Abstract
Introduction
Neurosurgery of the supplementary motor area (SMA) is associated with transient speech defects. We investigated whether SMA laterality correlates with postoperative speech defects.
Materials and methods
The authors reviewed 17 patients with SMA-area lesion resection and preoperative language fMRI. SMA laterality was calculated by comparison of voxel activation in paired SMAs by hand-drawn regions of interest (ROIs) (drawn by a neuroradiologist), and compared with qualitative assessment by two neuroradiologists. Postoperative speech defects before and after surgery were assessed by chart review.
Results
Six patients developed new speech defects that resolved within several months. Two of the patients had a pre-existing speech defect that had developed after prior SMA-area surgery. All these patients had left-sided lesions, while none of the four patients with a right-sided lesion developed a speech defect. Neuroradiologists’ assessment of SMA laterality agreed with ROI calculation for the SMAs that were lateralized. However, for the SMAs in the “codominant” range by ROI, the neuroradiologists felt that all but one of the cases clearly lateralized, with the exception deemed indeterminate or codominant. No correlation between laterality of SMA and speech defect was identified. Twelve patients showed lateralization contralateral to the lesion.
Conclusions
fMRI lateralization does not correlate with transient speech defects that developed from SMA-area surgery. Qualitative/visual assessment of SMA laterality was superior to ROI calculation because of the close proximity and possible overlap of signal from midline SMA. A majority of patients showed SMA lateralization contralateral to the SMA lesion.
Keywords: speech, fMRI, neurosurgery, SMA, language
Introduction
The supplementary motor area (SMA) is a secondary motor structure implicated by lesioning studies and intra-and postoperative experience in motor and speech production.1 Studies on the effects of lesioning the SMA have shown unpredictable transient speech problems ranging from slowness of speech and word choice difficulties, to complete mutism, though the majority of reported problems have been described as transient.1–3 The SMA is within the superior frontal gyrus, anterior to the parasagittal precentral gyrus (the motor foot homunculus), and along the medial left frontal lobe cephalad to the cingulated gyrus.4 The precise location of the SMA may vary and cannot be readily discerned from cortical morphology or cytoarchitecture alone, and as well, the SMA may be divided into multiple regions by functionality (motor, language).5 The SMA can, however, be reliably localized on functional magnetic resonance imaging (fMRI) using productive language and motor tasks.
The SMA is thought to be composed of at least two functional components: the SMA-proper, and more anteriorly, the pre-SMA. More complex higher-order processing is attributed to the pre-SMA, with motor-related tasks to the SMA-proper. Peck et al. have also recently shown that the central SMA is activated for both language and motor tasks.6 Some of the language-related functions of the higher-order SMA include quickly constructing and coordinating sequences of motor outputs in preparation for a potential action (or word pattern), priming the motor areas in anticipation of the most likely potential action patterns, and likely inhibition of actions that are deemed unnecessary.4,7,8
Lesion studies of the primary language centers (Broca and Wernicke), either through surgery or an insult, and correlation with Wada testing have validated the concept of language dominance.9,10 Determination of the hemispheric dominance for language has become part of the preoperative evaluation for neurosurgery to areas near the primary language centers, to optimize preoperative planning, guide the resection and counsel patients of the risks of their surgery.11,12 fMRI shows excellent correlation with Wada testing for assessment of language dominance, though with the considerable added benefits of being noninvasive, availability, and speed.13 Quantitative assessment of laterality by measuring the activated voxels within regions of interest (ROIs) over the bilateral language centers has been validated as a way to determine hemispheric dominance for language on fMRI.13
Krainik et al. have reported the use of fMRI to correlate SMA activity with speech defects following SMA-area surgery in 12 patients, observing that the SMA involved by tumor often had less activity than the contralateral healthy SMA, and further that the higher the activation in the SMA not involved by the tumor, the less the chance of speech defect following resection of the tumor-involved SMA. They found that the severity of acquired speech defect also appeared to correlate with the size of the resection around the SMA.14,15 The purpose of this study is to build on their observations by studying 17 patients with SMA-area surgery. We hypothesize that assessment of laterality of the language SMA can help predict postoperative speech defects and further, that lesioning of a “dominant” SMA increases risks of speech defects versus lesioning of a non-dominant SMA.
Methods
Patient selection
Institutional review board waiver of authorization was granted prior to the study. Seventeen patients with SMA-area lesions were retrospectively identified by search of the fMRI database who had received a preoperative functional MRI and subsequent surgical resection of the SMA area without resection extending to the Broca’s area. The patients were treated between 2006 and 2009 at our institution. The fMRI scans had been performed for localization of primary speech or motor centers, including the Broca’s, Wernicke’s, and motor gyri, though not specifically for localization of the SMA.
Demographics
As summarized in Table 1, seven of the patients were male and 10 were female, with an average age of 46 years (range: 29–64) at the time of surgery. Fourteen of the patients were 100% right handed following criteria of the Edinburgh Handedness Inventory, and three were left handed. Thirteen of the patients had a left-sided lesion, and four of them a right-sided lesion. Pathology included seven low-grade primary brain tumors (five astrocytomas, two oligodendrogliomas), four recurrent high-grade primary brain tumors (two anaplastic oligodendroglioma, one anaplastic oligodendroglioma, one glioblastoma (GBM)), and two high-grade primary brain tumors (one anaplastic oligoastrocytoma, one anaplastic astrocytoma). Three lesions were metastases (one breast cancer, two non-small-cell lung cancer), and one lesion was radiation necrosis following stereotactic radio surgery (SRS) for a metastasis.
Table 1.
Demographics.
| Patient | Age | Sex | Handedness | Broca’s by fMRI | Pathology |
|---|---|---|---|---|---|
| 1 | 44 | M | R | L | Anaplastic astrocytoma |
| 2 | 62 | M | R | L | Radiation necrosis (status post-SRS for a metastasis) |
| 3 | 45 | F | R | L | Breast cancer metastasis |
| 4 | 29 | F | R | L | Low-grade oligodendroglioma |
| 5 | 31 | F | R | L | Low-grade astrocytoma |
| 6 | 62 | M | L | L | Recurrent oligodendroglioma |
| 7 | 50 | F | R | L | Low-grade oligodendroglioma |
| 8 | 42 | F | L | L | Low-grade glioma |
| 9 | 39 | F | L | L | Recurrent anaplastic oligodendroglioma |
| 10 | 45 | M | R | L | Anaplastic oligoastrocytoma |
| 11 | 47 | M | R | Codominant | Low-grade oligodendroglioma |
| 12 | 44 | M | R | L | Poorly differentiated NSCLC metastasis |
| 13 | 64 | F | R | L | NSCLC metastasis |
| 14 | 43 | F | R | L | Anaplastic oligodendroglioma |
| 15 | 44 | M | R | L | Low-grade oligodendroglioma |
| 16 | 53 | F | R | L | Low-grade oligodendroglioma |
| 17 | 46 | F | R | L | Glioblastoma |
fMRI: functional magnetic resonance imaging; SRS: stereotactic radio surgery; NSCLC: non-small-cell lung cancer; M: male; F: female; R: right; L: left.
Assessment of postoperative speech defects
Presence or absence of pre- and postoperative speech defects was assessed through review of the patient’s chart and physician’s notes. All of the patients received neuropsychological and/or neurological language evaluation prior to and following surgery and during subsequent rehabilitation and follow-up office visits, as documented by the neurosurgeon, neurologist, and occupational therapist/rehabilitation specialist.
Image acquisition
The scans were performed on 1.5 T TwinSpeed Excite and 3 T Signa HDxt scanners (GE Healthcare, Milwaukee, WI), using standard quadrature head coils. Functional images were gathered by using a T2*-weighted gradient-echo echo-planar imaging sequence (for both 1.5 T and 3 T: echo time (TE) = 40 ms, repetition time (TR) = 4000 ms, section thickness = 4.5 mm with no gap, matrix size = 128 × 128, field of view (FOV) = 240 mm, 21 sections). T1-weighted spin-echo images (for 1.5 T: TR = 600 ms, TE = 8 ms, 256 × 256 matrix, 90-degree flip angle, 4.5 mm in thickness with no gap, 240 mm FOV, 21 sections; for 3 T: TR = 400 ms, TE = 14 ms, 256 × 256 matrix, 90-degree flip angle, 4.5 mm in thickness with no gap, 240 mm FOV, 28–32 sections) were also acquired. Three-dimensional (3D) T1-weighted anatomic images were acquired with a spoiled gradient-recalled-echo sequence (for 1.5T: TR = 22 ms, TE = 4 ms, 256 × 256 matrix, 30-degree flip angle, 1.5 mm in thickness, 240 mm FOV; for 3 T: TR = 6.9 ms, TE = 3 ms, 256 × 256 matrix, 15-degree flip angle, 1.5 mm in thickness, 240 mm FOV, 124 sections). The patient’s head motion was minimized by using straps and foam padding.
fMRI paradigms
Four patients performed all the language tasks, eight patients performed two tasks, and five patients performed one task, with the variation explained by clinical necessity and patient’s ability to continue with the tasks and to perform the tasks correctly (Table 2). Participants were asked to perform the tasks silently, avoiding mouth and tongue movement. In verb generation, individuals were told a noun and asked to generate action words associated with the noun. For the letter task, the patient was asked to silently generate words that began with a letter. During the category task, participants were given a supraordinate category and were told to generate subordinate words that fit the category. Patients were pretested on each task outside the scanner to ensure that they could perform the task correctly. The paradigm was presented as a block design and consisted of paradigm execution alternating with a baseline of rest. At least one additional non-language motor task was performed, such as bilateral finger and toe tapping or tongue movement. The patient’s brain activity and head motion were monitored using software (Brainwave, Medical Numerics) that permits the observation of real time.
Table 2.
Results by SMA laterality index.
| Patient | Tumor side | SMA resected? | Laterality ROI | Laterality readers | Speech deficit? |
|---|---|---|---|---|---|
| 1 | Left | Partial | Right | Right | No |
| 2 | Left | Partial | Right | Right | Transient |
| 3 | Left | Partial | Right | Right | No |
| 4 | Left | Yes | Codominant | Right | No |
| 5 | Left | Yes | Codominant | Right | No |
| 6 | Left | Yes | Codominant | Right | No |
| 7 | Left | Yes | Codominant | Right | Transient |
| 8 | Right | Partial | Codominant | Left | No |
| 9 | Right | Yes | Codominant | Left | No |
| 10 | Left | Partial | Codominant | Right | Transient |
| 11 | Right | Yes | Left | Left | No |
| 12 | Left | Partial | Left | Left | Transient |
| 13 | Left | Yes | Left | Left | Transient |
| 14 | Left | Partial | Left | Left | Pre-existing, no change |
| 15 | Right | Yes | Left | Left | No |
| 16 | Left | Partial | Left | Left | Transient |
| 17 | Left | Partial | Left | Not sure | Pre-existing, no change |
SMA: supplementary motor area; ROI: region of interest.
fMRI data analysis
Image processing and analysis was performed using AFNI software, with head motion correction and spatial smoothing using a Gaussian kernel (full width at half maximum (FWHM) = 4 mm). Cross-correlation analysis was with Gaussian convolved waveforms. ROIs were hand-drawn around each of the bilateral SMAs by a fellowship-trained, certificate of added qualification (CAQ)-certified neuroradiologist with three years’ experience in fMRI using anatomic and fMRI correlation. The number of activated voxels in each ROI was determined using an uncorrected p value <0.001; laterality indices (LIs) were calculated on the basis of left (L) and right (R) activation volume ROIs using the formula: LI = (L–R)/(L + R). The LI ranged from –1 (completely lateralized to the right) to +1 (completely lateralized to the left). Participants were defined as having bilateral language representation if their values fell within the well-established LI range of –0.2 to 0.2.
In addition, the laterality of SMAs’ fMRI activation was also assessed by visual inspection by two neuroradiologists by consensus.
The localization and dominance of the primary language centers was determined by a fellowship-trained, CAQ-certified neuroradiologist (with at least five years’ experience in fMRI) at the time of the original fMRI scanning prior to surgery. Sixteen of the patients were determined to have a dominant left-sided Broca’s area on fMRI. One right-handed patient exhibited a codominant Broca.
Results
Speech deficits
Fifteen of the patients had normal speech at baseline, while two of the patients had word-finding difficulty at baseline. The two patients with preoperative speech deficit were presented for re-resection of tumor and had developed speech deficit following remote prior SMA-area surgery and treatment course. Of note, the SMA was mostly preserved during tumor resection for the two patients with persisting speech deficit, and there was no appreciable improvement or worsening of speech after the repeat surgeries. Six patients developed a new speech deficit postoperatively, but all exhibited recovery of speech to baseline within several months following surgery. All of the patients who developed postoperative speech deficits had a left-dominant Broca’s area determined by preoperative fMRI; the codominant Broca’s patient showed no pre- or postoperative speech dysfunction.
SMA lateralization
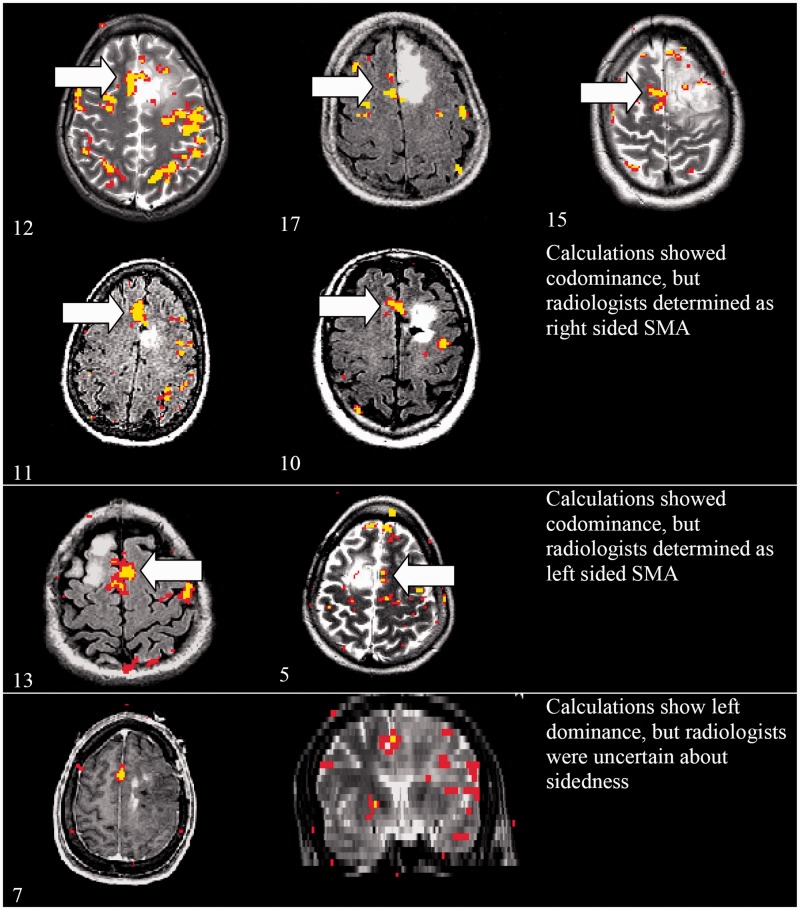
The average values of the fMRI-determined SMA LIs for language tasks were within the codominant range of –0.2 to +0.2 for seven of the patients (Figure 1). However, the neuroradiologists felt that all seven of these patients clearly lateralized, with five of them lateralizing to the right, and two lateralizing to the left (Table 2). Of the remaining patients, seven had left-lateralized SMA by calculation from ROIs (LI >0.2), and three had right-lateralized SMA based on the calculations (LI <0.2); for these cases, the neuroradiologists were in agreement.
Figure 1.
Cases for which radiologist-determined laterality was deemed superior to ROI-calculated laterality (Patient # to lower left of each axial image). SMA: supplementary motor area; ROI: region of interest.
SMA lateralization and tumor sidedness
When using the radiologist-determined SMA laterality, 12 patients demonstrated increased SMA activity on the side opposite the tumor. Only four of the 17 patients demonstrated SMA activity within the SMA-area tumor that was visually greater than the contralateral side. One patient demonstrated indeterminate midline SMA activity (which could have been either in the left or right or both SMAs). Of the right-sided SMA-area tumors, all four showed asymmetrically increased SMA activity on the contralateral side. The ROI-based lateralities, in contrast, were similar except that of the 12 patients that the radiologists rated as having SMA activity more contralateral to the tumor, seven of these were in the “codominant” category.
SMA lateralization and speech deficits
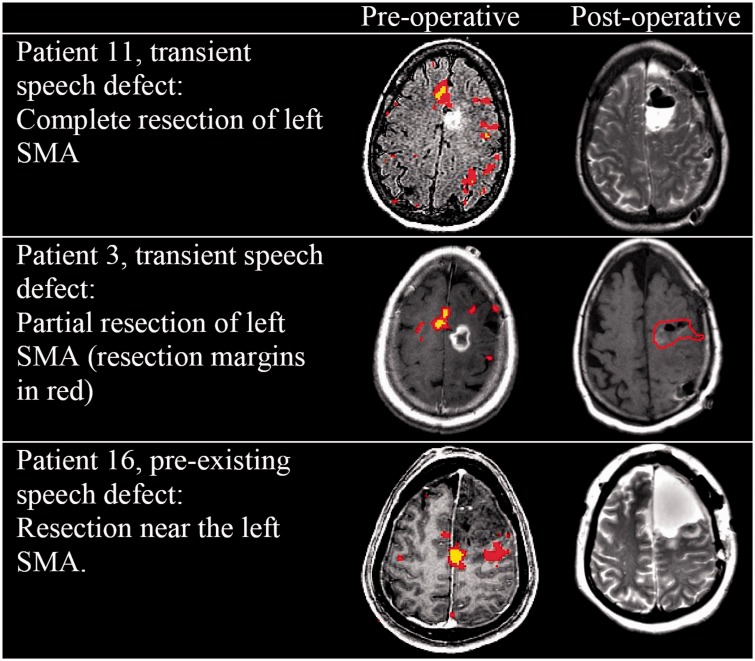
All of the patients who developed new speech deficits had a left-sided SMA resection for left-sided tumor, and the two patients with prior speech deficits also had had left-sided surgery (Figure 2). Of the six patients with new speech deficits, half (three) had a right-lateralized SMA and the other half had left-lateralized SMA, indicating that the fMRI lateralization of the SMA was not significantly correlated with postoperative speech defects. In addition, four of these six patients had a partial SMA resection or resection near the SMA (where the area of the SMA defined by fMRI was not resected). Only two of the patients who developed transient speech deficit had a complete SMA resection.
Figure 2.
Partial resection of the SMA can lead to transient speech defects. SMA: supplementary motor area.
Discussion
Speech deficits following SMA-area surgery are not infrequent and are usually transient. Significantly, the deficits can develop with partial resection of the SMA, and with surgery near but not including the fMRI-defined SMA. This may reflect surgery-related edema or microtrauma on the adjacent SMA. It could also be due to damage or edema affecting the-white matter tracts that link the SMA to other language centers. The “anatomic” SMA also likely encompasses a region larger than that visible on fMRI as the focal signal uptake, and indeed the full extent of the SMA has been difficult to visualize on fMRI and on pathology specimens and its precise margins are unclear.
The four patients with a right SMA tumor did not develop a speech deficit; whether this indicates that the ipsilateral left SMA (for these left language-dominant patients) was more important for speech function, or whether it relates to the small sample size of right SMA tumor patients, cannot be confidently determined without additional study of right SMA tumor patients. It is also not known if with right language dominance and right or left SMA tumor resection if transient speech deficits can be replicated on the right side in a situation analogous to what was seen on the left (SMA-area resection ipsilateral to language dominance), or if left SMA damage could cause speech deficit even with right language dominance.
In most of the cases of SMA-area tumor, the amount of fMRI signal within the tumor-infiltrated SMA was less than on the contralateral hemisphere, whether the tumor was on the right or the left. This highly suggests that tumor in and about the SMA leads to a decrease in the SMA signal on fMRI, which is consistent with what other authors have reported for tumor near primary motor areas.16,17 The relative decrease in fMRI blood oxygen level-dependent (BOLD) signal may be related to alterations in vessel autoregulation by the infiltrative tumor, “neurovascular uncoupling,” as the BOLD effect requires intact ability of the SMA-area vessels to vasodilate and allow increased blood flow. If the vessels were already maximally dilated or if they lost the ability to alter flow through vasodilatory response, there would be less or no fMRI signal seen. Interestingly, this decrease in fMRI activation did not necessarily compromise SMA function as shown by the potential to cause speech deficits by lesioning the tumor-infiltrated, less fMRI-active SMA.
Use of ROIs to study the SMA is limited, as a comparison of the calculated literalities (by ROI) and the neuroradiologist result by visual inspection showed disparities mainly for cases where the SMA signal in the bilateral hemispheres was near the midline or crossed into the midline. In these cases, ROI calculations often resulted in a “codominant” categorization, though the neuroradiologists felt that there was clear “subjective” lateralization to either right or left. They observed that slight shifts of the drawing of the borders of the ROI down the midline between the SMAs could cause alteration of the calculated laterality in these cases, and it was felt that for these cases a visual determination was superior to hand-drawn ROI. Representative axial images from these cases where the calculated laterality was somewhat discordant from the neuroradiologist opinion are shown in the Figure 1.
In addition to the relatively low signal produced by language tasks in the SMA are other limitations such as difficulty of standardizing for poor patient effort/concentration, patient motion during language tasks, and contamination from venous flow from the adjacent interhemispheric fissure.
There have been suggestions that increased fMRI activity within a tumor-infiltrated SMA leads to increased risk for a postoperative speech defect, using comparison with the concept of laterality developed for the primary language centers. However, our results show that there was little correlation with speech defects and whether the resected/damaged SMA had high or low fMRI activity relative to the healthy side. All of the patients with speech defects had a left SMA surgery, though nearly half showed SMA activity greater on the normal, contralateral side. These results are partially consistent with the results of Krainik et al.14 in that patients with SMA resection frequently exhibit transient speech defects and that many patients exhibited apparent “translocation” of SMA fMRI activity to the normal hemisphere.
Translocation of primary language center lateralization has been observed for infiltrative glioma in the Wernicke’s area by Petrovich et al. and by Holodny et al. for the Broca’s area.18,19 However, it has also been noted that a limitation of the assessment of BOLD fMRI function in close proximity to infiltrative brain tumors is the neurovascular uncoupling effect that may erroneously lead to appearance of actual translocation (pseudo-dominance) of the functioning language center.20
Further research to increase the number and types of patients and to validate methods for improving signal-to-noise measurements for language SMA activation would be helpful. In addition, intraoperative stimulation of the SMA area prior to tumor resection to assess for awake speech arrest could be useful to determine presence of a “BOLD silent” SMA within an area infiltrated by tumor. Transcranial magnetic stimulation (TMS) could also be used to achieve the same purpose, and early studies to this effect have already been performed.21 Diffusion tractography (DTI) would be a useful adjunct, as the speech deficits could conceivably be caused by disruption of the tracts running near the SMA or leading to/from the SMA, as a recent DTI study suggested a single axonal bundle between the SMA and Broca’s area.22 These findings have important implications in counseling patients on their risks for speech arrest following SMA-area surgery, and furthermore, in assuring them that the speech arrests will be transient.
Limitations
First, a possible limitation of the quantitative ROI-based approach used in our study is that the ROIs were drawn by a single rater and therefore it could lead to subjectivity and operator dependence of ROI designation. Second, the sample of patients is somewhat heterogeneous, comprising low-grade and high-grade gliomas, diverse metastatic lesions, and even a case of radiation necrosis. The diverse nature of these lesions and their particular biological behavior, as well as differences between these subgroups with respect to infiltration, destruction or displacement of eloquent cortex, and neurovascular uncoupling could be an issue in some cases. This fact could affect LI computation via false-negative activation. Also, use of a more histologically homogeneous sample would have been useful in order to reduce confounding variables. Third, in some cases only partial resection of the SMA was performed. This could represent a potential confounding variable in the overall assessment of correlation of preoperative LIs with postoperative outcome since the percentage of the volume of SMA activation is not considered; i.e. even if eloquent cortex is resected, loss of function may be related to the amount of tissue resected, and a 25% resection may yield different clinical consequences than a 75% or 100% resection because cortical reorganization/plasticity may play a greater role in one scenario than in the other. Fourth, almost all patients presented with left hemispheric lesions; different results may have been obtained if equal groups of left and right hemispheric lesions were considered. However, since this was a retrospective analysis, only the available data based on referral patterns could be reported.
Conclusion
There was no significant correlation between fMRI lateralization and transient speech defects that had been observed following SMA-area surgery. In addition, during the analysis of the fMRI data, we found that qualitative/visual assessment of SMA laterality was superior to LI as calculated via ROI, and hypothesize that this may be due to the close proximity and possible overlap of signal from midline SMA. Moreover, a majority of patients showed SMA lateralization contralateral to the SMA lesion.
Acknowledgment
Julio Arevalo Perez was supported by a grant from the Spanish foundation Fundación Alfonso Martín Escudero.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Bannur U, Rajshekhar V. Post operative supplementary motor area syndrome: Clinical features and outcome. Br J Neurosurg 2000; 14: 204–210. [DOI] [PubMed] [Google Scholar]
- 2.Laplane D, Talairach J, Meininger V, et al. Clinical consequences of corticectomies involving the supplementary motor area in man. J Neurol Sci 1977; 34: 301–314. [DOI] [PubMed] [Google Scholar]
- 3.Zentner J, Hufnagel A, Pechstein U, et al. Functional results after resective procedures involving the supplementary motor area. J Neurosurg 1996; 85: 542–549. [DOI] [PubMed] [Google Scholar]
- 4.Nachev P, Kennard C, Husain M. Functional role of the supplementary and pre-supplementary motor areas. Nat Rev Neurosci 2008; 9: 856–869. [DOI] [PubMed] [Google Scholar]
- 5.Geyer S, Matelli M, Luppino G, et al. Receptor autoradiographic mapping of the mesial motor and premotor cortex of the macaque monkey. J Comp Neurol 1998; 397: 231–250. [DOI] [PubMed] [Google Scholar]
- 6.Peck KK, Bradbury M, Psaty EL, et al. Joint activation of the supplementary motor area and presupplementary motor area during simultaneous motor and language functional MRI. Neuroreport 2009; 20: 487–491. [DOI] [PubMed] [Google Scholar]
- 7.Dinomais M, Minassian AT, Tuilier T, et al. Functional MRI comparison of passive and active movement: Possible inhibitory role of supplementary motor area. Neuroreport 2009; 20: 1351–1355. [DOI] [PubMed] [Google Scholar]
- 8.Tremblay P, Gracco VL. Contribution of the pre-SMA to the production of words and non-speech oral motor gestures, as revealed by repetitive transcranial magnetic stimulation (rTMS). Brain Res 2009; 1268: 112–124. [DOI] [PubMed] [Google Scholar]
- 9.Baxendale S. The Wada test. Curr Opin Neurol 2009; 22: 185–189. [DOI] [PubMed] [Google Scholar]
- 10.Lehéricy S, Cohen L, Bazin B, et al. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology 2000; 54: 1625–1633. [DOI] [PubMed] [Google Scholar]
- 11.Medina LS, Bernal B, Ruiz J. Role of functional MR in determining language dominance in epilepsy and nonepilepsy populations: A Bayesian analysis. Radiology 2007; 242: 94–100. [DOI] [PubMed] [Google Scholar]
- 12.Klöppel S, Büchel C. Alternatives to the Wada test: A critical view of functional magnetic resonance imaging in preoperative use. Curr Opin Neurol 2005; 18: 418–423. [DOI] [PubMed] [Google Scholar]
- 13.Springer JA, Binder JR, Hammeke TA, et al. Language dominance in neurologically normal and epilepsy subjects: A functional MRI study. Brain 1999; 122(Pt 11): 2033–2046. [DOI] [PubMed] [Google Scholar]
- 14.Krainik A, Lehericy S, Duffau H, et al. Postoperative speech disorder after medial frontal surgery: Role of the supplementary motor area. Neurology 2003; 60: 587–594. [DOI] [PubMed] [Google Scholar]
- 15.Fontaine D, Capelle L, Duffau H. Somatotopy of the supplementary motor area: Evidence from correlation of the extent of surgical resection with the clinical patterns of deficit. Neurosurgery 2002; 50: 297–303. discussion 303–305. [DOI] [PubMed] [Google Scholar]
- 16.Ulmer JL, Krouwer HG, Mueller WM, et al. Pseudo-reorganization of language cortical function at fMR imaging: A consequence of tumor-induced neurovascular uncoupling. AJNR Am J Neuroradiol 2003; 24: 213–217. [PMC free article] [PubMed] [Google Scholar]
- 17.Holodny AI, Schulder M, Liu W, et al. The effect of brain tumors on BOLD functional MR imaging activation in the adjacent motor cortex: Implications for image-guided neurosurgery. AJNR Am J Neuroradiol 2000; 21: 1415–1422. [PMC free article] [PubMed] [Google Scholar]
- 18.Petrovich NM, Holodny AI, Brennan CW, et al. Isolated translocation of Wernicke’s area to the right hemisphere in a 62-year-man with a temporo-parietal glioma. AJNR Am J Neuroradiol 2004; 25: 130–133. [PMC free article] [PubMed] [Google Scholar]
- 19.Holodny AI, Schulder M, Ybasco A, et al. Translocation of Broca’s area to the contralateral hemisphere as the result of the growth of a left inferior frontal glioma. J Comput Assist Tomogr 2002; 26: 941–943. [DOI] [PubMed] [Google Scholar]
- 20.Ulmer JL, Hacein-Bey L, Mathews VP, et al. Lesion-induced pseudo-dominance at functional magnetic resonance imaging: Implications for preoperative assessments. Neurosurgery 2004; 55: 569–579; discussion 580–581. [DOI] [PubMed] [Google Scholar]
- 21.Ramsey NF, Sommer IE, Rutten GJ, et al. Combined analysis of language tasks in fMRI improves assessment of hemispheric dominance for language functions in individual subjects. Neuroimage 2001; 13: 719–733. [DOI] [PubMed] [Google Scholar]
- 22.Morgan VL, Mishra A, Newton AT, et al. Integrating functional and diffusion magnetic resonance imaging for analysis of structure-function relationship in the human language network. PLoS One 2009; 4: e6660. [DOI] [PMC free article] [PubMed] [Google Scholar]