneurysm patients who were followed for more than six months showed any change in their anatomic outcome after six months.
Anatomic outcome: Subgroups FD alone vs. adjunctive coiling
Table 4 summarizes the late results for these subgroups: Six of 10 (60%) aneurysms treated with FD alone were completely occluded at their individual maximum FU interval. This was also true for eight of 14 (57%) aneurysms with adjunctive coiling.
Postinterventional recanalizations were seen in three of 13 (23%) aneurysms treated with FD alone; none were observed in 15 aneurysms treated with FD and coiling.
Overall clinical outcome
As summarized in Table 3, the overall rate of adverse effects during hospitalization and FU was 48% (12/25). Two of 25 patients suffered from an immediate procedure-related, persistent neurological deficit, resulting in a procedural morbidity of 8%. Two of 24 patients died during FU; one patient (no. 2) died from a procedure-related complication (in-stent thrombosis) after six months, resulting in a 4% procedure-related mortality. The other patient (no. 24) died from myocardial infarction at the fourth postinterventional month before having any control exam. Final clinical outcome in 22 patients in terms of mRS included 13 with mRS = 0, six with mRS = 1, two with mRS = 2 and one with mRS = 3. Compared to their individual pretreatment status, four patients improved, 18 remained unchanged and one patient worsened because of a procedural complication.
Discussion
The mid- and long-term outcome in patients treated by flow diversion with and without adjunctive coiling and potential adverse effects of this therapy are not yet well known and understood; especially the understanding of the pathophysiology of late ruptures, which have been reported for the Silk and the Pipeline device,7,16–20 is fragmentary, except for cases with FD migration into the aneurysm sac, where the underlying etiology is hemodynamic.7,13 Owing to reports of fatal hemorrhages Balt Extrusion published an Urgent Field Safety Notice on March 9, 2010, recommending the use of coils in conjunction with the Silk, especially in giant aneurysms. This has not led to a change in paradigm yet, as the majority of published Silk cases are still treated without adjunctive coiling.7,16,21 The vast majority of published Pipeline cases are also treated without adjunctive coiling.17,19 Interestingly, all reported late ruptures were observed in aneurysms treated with FD placement alone; Szikora et al. reported their experience with the Pipeline in 19 cases with nearly half of them with coiling observed to have no bleedings in six months.22 The same observation was recently reported by Maimon et al. in a series of 28 patients with FU of two years, where coils were used in aneurysms with a diameter ≥15 mm.21 These findings are supported by a Silk study by Berge et al., where delayed rupture was seen in three of 77 aneurysms, all of them treated without coils;16 as only 13% of aneurysms were treated with coils though, this finding was not significant. The results of the present study are ambiguous in light of these reports as we observed neither procedural nor delayed ruptures in the subgroups with and without adjunctive coiling, whereby patient numbers and FU intervals were comparable. Unlike Maimon et al., we did not refrain from using coils in aneurysms smaller than 15 mm in diameter; this was true in four patients, the smallest diameter being 11 mm.
Technically, a misdeployment or a poor apposition is still a concern in FD treatment. This is true for both devices, Pipeline and Silk.16,17 These instances may be corrected by the additional use of a balloon or a stent. The reported rates of poor apposition range between 0 and 17%.13,16,17,19,21,23–27 In the present cohort we saw this phenomenon in 40% of cases. An adjunctive PTA led to a correct apposition of the Silk in all cases. This is not without potential side effects though, as we noticed that the PTA led to a significant contraction of the Silk in one case, leading almost to an uncovering of one of the treated aneurysms. Persistent misdeployment may be an important factor in acute PAO peri-interventionally, despite a proper antiplatelet regimen; the reported rates vary between 4% and 10% for the Silk and between 0 and 2.4% for the Pipeline.13,16,17,19,22–24
It has been reported that FD placement does not provide immediate aneurysm occlusion in the majority of cases.7,13,16,17,19,21–23 Fischer et al. experienced in a large series of 101 treated aneurysms or dissections that the Pipeline device did not lead to an immediate complete occlusion in any of their cases.17 Concurrently, in our study we observed an immediate complete occlusion of the treated aneurysms in 14% of cases; the majority of 64% showed a residual filling of ≥50% or remained unaltered. As depicted in Table 4, there might be a favorable tendency in our cohort for aneurysms treated with adjunctive coiling, although patient numbers are too small for any statistically relevant effect. A mid-term FU of all patients treated by FD placement is mandatory, because aneurysm occlusion seems to be a progressive process. Pertaining to the Silk, between 50% and 68% of aneurysms were completely occluded after three to six months;7,16,23,24 the respective reported occlusion rates for the Pipeline range between 52% and 94% at six months.17,19,22 In our cohort the rate of complete occlusions at three to six months was 36%, which is lower than in the literature; a potential explanation may be that we performed a DSA rather than MRI or CT in all but one patient who refused, leading probably to a higher detection rate of incomplete occlusions. We also found one recanalization and one endoleak within three months after FD placement in previously completely occluded aneurysms and a recanalization of an aneurysm after six months, which had been completely occluded in the first DSA control after three months. These recanalizations occurred only in aneurysms treated without adjunctive coiling. There is a case report in the literature supporting our findings, thus increasing the number of observed recanalizations to four cases.25 In contrast to our experience, this other aneurysm presented by Wong et al. had previously been treated with coils, but showed a recurrence after one year, leading to the FD treatment. In conclusion, the question whether the absence of coils plays a role in recanalizations after FD placement cannot be properly answered because of small numbers; late recanalizations do though exist, although a current meta-analysis of major FD case series reported none.26
We would like to stress that in the present cohort all observed changes in anatomic outcome took place within six months; none of the 21 aneurysm patients who were followed for more than six months showed any change in their anatomic outcome after six months. Interestingly, the aforementioned recanalization reported by Wong et al. was observed three months after FD placement and had disappeared after six months. Based on these findings, we recommend that all patients treated with FD placement should be clinically and angiographically followed for at least six, preferably 12, months. Within six months, more than 60% of aneurysms improved in their respective occlusion rate, leading to 59% of complete occlusions at latest FU (mean FU interval 14.9 months, range 4–36 months) and 29% with only neck remnants. The rate of complete occlusion in our cohort is lower than in the literature, which ranges between 79 and 85% for Silk cases and between 74% and 95% for the Pipeline cases, respectively, in studies with comparable mid-term FU intervals.16,17,19,21,22 We found no obvious differences in occlusion rates at maximum FU between aneurysms treated with and without adjunctive coiling; this finding is supported by the data of Szikora et al., who had a distribution of aneurysms with and without adjunctive coiling similar to our cohort.
Conclusions
Flow diversion is a useful extension of therapeutic options in complex aneurysms; their anatomic presentation and location are key factors for successful FD treatment. Its potential for a regression of symptoms caused by aneurysmal mass effects is not dependent on the omission of adjunctive coiling. In the mid-term the occlusion rate does not seem to be influenced by the presence of coils, which may not be true in the early postinterventional phase. A rigorous monitoring concept postinterventionally for at least six months is mandatory, as the anatomic outcome is not reliably predictable; recanalizations may take place even in previously completely occluded aneurysms.
Key points
Flow-diverter treatment is technically feasible but challenging. Thus proper patient selection is of paramount importance, as complication rates are higher compared to conventional techniques.
Late recanalization can be observed during long-term follow-up. Thus a follow-up interval of at least six months is recommended, as the anatomic outcome is not reliably predictable.
Strengths
Strengths of the study are its multicentric design with prospectively collected data based on standardized treatment protocols and an antiplatelet regimen. Patients had DSAs for follow-up.
Limitations
Limitations of the study include a retrospective analysis of data. Some patients were treated previously with conventional stent and/or coiling. The sample size is too small to allow for definitive statistical analysis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Guglielmi G. The beginning and the evolution of the endovascular treatment of intracranial aneurysms: From the first catheterization of brain arteries to the new stents. J Neurointerv Surg 2009; 1: 53–55. [DOI] [PubMed] [Google Scholar]
- 2.Thornton J, Debrun GM, Aletich VA, et al. Follow-up angiography of intracranial aneurysms treated with endovascular placement of Guglielmi detachable coils. Neurosurgery 2002; 50: 239–249. discussion 249–250. [DOI] [PubMed] [Google Scholar]
- 3.Weber W, Siekmann R, Kis B, et al. Treatment and follow-up of 22 unruptured wide-necked intracranial aneurysms of the internal carotid artery with Onyx HD 500. AJNR Am J Neuroradiol 2005; 26: 1909–1915. [PMC free article] [PubMed] [Google Scholar]
- 4.Izar B, Rai A, Raghuram K, et al. Comparison of devices used for stent-assisted coiling of intracranial aneurysms. Plos One 2011; 6: e24875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kis B, Weber W, Berlit P, et al. Elective treatment of saccular and broad-necked intracranial aneurysms using a closed-cell nitinol stent (Leo). Neurosurgery 2006; 58: 443–450. discussion 443–450. [DOI] [PubMed] [Google Scholar]
- 6.Felber S, Henkes H, Weber W, et al. Treatment of extracranial and intracranial aneurysms and arteriovenous fistulae using stent grafts. Neurosurgery 2004; 55: 631–638. discussion 338–339. [DOI] [PubMed] [Google Scholar]
- 7.Leonardi M, Cirillo L, Toni F, et al. Treatment of intracranial aneurysms using flow-diverting silk stents (BALT): A single centre experience. Interv Neuroradiol 2011; 17: 306–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pumar JM, Castiñeira JA, Vazquez F, et al. Exclusion of a cavernous aneurysm by leo stent. Interv Neuroradiol 2006; 12: 57–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vanninen R, Manninen H, Ronkainen A. Broad-based intracranial aneurysms: Thrombosis induced by stent placement. AJNR Am J Neuroradiol 2003; 24: 263–266. [PMC free article] [PubMed] [Google Scholar]
- 10.Weber W, Bendszus M, Kis B, et al. A new self-expanding nitinol stent (Enterprise) for the treatment of wide-necked intracranial aneurysms: Initial clinical and angiographic results in 31 aneurysms. Neuroradiology 2007; 49: 555–561. [DOI] [PubMed] [Google Scholar]
- 11.Augsburger L, Farhat M, Reymond P, et al. Effect of flow diverter porosity on intraaneurysmal blood flow. Klin Neuroradiol 2009; 19: 204–214. [DOI] [PubMed] [Google Scholar]
- 12.Kulcsár Z, Ernemann U, Wetzel SG, et al. High-profile flow diverter (silk) implantation in the basilar artery: Efficacy in the treatment of aneurysms and the role of the perforators. Stroke 2010; 41: 1690–1696. [DOI] [PubMed] [Google Scholar]
- 13.Lubicz B, Collignon L, Raphaeli G, et al. Flow-diverter stent for the endovascular treatment of intracranial aneurysms: A prospective study in 29 patients with 34 aneurysms. Stroke 2010; 41: 2247–2253. [DOI] [PubMed] [Google Scholar]
- 14.Wong GK, Kwan MC, Ng RY, et al. Flow diverters for treatment of intracranial aneurysms: Current status and ongoing clinical trials. J Clin Neurosci 2011; 18: 737–740. [DOI] [PubMed] [Google Scholar]
- 15.Binning MJ, Natarajan SK, Bulsara KR, et al. SILK flow-diverting device for intracranial aneurysms. World Neurosurg 2011; 76: 477.e1-e6. [DOI] [PubMed] [Google Scholar]
- 16.Berge J, Biondi A, Machi P, et al. Flow-diverter silk stent for the treatment of intracranial aneurysms: 1-year follow-up in a multicenter study. AJNR Am J Neuroradiol 2012; 33: 1150–1155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fischer S, Vajda Z, Aguilar Perez M, et al. Pipeline embolization device (PED) for neurovascular reconstruction: Initial experience in the treatment of 101 intracranial aneurysms and dissections. Neuroradiology 2012; 54: 369–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kulcsár Z, Houdart E, Bonafe A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 2011; 32: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lubicz B, Collignon L, Raphaeli G, et al. Pipeline flow-diverter stent for endovascular treatment of intracranial aneurysms: Preliminary experience in 20 patients with 27 aneurysms. World Neurosurg 2011; 76: 114–119. [DOI] [PubMed] [Google Scholar]
- 20.Turowski B, Macht S, Kulcsár Z, et al. Early fatal hemorrhage after endovascular cerebral aneurysm treatment with a flow diverter (SILK-Stent): Do we need to rethink our concepts? Neuroradiology 2011; 53: 37–41. [DOI] [PubMed] [Google Scholar]
- 21.Maimon S, Gonen L, Nossek E, et al. Treatment of intra-cranial aneurysms with the SILK flow diverter: 2 years’ experience with 28 patients at a single center. Acta Neurochir (Wien) 2012; 154: 979–987. [DOI] [PubMed] [Google Scholar]
- 22.Szikora I, Berentei Z, Kulcsár Z, et al. Treatment of intracranial aneurysms by functional reconstruction of the parent artery: The Budapest experience with the pipeline embolization device. AJNR Am J Neuroradiol 2010; 31: 1139–1147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Byrne JV, Beltechi R, Yarnold JA, et al. Early experience in the treatment of intra-cranial aneurysms by endovascular flow diversion: A multicentre prospective study. Plos One 2010; 5: 9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: The Buenos Aires experience. Neurosurgery 2009; 64: 632–642. discussion 642–643; quiz N6. [DOI] [PubMed] [Google Scholar]
- 25.Wong GK, Yu SC, Siu DY, et al. Recanalization with subsequent near-total occlusion of an internal carotid artery aneurysm after immediate thrombotic occlusion using a flow-diverting stent. J Neurosurg 2012; 116: 888–891. [DOI] [PubMed] [Google Scholar]
- 26.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 27.Cirillo L, Leonardi M, Dall’olio M, et al. Complications in the treatment of intracranial aneurysms with silk stents: An analysis of 30 consecutive patients. Interv Neuroradiol 2012; 18: 413–425. [DOI] [PMC free article] [PubMed] [Google Scholar]