Abstract
Pediatric cerebral sinovenous thrombosis (CSVT) is associated with high morbidity and mortality. Severe long-term sequelae are reported in up to 48% of children. The most frequent location of CSVT in children is the superficial venous system. We present the neuroimaging findings using both computed tomography and magnetic resonance imaging (MRI) in a 10-year-old child with extensive superficial CSVT. Our report aims to stress the importance of awareness of risk factors in suspecting and rapidly diagnosing CSVT. The application of targeted conventional and advanced MRI sequences is the diagnostic tool of choice in children at risk of or with clinically suspected CSVT.
Keywords: sinovenous thrombosis, neuroimaging, children, magnetic resonance imaging, risk factor
Introduction
Cerebral sinovenous thrombosis (CSVT) is defined by thrombosis of the superficial (cortical veins, superior sagittal sinus, transverse sinus, sigmoid sinus, and jugular vein) or deep (inferior sagittal sinus, internal cerebral veins, vein of Galen, straight sinus) venous system.1 CSVT occurs in about 1 of 100,000 children per year and accounts for 1 in 4 cases of pediatric stroke.2 CSVT is associated with high mortality and morbidity. The mortality rate ranges between 8–19% and severe long-term neurological sequelae occur in up to 48% of children.2,3 Prognosis is related to the extent of vessel and brain parenchymal involvement as well to timeliness of diagnosis and institution of therapy. A rapid institution of therapy including anticoagulation may prevent thrombus propagation and involvement of a larger brain region (e.g. intraparenchymal venous stasis or venous stroke) and improve the long-term outcome.4 The diagnosis of CSVT must consequently be made as soon as possible after onset of symptoms or should be ruled out in children at high risk for thrombosis, even when only minor symptoms are present.
We report on a 10-year-old girl with Crohn’s disease who presented with headaches and neck pain and stiffness due to bacterial meningitis and CSVT. We discuss the key role of the clinician and neuroradiologist to be aware of the multiple risk factors that can increase the likability of CVST to develop and the diagnostic significance of head magnetic resonance imaging (MRI) in making a rapid diagnosis of CSVT.
Case report
A 10-year-old girl presented to the emergency department (ED) of our tertiary children’s hospital with worsening headache. Based on a normal neurological exam and transient improvement upon analgesic drugs, she was discharged home. Two days later, she presented again to the ED because of severe, pulsating headaches in the left temporal and periorbital regions. In addition, she developed neck pain and stiffness, photophobia, and phonophobia. The neurological exam revealed a positive Brudzinski sign.
On retrospect, the headaches had started about 4 weeks before the presentation at the ED, were intermittent, located in the left temporal region, and associated with dizziness and blurry vision. One week prior to presentation, the symptoms were worsening with episodes of nausea and vomiting. At that time, the patient had a head computed tomography (CT) scan at an outside hospital, which was reported as normal. In addition, she developed bilateral acute otitis media two weeks to presentation.
The patient’s past medical history is notable for atypical Crohn’s disease since the age of 5 years. The medical management of Crohn’s disease was difficult and remission was obtained only under continuous daily steroid therapy. The child became steroid dependent and developed a secondary adrenal insufficiency that needed hydrocortisone substitution. In addition, the child developed a secondary vitamin D deficiency that needed substitution with cholecalciferol. In an attempt to reduce the steroid dependency, an immunosuppressive therapy with thalidomide 50 mg daily was started. Immunosuppression caused recurrent otitis media and sinusitis requiring antibiotic therapy.
Neck pain and stiffness, photophobia, phonophobia, and a positive Brudzinski sign at the time of ED presentation were suggestive of meningitis, which was confirmed by a lumbar puncture and cerebrospinal fluid (CSF) exam. CSF as well as peripheral blood culture were positive for Streptococcus anginosus. Bacterial meningitis was considered secondary to otitis media in an immunosuppressed child. An antibiotic therapy with ceftriaxone was started. In addition, a brain MRI was performed that showed a T2 hyperintense and T1 isointense tubular structure with restricted diffusion within the bilateral proximal internal jugular vein as well as sigmoid and transverse sinus corresponding to an extensive thrombus (Figure 1). In addition, there was an area of restricted diffusion noted within the left cavernous sinus suggesting partial left sided cavernous sinus thrombosis. There was no leptomeningeal enhancement or evidence of infarction or hemorrhage. A complementary head CT scan revealed bilateral otomastoiditis and a large area of osseous dehiscence in the left mastoid complex communicating with the left middle cranial fossa (Figure 2).
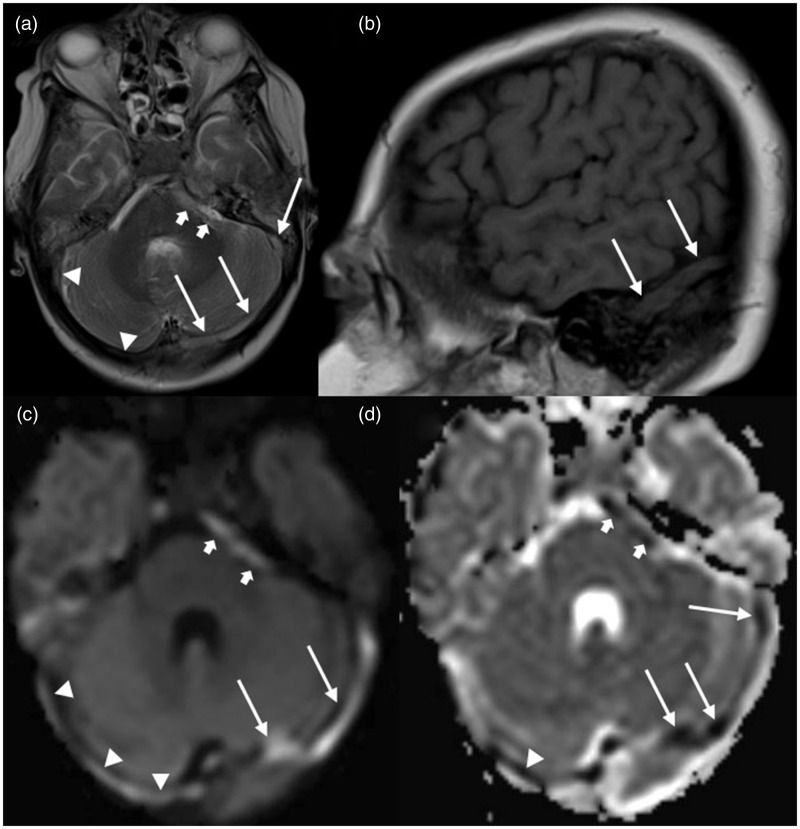
Figure 1.
MRI of a 10-year-old girl with extensive CSVT: (a) axial T2-weighted image shows hyperintense signal in the left (long arrows) and right (arrowheads) transverse and sigmoid sinus and mixed hyper- and isointense signal in the left cavernous sinus (short arrows); (b) sagittal T1-weighted image reveals isointense signal in the left transverse sinus (long arrows); (c) axial trace of diffusion and (d) apparent diffusion coefficient (ADC) maps show DWI-bright signal in the left (long arrows) and right (arrowheads) transverse and sigmoid sinus and the left cavernous sinus (short arrows) with matching low ADC values.
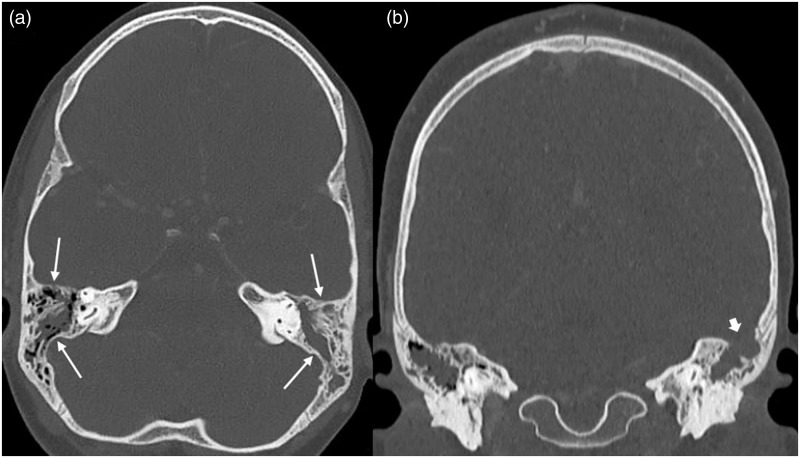
Figure 2.
CT of a 10-year-old girl with extensive CSVT: axial (a) and coronal (b) CT images of the skull base reconstructed in bone algorithm show bilateral mastoid effusion, left > right (long arrows) and an area osseous dehiscence in the left mastoid complex communicating with the left middle cranial fossa (short arrow).
Because of the diagnoses of bacterial meningitis and extensive CSVT, the child was transferred to the pediatric intensive care unit and an anticoagulation therapy with enoxaparin was started. Although the child had multiple risk factors for CSVT (otitis media, mastoiditis, meningitis, and Crohn’s disease), a thrombophilia workup including cardiolipin, B2 glycoprotein, protein C/S, homocysteine, antithrombin III, and antiphospholipid a/b level and MTHFR and prothrombin 20212 gene mutation was performed and revealed normal results.
The antibiotic therapy was continued for 6 weeks. A clinical follow-up exam 6 weeks after diagnosis was unremarkable. A follow-up brain MRI at the same time showed recanalization of the right proximal jugular vein, and sigmoid and transverse sinus, while there was persistent thrombosis of the left transverse and sigmoid sinus.
Discussion
Epidemiology studies suggest that CSVT affects about 1 of 100,000 children per year and accounts for 1 in 4 cases of childhood ischemic stroke.2 A prompt diagnosis of CSVT in children is clinically challenging because of the sometimes subtle and usually non-specific clinical presentation. An early diagnosis however is essential to effecting the best possible outcome. A high index of suspicion is needed for clinicians and radiologists who need to be aware of the various risk factors for CSVT in children.
Risk factors are definable in more than 98% of children with CSVT.5 They are frequently multiple and are age-related. In newborns and infants, dehydration and perinatal events are common. Head and neck infections are present in about one-third of preschool children, while other associations, including systemic disease and trauma, are clustered in the older age groups. Infection is a major risk factor for childhood CSVT.5 Septic CSVT is defined as thrombosis of venous sinuses associated with head and neck infections, including otitis media, mastoiditis, sinusitis, and meningitis as in our patient. Septic CSVT may account for 18–50% of childhood CSVT.5 The common pathomechanism involves spread of infection from adjacent tissues into venous sinuses leading to thrombophlebitis. In addition, acute bacterial infection and sepsis impart other risk factors such as dehydration, systemic inflammation, and coagulopathy. Chronic systemic diseases also increase the risk of childhood CSVT, especially in older school-age children. In inflammatory bowel disease such as Crohn’s disease, systemic inflammation may combine with gastrointestinal disease factors, such as dehydration, medications, and iron deficiency.6 In pediatric inflammatory bowel diseases, neurologic manifestations are rare, occur in about 3% of the children, and are dominated by vascular complications, including arterial occlusion, CSVT, and vasculitis.6 In addition, mononeuropathies and abnormalities of the white matter have been reported.7 Our patient displayed three risk factors for CSVT: (1) a chronic systemic disease (Crohn’s disease) treated with (2) a procoagulant drug (thalidomide);8 and (3) an acute infectious disease of the head or neck (bilateral otomastoiditis and meningitis). Awareness of these risk factors led to early diagnosis, therapy, and determined the good outcome of the extensive CSVT in our patient.
After the identification of risk factors, neuroimaging plays the key role in the diagnosis of CSVT.5 The primary goal of neuroimaging is (1) to visualize and characterize the thrombus, (2) to identify the degree of impaired flow within the affected venous system, and (3) to rule out secondary complications such as venous ischemia or hemorrhage. A number of different techniques have been applied in the diagnostic work-up of CSVT: CT with or without venography, MRI with or without venography, conventional angiography, and trans-fontanel Doppler ultrasonography.5 In most circumstances, MRI is the diagnostic modality of choice in pediatric CSVT. MRI lacks radiation and may provide additional details of both brain parenchyma and the venous system combining conventional and advanced MRI sequences such as diffusion weighted/tensor imaging (DWI/DTI) and susceptibility weighted imaging (SWI). MRI sequences sensitive to vasogenic edema (e.g. fluid attenuation inversion recovery (FLAIR), DWI, and DTI), cytotoxic edema (e.g. DWI and DTI), and blood products (e.g. SWI) can confirm and characterize CSVT-related parenchymal changes. MRI may also image thrombosis itself, possibly providing more information about the age and nature of the thrombus/lesion. Subacute thrombi often appear hyperintense on T1- and T2-weighted images.
A number of potential pitfalls may challenge the MRI diagnosis of CSVT.2 Common pitfalls are congenital asymmetry of the transverse sinuses without cerebral venous pathology,2 and atretic segments in the transverse sinus with otherwise normal brain MRI studies. Such “flow-gaps” occur more frequently in the smaller or non-dominant sinus.2 Another common pitfall is arachnoid granulations. They are physiologic small filling defects within the dural sinuses and have a density (CT)/signal intensity (MRI) identical to cerebrospinal fluid. They can be isointense on T1-weighted sequences and on FLAIR sequences, but should not be hyperintense on either of these sequences.2,9
A high index of suspicion as well as familiarity with the neuroimaging findings is important for an early, sensitive, and specific diagnosis of CSVT in children.
In conclusion, our case demonstrates that the awareness of risk factors is the most important premise for an early and accurate diagnosis of CSVT in children and that neuroimaging is the diagnostic tool of choice to confirm CSVT and to follow-up patients with confirmed diagnosis.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The authors report that there are no conflicts of interest.
References
- 1.deVeber G. Cerebral sinovenous (venous sinus) thrombosis. In: Ganesan V, Kirkham FJ. (eds). Stroke and cerebrovascular disease in childhood, International Review of Child Neurology Series, London: Mac Keith Press, 2011, pp. 145–159. [Google Scholar]
- 2.Bracken J, Barnacle A, Ditchfield M. Potential pitfalls in imaging of paediatric cerebral sinovenous thrombosis. Pediatr Radiol 2013; 43: 219–231. [DOI] [PubMed] [Google Scholar]
- 3.Berfelo FJ, Kersbergen KJ, van Ommen CH, et al. Neonatal cerebral sinovenous thrombosis from symptom to outcome. Stroke 2010; 41: 1382–1388. [DOI] [PubMed] [Google Scholar]
- 4.Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a Special Writing Group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke 2008; 39: 2644–2691. [DOI] [PubMed] [Google Scholar]
- 5.deVeber G, Andrew M, Adams C, et al. Cerebral sinovenous thrombosis in children. N Engl J Med 2001; 345: 417–423. [DOI] [PubMed] [Google Scholar]
- 6.Standridge S, de los Reyes E. Inflammatory bowel disease and cerebrovascular arterial and venous thromboembolic events in 4 pediatric patients: a case series and review of the literature. J Child Neurol 2008; 23: 59–66. [DOI] [PubMed] [Google Scholar]
- 7.Lossos A, River Y, Eliakim A, et al. Neurologic aspects of inflammatory bowel disease. Neurology 1995; 45(3 Pt 1): 416–421. [DOI] [PubMed] [Google Scholar]
- 8.El Accaoui RN, Shamseddeen WA, Taher AT. Thalidomide and thrombosis. A meta-analysis. Thromb Haemost 2007; 97: 1031–1036. [PubMed] [Google Scholar]
- 9.Leach JL, Meyer K, Jones BV, et al. Large arachnoid granulations involving the dorsal superior sagittal sinus: findings on MR imaging and MR venography. Am J Neuroradiol 2008; 29: 1335–1339. [DOI] [PMC free article] [PubMed] [Google Scholar]