Abstract
Epiglottic masses may be cystic, granulomatous, infectious, benign or malignant neoplastic, or manifestations of a systemic disease. When large in size, the airway may become obstructed, and when accompanied by suspicious features such as cartilaginous invasion, extension to the pre-epiglottic or para-glottic spaces, or lymphadenopathy, the radiologist must consider malignancy as a primary differential diagnosis. However, when only benign features are identified, the differential diagnosis is broad. We present a 65-year-old female with an incidental 1 cm exophytic, pedunculated, papillomatous lesion on the laryngeal surface of the epiglottis discovered upon endoscopic evaluation for dyspepsia and heartburn. Because of her risk factors for malignancy, CT scan was requested and revealed only benign features. Subsequent excisional biopsy revealed a benign squamous papilloma; however, multiple additional differential considerations were entertained preoperatively.
Keywords: epiglottis, CT imaging, larynx, mass
Introduction
Symptoms related to laryngeal lesions are broad and may include hoarseness, dysphagia, odynophagia, globus, and difficulty breathing, among others.1–6 According to the National Institute for Health Care Excellence (NICE) guidelines, which are based on expert committee reports, opinions, and clinical experience of respected authorities, patients with such symptoms should be referred to a head and neck specialist for evaluation.7 Evaluation typically encompasses a full history and physical exam, including direct visualization of the larynx by laryngoscopy.8,9 When an epiglottic lesion is seen on laryngoscopy, the treatment plan may include operative or nonoperative options depending on the patient’s symptoms and the size of the lesion. While small, benign-appearing lesions may be amenable to nonoperative management, excision may be elected for masses with indeterminate features and for larger masses causing laryngeal obstruction.1,4,10
Cross-sectional imaging by computed tomography (CT) or magnetic resonance imaging (MRI) tends to be reserved for clinically malignant or possibly malignant lesions, in order to further characterize the process and the extent of involvement.1 In our experience, benign-appearing epiglottic masses tend to be encountered by the radiologist as incidental findings on a cross-sectional study performed for another reason, or for indeterminate findings on physical exam.
The purpose of this paper is to introduce the radiologist to the broad differential diagnosis of polypoid epiglottic masses. We describe a patient with a benign squamous papilloma of the epiglottis and discuss the clinical presentation and imaging findings in a vast array of epiglottic masses, providing clues to narrow the differential diagnosis, and instilling the importance of diagnosis as it may affect clinical management.
Case report
A 65-year-old female was referred to a gastroenterologist for a several-month history of dyspepsia and heartburn. Upper endoscopy revealed a non-bleeding erosion in the stomach with biopsy yielding mild chronic gastritis, and an incidental medium-sized polypoid mass on the epiglottis for which she was referred to an otolaryngologist. The patient denied sore throat, difficulty swallowing, voice changes, or pain in the neck or referred to her ears. She reported a past history of heavy alcohol intake and smoking one pack of cigarettes daily for 30 years but quit smoking 12 years ago and has since reduced her alcohol intake. Fiberoptic laryngeal exam revealed a 1 cm exophytic, pedunculated, papillomatous lesion close to midline on the laryngeal surface of the suprahyoid epiglottis. The clinical differential diagnosis included a benign papilloma or a papillomatous-appearing, well-differentiated laryngeal malignancy. Although benign-appearing, the patient’s tobacco history risk factor was sufficient to warrant a CT scan to exclude deeper involvement of possible malignancy, as up to 95% of patients with laryngeal cancer have a history of tobacco use.11 CT did not show any malignant features, and the patient thereafter underwent excisional biopsy of the lesion at its base of attachment using a CO2 laser, with pathologic examination revealing a benign squamous papilloma. At the time of surgery, the lesion appeared superficial without apparent involvement of the underlying cartilage (Figure 1).
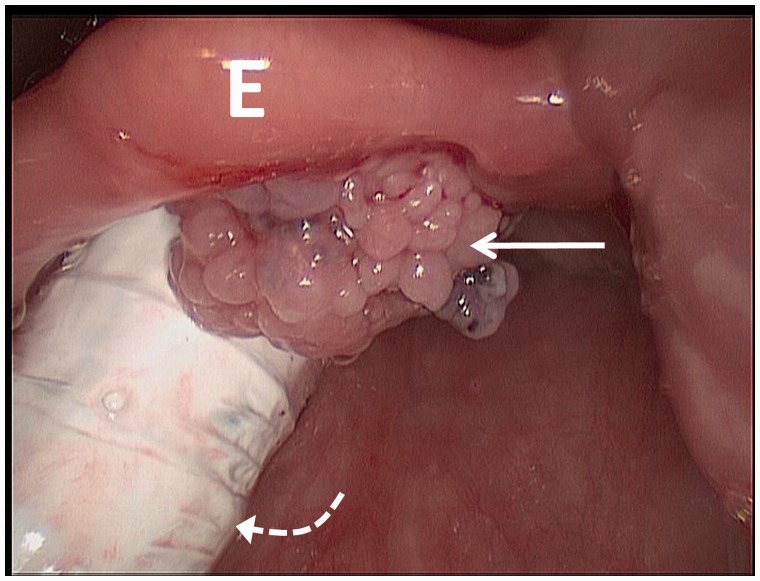
Figure 1.
Intra-operative photograph from laryngoscopy reveals an approximately 1 cm exophytic, pedunculated, papillomatous lesion (arrow) on the laryngeal surface of the suprahyoid epiglottis (E), close to midline, without apparent involvement of the underlying cartilage. The endotracheal tube is seen posteriorly (curved dotted arrow).
Imaging findings
CT scan revealed a 0.7 cm × 0.5 cm × 0.5 cm (transverse × anterior-posterior × craniocaudal) circumscribed structure of approximately 50 Hounsfield units, inseparable from the posterior (laryngeal) surface of the right parasagittal epiglottis (Figure 2). The epiglottis was not thickened. The remainder of the supraglottic larynx, including the aryepiglottic folds, false vocal cords, vestibule, pre-epiglottic and para-epiglottic spaces, and the arytenoid cartilages, appeared normal.11 The glottic larynx, including the true vocal cords and anterior and posterior commissures, and the subglottic larynx, extending from the inferior aspect of the true vocal cords to the inferior aspect of the cricoid cartilage, both appeared normal. Of note, while the radiological definition of the subglottic larynx is simply inferior to the lower margin of the true vocal folds, the surgical definition of the subglottic larynx is 1 cm below the true vocal cords anteriorly and 1.5 cm below the true vocal cords posteriorly (personal correspondence).
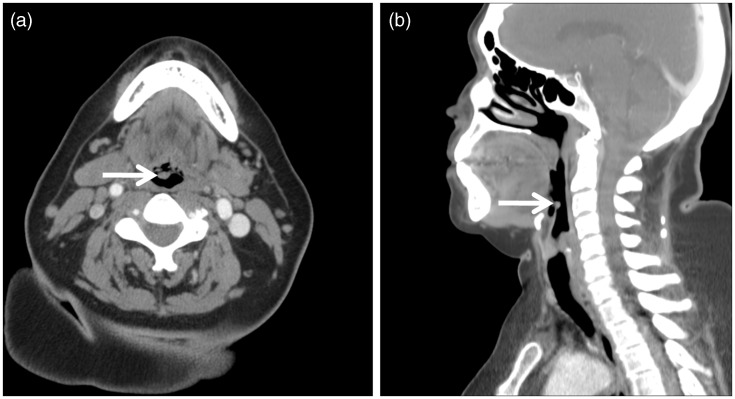
Figure 2.
Axial (a) and sagittal (b) contrast-enhanced computed tomography (CT) images of our 65-year-old female patient revealed a 0.7 cm × 0.5 cm × 0.5 cm (transverse × anterior-posterior × craniocaudal) circumscribed structure (arrow) of approximately 50 Hounsfield units, inseparable from the posterior (laryngeal) surface of the right parasagittal epiglottis. The epiglottis was not thickened, and the remainder of the larynx appeared normal.
Pathologic findings
The resection margin was inked black, and the entire specimen was submitted for histologic examination. The papillary cauliflower-appearing excisional biopsy specimen measured 0.8 cm × 0.8 cm × 0.6 cm. Microscopy revealed a benign lesion composed of fibrovascular cores covered by mature keratinizing stratified squamous epithelium (Figure 3). No epithelial dysplasia, atypical mitoses, or areas suspicious of invasion were seen.
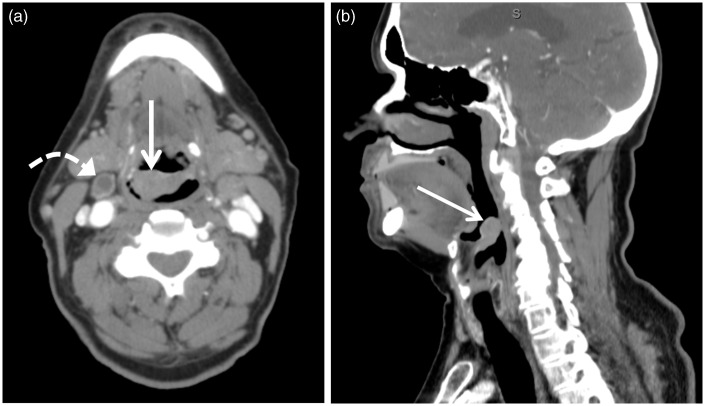
Figure 4.
Axial (a) and sagittal (b) contrast-enhanced computed tomography (CT) images of a 73-year-old male patient with foreign body sensation revealed a 2.6 cm × 1.5 cm × 2.0 cm (transverse × anterior-posterior × craniocaudal) epiglottic mass (arrow) of approximately 70 Hounsfield units biopsy proven squamous cell carcinoma. A necrotic lymph node was also identified (curved arrow).
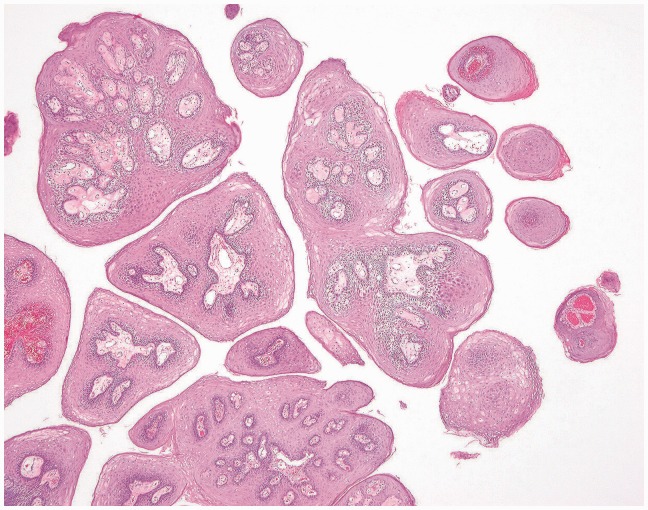
Figure 3.
Low-power magnification showing central fibrovascular cores covered by benign keratinizing squamous epithelium.
Discussion
Masses of the epiglottis may be cystic, granulomatous, infectious, neoplastic, or manifestations of a systemic disease.1 The radiologist may greatly contribute to patient care by being able to narrow the differential diagnosis. Acute epiglottitis is an emergency diagnosis, which is often made on a clinical basis, as patients may present with rapid-onset stridor and dysphagia with a high fever, and clinically appear toxic and may be drooling due to the inability to swallow. CT findings include an enlarged and edematous epiglottis possibly with edema of the entire supraglottic larynx.12 As we are concerned with the differential diagnosis of epiglottic masses and not diffuse epiglottic thickening, we will not consider etiologies that traditionally involve epiglottic thickening such as radiation treatment or acute epiglottitis. Nevertheless, if an epiglottic mass is identified concurrently with diffuse epiglottic thickening, the radiologist must consider the possibility of a superimposed acute epiglottitis and convey this to the treating physician.13
Benign neoplastic and non-neoplastic proliferations can present as an epiglottic mass. Benign squamous papillomas are noninvasive and slow-growing epithelial tumors that tend to form fine papillary, well-defined masses that may be pedunculated. While often arising from the soft palate, tonsils, or pharyngeal wall, laryngeal involvement is common and may produce symptoms when the epiglottis is involved.14 Squamous papillomas tend to be exophytic with multiple papillary fronds, covered by mature stratified squamous epithelium.15 It has been known for nearly three decades that the human papilloma virus (HPV) is linked to the development of squamous papilloma and to the risk of developing laryngeal squamous cell carcinoma.16–18 Fibroepithelial polyps, although noninvasive like papillomas, are composed of a larger solid fibrous core rather than multiple fine papilla and lack squamous epithelial overgrowth. On MRI, they tend to be T2 hyperintense, mildly T1 hyperintense, without enhancement after gadolinium contrast administration.1
Laryngeal cysts comprise 5% of all benign laryngeal lesions, and the majority of ductal cysts arise from the epiglottis.19,20 Fang et al. (2002) described a 64-year-old female with progressive stridor and foreign body sensation who had a 2.5 cm × 1.8 cm epiglottic cyst seen on CT as a low-density structure attached to the lingual surface of the epiglottis. The authors warn that epiglottic cysts may become secondarily infected and present with epiglottitis, which may demonstrate ring enhancement on post-contrast CT scan. They further caution that abscess must not be mistaken for a small amount of normal air that may be present within an epiglottic cyst.20 Henderson et al. (1985) presented a 43-year-old male with progressive dysphagia and hoarseness who presented with airway obstruction from a large epiglottic cyst.4
Other unusual tumors may also present as epiglottic masses. Extracardiac rhabdomyomas have been reported to demonstrate isointensity or slight hyperintensity on T1- and T2-weighted imaging with mild diffuse contrast enhancement, in a submucosal location without invasion into surrounding tissues.21 While solitary fibrous tumors usually arise in the pleura or other serosal surfaces such as the peritoneum, they can arise in other sites such as the larynx. Safneck et al. (1993) reported a 13-year-old male with foreign body sensation and difficulty breathing while supine who underwent CT scan revealing a 2 cm mixed attenuation mass on the laryngeal surface of the epiglottis with subsequent excision yielding solitary fibrous tumor.22 Neurogenic tumors such as schwannomas, neurofibromas, and malignant peripheral nerve sheath tumors are extremely rare in the larynx, with schwannomas accounting for 0.1% of all benign laryngeal masses. Of all laryngeal tumors of mesenchymal origin, the neurogenic tumors are least common and can range in size from a few millimeters to a few centimeters, causing obstruction.23 Laryngeal schwannomas tend to arise in the aryepiglottic folds or true vocal folds and may present with hoarseness, dysphagia, and foreign body sensation. Martin et al. (2002) reported an incidental 2.5 cm pedunculated, smooth schwannoma arising from the lingual surface of the epiglottis, discovered during intubation for an orthopedic procedure in a 79-year-old male with only mild globus sensation.24 Hamartomas are benign proliferations of epithelial and mesenchymal tissue that, unlike teratomas, arise in orthotopic locations. Although laryngeal hamartomas are rare, Eustace and Suojanen (1995) reported a hamartomatous large exophytic heterogeneous mass containing fat density on CT arising from the tip of the epiglottis in a 58-year-old man with dysphagia and hoarseness. The authors noted that recurrence of laryngeal hamartomas often occurs owing to the lack of a capsule or defined margin.6 Ohsawa et al. (1983) described a 2.8 cm malignant paraganglioma arising from the epiglottis submucosa in a 47-year-old man who presented with laryngeal discomfort.25
Granulomatous diseases that may cause an epiglottis mass include tuberculosis, sarcoidosis, and Wegener’s granulomatosis. When sarcoidosis involves the larynx, it most often involves the epiglottis and manifests with erythema, edema, punctate nodules, and mass lesions.26 Laryngeal tuberculosis occurs in less than 1% of all patients with tuberculosis, and laryngeal tuberculosis most commonly involves the posterior commissure.2,27,28 Epiglottis involvement by tuberculosis is extremely rare. In a series of 31 patients with laryngeal tuberculosis, Agarwal and Bais (1998) found only one to have epiglottic involvement.29 Tuberculosis involving the epiglottis may manifest with thickening or less likely a discrete mass.30 Fernandez et al. (2007) described a 32-year-old male with asthenia, weight loss, cough, and dysphagia, who was found to have tuberculous involvement of the epiglottis manifesting as a bulky mass on laryngoscopy.31 Gupta et al. (2008) described a 60-year-old male patient with hoarseness, cough, and difficulty breathing and swallowing who was found to have tuberculous involvement of the epiglottis manifesting as a proliferative growth on the epiglottis with associated aryepiglottic fold thickening. Concordant with reports that virtually all patients with laryngeal tuberculosis have concurrent active pulmonary tuberculosis, their patient was also found to have miliary pulmonary tuberculosis upon high-resolution chest CT.27,30 Khan et al. (2009) presented a 66-year-old female with sore throat, hoarseness, and cough who was on steroids for nephrotic syndrome who was found to have a supraglottic tuberculous mass involving the epiglottis, aryepiglottic fold, and true and false cords.2 Despite the possibility of epiglottic tuberculosis presenting as a discrete mass, tuberculosis of the epiglottis typically presents with thickened free margins associated with thickening of the aryepiglottic folds. The pre-epiglottic fat spaces are typically preserved, in contradistinction to laryngeal carcinoma, which tends to infiltrate the pre-epiglottic fat and can involve nearly any laryngeal structure including the epiglottis, aryepiglottic folds, and true and false vocal cords.11,27,32 Also differentiating tuberculosis from carcinoma, the most common symptom of tuberculosis is hoarseness, with odynophagia present in 45%–90% of patients. Laryngeal carcinoma, on the other hand, more often presents with a foreign body sensation and referred otalgia.2 Additionally, despite the possibility of presenting as a small midline mass, epiglottic squamous cell carcinoma tends to present with bilateral lymphadenopathy (Figure 4).11 Nevertheless, the symptoms of tuberculosis and carcinoma may overlap, and patients have been misdiagnosed.27 It is important to identify indirect signs of invasive disease such as cartilaginous erosion or involvement of the pre-epiglottic or paraglottic fat spaces, as the latter often correlates with vocal cord paralysis on fiberoptic examination.33,34
Infections such as histoplasmosis may also present as an epiglottic mass. O’Hara et al. (2004) described a 78-year-old retired soil science professor with an extensive travel history who had progressive odynophagia with weight loss and night sweats. On laryngoscopy, an epiglottic mass with erosion at the free edge was biopsy-proven histoplasmosis. Histoplasmosis involving the larynx and oropharynx is common, present in 66% of chronic disseminated histoplasmosis, in 31% of subacute progressive disseminated histoplasmosis, and in 19% of acute histoplasmosis.28 Laryngeal histoplasmosis tends to involve the anterior larynx; however, histoplasmosis of the epiglottis is less common, present in only one of 58 patients in a series of patients with histoplasmosis.28,35
Children may also present with polypoid epiglottic masses. Laryngeal malignancies are rare in children, and when present, are usually squamous cell carcinomas. Mitchell et al. (1988) reported a child who was incidentally found to have a papillomatous lesion at the tip and left lateral border of the epiglottis, biopsy-proven to be mucoepidermoid carcinoma. The patient subsequently underwent epiglottectomy with good results. In adults, epiglottic mucoepidermoid carcinoma is less common than squamous cell carcinoma but tends to arise in the supraglottic larynx.36 Other rare malignancies have also been reported, including a laryngeal small cell carcinoma presenting as a smooth-surfaced mass on the lingual surface of the epiglottis.37 Lymphoma originating in the larynx is very rare, accounting for less than 1% of all laryngeal neoplasms, and is typically of the non-Hodgkin B-cell subtypes and typically presents with hoarseness and dysphagia. Laryngeal lymphoma tends to occur in the supraglottic larynx, including the epiglottis and aryepiglottic folds, and is isointense on T1- and T2-weighted MRI with moderate homogeneous gadolinium contrast enhancement.38 Solitary plasmacytoma, another rare malignancy, although usually presenting in the nasopharynx, nasal cavity, paranasal sinuses, or tonsils, was diagnosed in a 59-year-old male with a dry cough and shortness of breath, who had a 1 cm spherical submucosal mass on the laryngeal surface of the epiglottis.39 Laryngeal involvement of the epiglottis, glottis, false vocal folds, aryepiglottic folds, and subglottic airway has been reported in 6%–18% of patients with solitary plasmacytoma.40–42 Unlike solitary plasmacytoma, metastatic multiple myeloma presenting as a laryngeal mass is extremely rare.41 Metastases to the larynx are extremely rare, usually from renal cell carcinoma or melanoma, but have been reported from pancreatic adenocarcinoma as well.43
The epiglottis is composed of elastic cartilage and thus is susceptible to lesions of cartilaginous origin as well.44 Of all tumors of mesenchymal origin, the cartilaginous tumors are most common.23 Typically, chondromas involve the thyroid cartilage or the posterior lamina of the cricoid cartilage, and epiglottic chondromas are extremely rare.3 When chondromas involve the epiglottis, they may arise from the anterior or posterior surfaces and can lead to dysphagia or dyspnea.45 Yang et al.3 (2005) presented a 52-year-old female with foreign body sensation who had a small mass at the tip of the epiglottis corresponding to biopsy proven chondroma. Laryngeal chondroid lesions usually present with calcifications and are T2 hyperintense.46–48 Chondromas, which tend to be less than 2 cm in size, must be distinguished from chondrosarcomas, which tend to be greater than 3 cm. Both chondromas and low-grade chondrosarcomas may be round, smooth, and lobular, confusing imaging and histological diagnosis.3 Laryngeal chondrosarcoma arises from the cricoid cartilage in 72%–75% of patients and only involves the epiglottis in 1%–2% of patients.5
Lymphangioma, or lymphatic malformations, must also be considered in the differential diagnosis of an epiglottic mass particularly in the pediatric population, as 90% are detected by the second year of age. Laryngeal lymphangiomas typically arise in the supraglottic larynx within the epiglottis, aryepiglottic folds, and arytenoid cartilages, and are hyperintense on T2-weighted MRI.13,49 Despite the predominance in the pediatric population, Seven et al. (2004) reported a 37-year-old male who initially presented with acute epiglottitis. Upon resolution of the acute symptoms, laryngoscopy revealed a 3 cm pedunculated mass arising from the lingual aspect of the epiglottis, biopsy-proven lymphangioma.13
Systemic conditions such as systemic lupus erythematosus, rheumatoid arthritis, relapsing polychondritis, and amyloidosis may manifest with epiglottic masses.1 Focal amyloidosis has been reported in various sites in the head and neck, but is more than twice as common in the larynx as in any other site in the head and neck, particularly the supraglottic false cord region. Nevertheless, focal amyloidosis accounts for less than 1% of all benign laryngeal masses. Unlike osseous amyloidosis, laryngeal amyloidosis rarely causes osteolysis. Patients with focal amyloidosis tend to be 40 - to 60-year-old males who present with hoarseness, breathing difficulty, and pain. Rodriguez-Romero et al. (1996) described a non-enhancing soft tissue mass with stippled calcification arising in the epiglottis in a 39-year-old male with dysphagia and hoarseness, without extension to the aryepiglottic folds, false cords, or pre-epiglottic space. While the presence of calcifications raised the possibility of a chondrosarcoma or tuberculosis, amyloidosis tends to be isointense on T1-weighted MRI and slightly hyperintense on T2-weighted MRI, while chondrosarcoma tends to be T1 hypo- or isointense and markedly T2 hyperintense.44 Badr et al. (2008) reported a case of Kimura disease, a benign chronic inflammatory disease that is likely immune driven, manifesting as a single polypoid epiglottic mass in a 37-year-old female who presented with difficulty breathing and swallowing. While usually presenting as a subcutaneous mass, the epiglottis may be involved.50
Conclusion
Epiglottic masses are more often seen clinically by the otolaryngologist than by the radiologist on CT or MRI, as most patients will not undergo imaging of benign-appearing epiglottic masses. Nevertheless, the radiologist must be vigilant for their presence and aware of the differential diagnosis, as epiglottic masses may simply be incidental findings without symptoms, as in our patient, or they may present with airway obstruction.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Farzal Z, Ulualp SO, Rakheja D. Fibroepithelial polyp of the epiglottis. Am J Case Rep 2014; 15: 340–342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Khan NU, Wallis S, Siddiqui N. Laryngeal tuberculosis: A diagnosis not to be missed. BMJ Case Rep 2009; 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yang SW, Lin CY. A peculiar site of chondroma: The epiglottis. Acta Otolaryngol 2005; 125: 906–909. [DOI] [PubMed] [Google Scholar]
- 4.Henderson LT, Denneny JC, 3rd, Teichgraeber J. Airway-obstructing epiglottic cyst. Ann Otol Rhinol Laryngol 1985; 94(5 Pt 1): 473–476. [DOI] [PubMed] [Google Scholar]
- 5.Mesolella M, Motta G, Galli V. Chondrosarcoma of the epiglottis: Report of a case treated with CO2 laser epiglottectomy. Acta Otorhinolaryngol Belg 2004; 58: 73–78. [PubMed] [Google Scholar]
- 6.Eustace S, Suojanen J. Computed tomography of hamartoma of the epiglottis. Clin Imaging 1995; 19: 237–239. [DOI] [PubMed] [Google Scholar]
- 7.Referral guidelines for suspected cancer in adults and children. Clinical Governance Research and Development Unit (CGRDU), Department of Health Sciences, University of Leicester. London: Royal College of General Practitioners (UK); 2005 June. (NICE Clinical Guidelines, No. 27.) 17, Head and Neck cancer, http://www.ncbi.nlm.nih.gov/books/NBK45770/ (accessed 25 December 2014).. [PubMed]
- 8.Hegde MC, Kamath MP, Bhojwani K, et al. Benign lesions of larynx—A clinical study. Indian J Otolaryngol Head Neck Surg 2005; 57: 35–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Johnson JT and Clayburgh D. Malignant tumors of the larynx. Medscape, http://emedicine.medscape.com/article/848592 (accessed 25 December 2014).
- 10.Doloi PK, Khanna S. A study of management of benign lesions of the larynx. IJOPL 2011; 1: 61–64. [Google Scholar]
- 11.Landry D, Glastonbury CM. Squamous cell carcinoma of the upper aerodigestive tract: A review. Radiol Clin North Am 2015; 53: 81–97. [DOI] [PubMed] [Google Scholar]
- 12.Capps EF, Kinsella JJ, Gupta M, et al. Emergency imaging assessment of acute, nontraumatic conditions of the head and neck. Radiographics 2010; 30: 1335–1352. [DOI] [PubMed] [Google Scholar]
- 13.Seven H, Topuz E, Turgut S. Isolated laryngeal lymphangioma showing the symptoms of acute epiglottitis. Eur Arch Otorhinolaryngol 2004; 261: 548–550. [DOI] [PubMed] [Google Scholar]
- 14.Desai S, Rajaratnam K. Pedunculated squamous papilloma of the laryngopharynx. J Laryngol Otol 1989; 103: 209–210. [DOI] [PubMed] [Google Scholar]
- 15.Devi RS, Rajsekhar B, Srinivas GV, et al. Unusual length of pedicle: Pedunculated squamous papilloma of uvula causing unusual dysphagia of long duration in a child of 10 years. Case Rep Dent 2014; 2014: 313506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gissmann L, Wolnik L, Ikenberg H, et al. Human papillomavirus types 6 and 11 DNA sequences in genital and laryngeal papillomas and in some cervical cancers. Proc Natl Acad Sci U S A 1983; 80: 560–563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Syrjanen KJ, Surjanen SM. Histological evidence for the presence of condylomatous epithelial lesions in association with laryngeal squamous cell carcinoma. ORL J Otorhinolaryngol Relat Spec 1981; 43: 181–194. [DOI] [PubMed] [Google Scholar]
- 18.Mounts P, Shah KV, Kashima H. Viral etiology of juvenile- and adult-onset squamous papilloma of the larynx. Proc Natl Acad Sci U S A 1982; 79: 5425–5429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.DeSanto LW, Devine KD, Weiland LH. Cysts of the larynx—classification. Laryngoscope 1970; 80: 145–176. [DOI] [PubMed] [Google Scholar]
- 20.Fang TJ, Cheng KS, Li HY. A huge epiglottic cyst causing airway obstruction in an adult. Chang Gung Med J 2002; 25: 275–278. [PubMed] [Google Scholar]
- 21.Liess BD, Zitsch RP, 3rd, Lane R, et al. Multifocal adult rhabdomyoma: A case report and literature review. Am J Otolaryngol 2005; 26: 214–217. [DOI] [PubMed] [Google Scholar]
- 22.Safneck JR, Alguacil-García A, Dort JC, et al. Solitary fibrous tumour: Report of two new locations in the upper respiratory tract. J Laryngol Otol 1993; 107: 252–256. [DOI] [PubMed] [Google Scholar]
- 23.Saita V, Azzolina A, Galia A, et al. Schwannoma of the epiglottis: Case report focusing on clinico-pathological aspects. Acta Otorhinolaryngol Ital 2005; 25: 378–380. [PMC free article] [PubMed] [Google Scholar]
- 24.Martin PA, Church CA, Chonkich G. Schwannoma of the epiglottis: First report of a case. Ear Nose Throat J 2002; 81: 662–663. [PubMed] [Google Scholar]
- 50.Ohsawa M, et al. Malignant chemodectoma (paraganglioma) of the larynx. A case report with electron microscopy and biochemical assay. Acta Pathol Jpn 1983; 33(6): 1279–1288. [DOI] [PubMed]
- 25.Bower JS, Belen JE, Weg JG, et al. Manifestations and treatment of laryngeal sarcoidosis. Am Rev Respir Dis 1980; 122: 325–432. [DOI] [PubMed] [Google Scholar]
- 26.Gupta R, Fotedar S, Sansanwal P, et al. Obstructing mass lesion of epiglottis: It can be tubercular. Indian J Tuberc 2008; 55: 100–103. [PubMed] [Google Scholar]
- 27.O’Hara CD, Allegretto MW, Taylor GD, et al. Epiglottic histoplasmosis presenting in a nonendemic region: A clinical mimic of laryngeal carcinoma. Arch Pathol Lab Med 2004; 128: 574–577. [DOI] [PubMed] [Google Scholar]
- 28.Agarwal P, Bais AS. A clinical and videostroboscopic evaluation of laryngeal tuberculosis. J Laryngol Otol 1998; 112: 45–48. [DOI] [PubMed] [Google Scholar]
- 29.Moon WK, Han MH, Chang KH, et al. Laryngeal tuberculosis: CT findings. AJR Am J Roentgenol 1996; 166: 445–449. [DOI] [PubMed] [Google Scholar]
- 30.Fernandez P, Guyot M, Lazaro E, et al. Systemic tuberculosis presenting as an epiglottic mass detected on F-18 FDG PET/CT. Clin Nucl Med 2007; 32: 719–724. [DOI] [PubMed] [Google Scholar]
- 31.Kim MD, Kim DI, Yune HY, et al. CT findings of laryngeal tuberculosis: Comparison to laryngeal carcinoma. J Comput Assist Tomogr 1997; 21: 29–34. [DOI] [PubMed] [Google Scholar]
- 32.Becker M, Burkhardt K, Dulguerov P, et al. Imaging of the larynx and hypopharynx. Eur J Radiol 2008; 66: 460–479. [DOI] [PubMed] [Google Scholar]
- 33.Baugnon KL, Beitler JJ. Pitfalls in the staging of cancer of the laryngeal squamous cell carcinoma. Neuroimaging Clin N Am 2013; 23: 81–105. [DOI] [PubMed] [Google Scholar]
- 34.Goodwin RA, Jr, Shapiro JL, Thurman GH, et al. Disseminated histoplasmosis: Clinical and pathologic correlations. Medicine (Baltimore) 1980; 59: 1–33. [PubMed] [Google Scholar]
- 35.Mitchell DB, Humphreys S, Kearns DB. Mucoepidermoid carcinoma of the larynx in a child. Int J Pediatr Otorhinolaryngol 1988; 15: 211–215. [DOI] [PubMed] [Google Scholar]
- 36.Pérez Fernández F, Delgado Moreno F, Gandul Merchán A, et al. Neuroendocrine carcinoma of the larynx [article in Spanish]. Acta Otorrinolaringol Esp 1996; 47: 484–486. [PubMed] [Google Scholar]
- 37.Takayama F, Takashima S, Momose M, et al. MR imaging of primary malignant lymphoma in the larynx. Eur Radiol 2001; 11: 1079–1082. [DOI] [PubMed] [Google Scholar]
- 38.Welsh J, Westra WH, Eisele D, et al. Solitary plasmacytoma of the epiglottis: A case report and review of the literature. J Laryngol Otol 1998; 112: 174–176. [DOI] [PubMed] [Google Scholar]
- 39.Van Dyke CW, Masaryk TJ, Lavertu P. Multiple myeloma involving the thyroid cartilage. AJNR Am J Neuroradiol 1996; 17: 570–572. [PMC free article] [PubMed] [Google Scholar]
- 40.Grobman AB, Vivero RJ, Campuzano-Zuluaga G, et al. Laryngeal involvement of multiple myeloma. Case Rep Oncol Med 2012; 2012: 257814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nofsinger YC, Mirza N, Rowan PT, et al. Head and neck manifestations of plasma cell neoplasms. Laryngoscope 1997; 107: 741–746. [DOI] [PubMed] [Google Scholar]
- 42.Oku T, Hasegawa M, Watanabe I, et al. Pancreatic cancer with metastasis to the larynx. J Laryngol Otol 1980; 94: 1205–1209. [DOI] [PubMed] [Google Scholar]
- 43.Rodríguez-Romero R, Vargas-Serrano B, Cortina-Moreno B, et al. Calcified amyloidoma of the larynx. AJNR Am J Neuroradiol 1996; 17: 1491–1493. [PMC free article] [PubMed] [Google Scholar]
- 44.Swerdlow RS, Som ML, Biller HF. Cartilaginous tumors of the larynx. Arch Otolaryngol 1974; 100: 269–272. [DOI] [PubMed] [Google Scholar]
- 45.Baatenburg de Jong RJ, van Lent S, Hogendoorn PC. Chondroma and chondrosarcoma of the larynx. Curr Opin Otolaryngol Head Neck Surg 2004; 12: 98–105. [DOI] [PubMed] [Google Scholar]
- 46.Burggraaff BA, Weinstein GS. Chondrosarcoma of the larynx. Ann Otol Rhinol Laryngol 1992; 101(2 Pt 1): 183–184. [DOI] [PubMed] [Google Scholar]
- 47.Obenauer S, Kunze E, Fischer U, et al. Unusual chondrosarcoma of the larynx: CT findings. Eur Radiol 1999; 9: 1625–1628. [DOI] [PubMed] [Google Scholar]
- 48.Sundarapandian S, Mohamed HK, Murugesan S, et al. Haemolymphangioma of epiglottis. J Clin Diagn Res 2013; 7: 1753–1754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Badr A, Abdul-Haleem A, Carlsen E. Kimura disease of the epiglottis. Head Neck Pathol 2008; 2: 328–332. [DOI] [PMC free article] [PubMed] [Google Scholar]