Abstract
This study aimed to assess changes in the posterior cranial fossa (PCF) to shed light on the mechanism of cerebellar herniation in children with Costello syndrome (CS) and posterior fossa crowding. We performed a morphovolumetric PCF analysis on brain magnetic resonance imaging (MRI) in seven children with CS (mean age 31 ± 16 months) comparing the MRI scans with those of seven age-matched healthy subjects.
PCF volume (PCFV), PCF brain volume (PCFBV) and cerebellar volume (CeV) were assessed on axial T2-weighted MRI. Morphometric parameters (diameters of the foramen magnum, tentorial angle, basiocciput, supraocciput, basisphenoid and exocciput lengths) were measured on sagittal T1-weighted MRI. The volume of the cerebrospinal fluid (CSF) spaces was calculated as PCFV minus PCFBV.
Five out of seven CS children showed tonsillar herniation in the upper cervical canal; no child had hydrocephalus but three out of seven children showed ventriculomegaly. In addition, the PCFV/PCFBV ratio, PCFV, CSF spaces volume, basiocciput, basisphenoid and exocciput lengths and latero-lateral and antero-posterior diameters of the foramen magnum were significantly reduced, whereas no significant changes were found in supraocciput length, PCFBV, CeV or hindbrain volume
The volumetric reduction of the PCF due to bony posterior fossa hypoplasia is a predisposing factor for developing cerebellar tonsillar herniation through the foramen magnum in children with CS. The altered anatomy of the foramen magnum and upward expansion of the PCF secondary to an increased tentorial slope serves to explain the possible mechanism of cerebellar herniation in patients with CS.
Keywords: Magnetic resonance imaging, Chiari anomaly, posterior cranial fossa
Introduction
Costello syndrome (CS) is a rare genetic disorder associated with heterozygous germline mutations in the proto-oncogene HRAS.1–3 The HRAS protein is a key regulator of the mitogen-activated protein kinase (MAPK) pathway, and Costello-associated missense mutations result in constitutive activation of the mutant protein and increased MAPK signalling.4,5 Mutations affecting other components of the MAPK pathway cause neurofibromatosis type 1, Noonan syndrome and cardio-facio-cutaneous syndrome, among others. The shared mechanism of increased MAPK signalling represents the common denominator, resulting in the overlapping phenotypes of these genetic syndromes collectively referred to as RASopathies.6
The phenotype of CS in infancy and childhood has been fully described in over 100 cases.7 This complex developmental disorder involves characteristic craniofacial features, failure to thrive, developmental delay, and cardiac and skeletal anomalies with a predisposition to develop benign and malignant tumours.6 Tonsillar herniation (Chiari anomaly) is one of the main features of this syndrome, and has been implicated as a possible cause of neurological abnormalities,8–11 even if its aetiology is not fully understood.
Magnetic resonance imaging (MRI) is the main diagnostic imaging tool to investigate Chiari anomaly and syringomyelia even in the absence of clear-cut symptoms. Follow-up MRI performed every 2 years is useful to monitor the development of tonsillar herniation and syringomyelia.
This paper presents a posterior cranial fossa (PCF) analysis including a morphometric and volumetric analysis of MRI scans to shed light on the possible mechanism of cerebellar herniation in children with CS and PCF crowding.
Material and methods
Patient population and MRI protocol
We analysed the brain MRI of seven children with CS identified by both characteristic craniofacial abnormalities and germline mutations in the proto-oncogene HRAS. The ages of our study group ranged from 18 to 48 months (mean age 31 ± 16 months) at the time of the first MRI. Morphometric and volumetric measurements of the PCF were performed on MRI scans. All MRI examinations were performed according to a standard protocol on a 1.5-Tesla Signa unit (General Electric Healthcare; Milwaukee, WI) with a standard head coil. MRI consisted of high-resolution sagittal T1-weighted and T2-weighted images in the axial, sagittal and coronal planes. Brain MRI images of patients with CS were compared with those of seven age-matched healthy subjects with normal MRI who were examined for epilepsy, syncope or EEG abnormalities (Table 1).
Table 1.
Classification of seven children with Costello syndrome.
| Syndrome | Patients (n) | Sex (n) |
Mean age (months) | Cerebellar tonsillar herniation (I RM) | Cerebellar tonsillar herniation (II RM) | Hydrocephalus | |
|---|---|---|---|---|---|---|---|
| M | F | ||||||
| Costello | 7 | 3 | 4 | 31 ± 16 | 5 | 7 | 0 |
The study was approved by the institutional review board.
Volumetric analysis of the posterior cranial fossa
We used axial T2-weighted MRI to measure posterior cranial fossa volume (PCFV), posterior cranial fossa brain volume (PCFBV) and the entire cerebellar volume (CeV). Volumetric measurements were calculated automatically using the Cavalieri principle.12,13
PCFV was defined as the osseous anatomical area with boundaries formed by the supraoccipital portion of the occipital bone (posterior boundary of the fossa), the tentorium cerebelli (superior boundary of the fossa), the basisphenoid and basioccipital portions of the clivus (anterior boundaries of this fossa) and the extension of the posterior petroclinoid ligaments from the petrous ridges of the temporal bones to the posterior clinoids (anterolateral borders of this cavity).
PCFBV was defined as the volume of the neural structures contained within the PCF including the parts of the brain that herniate into the spinal canal. It included the entire cerebellum and brain stem without a cerebrospinal fluid (CSF) signal around the hindbrain in the posterior fossa. The volume ratio of PCFV to PCFBV was calculated to estimate overcrowding in the PCF.
CeV comprised the entire cerebellum (vermis and hemispheres). The volume of the CSF spaces around the hindbrain was calculated as PCFV minus PCFBV (Figure 1).
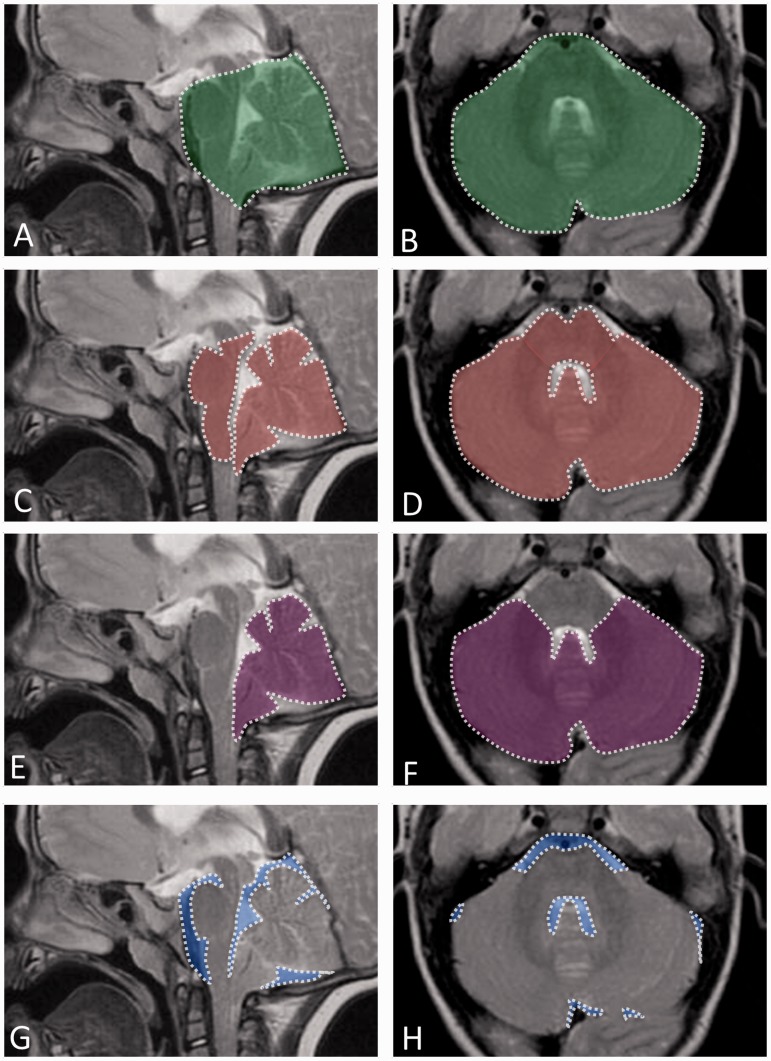
Figure 1.
Volumetric measurements of the posterior cranial fossa (PCFV) and cerebellum (PCFBV) in a representative child with Costello syndrome and posterior fossa crowding. T2-weighted sagittal (A, C, E, G) and axial (B, D, F, H) MR images. (A, B) Outlined in white are the boundaries of the posterior fossa which include the entire cerebellum, brain stem and extra-axial spaces. PCFV is shown in green-shaded areas. (C, D) Outlined in white are the boundaries of the neural structures contained within the posterior cranial fossa comprising the entire cerebellum including the parts herniating into the spinal canal and brain stem; extra-axial spaces are not included. PCFBV is shown in red-shaded areas. (E, F) Outlined in white are the boundaries of the cerebellum shown in purple shaded areas. (G, H) CSF spaces around the hindbrain are shown in blue-shaded areas.
Morphometric measurements of the PCF
Eight measurements from recently published morphometric studies were selected to evaluate PCF structures and were measured by an expert neuroradiologist. Six measurements were determined on the midsagittal T1-weighted image: (1) length of the basiocciput extending from the spheno-occipital synchondrosis to the basion; (2) length of the basisphenoid, extending from the spheno-occipital synchondrosis to the top of the dorsum sellae; (3) length of the supraocciput, extending from the internal occipital protuberance to the opisthion; (4) McRae’s line (antero-posterior diameter of the foramen magnum) from basion to opisthion; (5) tonsillar herniation (the distance between the tip of the cerebellar tonsils and McRae’s line); (6) the angle between the cerebellum tentorium and a line connecting the internal occipital protuberance and the opisthion (called Twining’s line) to estimate the steepness of the cerebellum tentorium. One measurement was determined on the coronal T1-weighted MRI: (7) the length of the exocciput of both sides (distance between the bottom of the occipital condyle and the top of the jugular tubercle). One additional measurement was obtained using axial T2-weighted MRI: (8) the maximum transverse diameter of the foramen magnum (Figure 2).
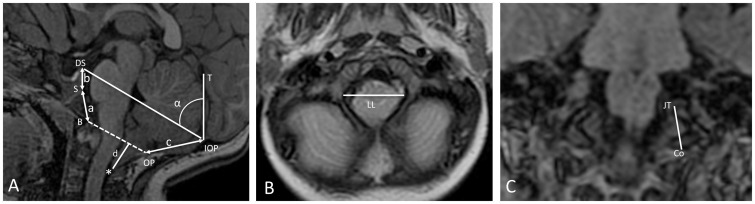
Figure 2.
Morphometric parameters of the posterior cranial fossa in a representative child with Costello syndrome. A) Midsagittal T1-weighted MRI; a = length of the basiocciput, between the spheno-occipital synchondrosis (S) and the basion (B); b = length of basisphenoid, between the top of the dorsum sellae (DS) and the spheno-occipital synchondrosis of the clivus (S); a + b = length of clivus; c = length of the supraocciput between the internal occipital protuberance (IOP) and the opisthion (OP); d = distance between the tip of the cerebellar tonsils (*) and McRae’s line (dashed line: B to OP); McRae’s line = antero-posterior diameter of the foramen magnum (AP); α = angle of the cerebellar tentorium (T) to Twining's line (TL: IOP to DS). B) Axial T2-weighted MRI; LL = maximum tranverse diameter of the foramen magnum. C) Coronal T1-weighted MRI; F = length of exocciput extends from the bottom of the occipital condyle (Co) to the top of the jugular tubercle (JT).
Statistical analysis
Descriptive statistics were expressed as the means ± SD for continuous variables. All statistical analyses were performed with Stat View version 5.0 (SAS Institute Inc.). Baseline characteristics were compared using the Student t-test. The level of significance was set at p < 0.05, and significance levels were adjusted according to the Bonferroni procedure to control the risk of α-inflation.
Results
Results of all measurements are listed in Tables 1 and 2. All studied children showed overcrowding of the PCF (ratio PCFV/PCFBV < 0.05). Five out of seven children examined showed tonsillar herniation in the cervical canal at the time of the first evaluation and two children showed tonsillar herniation after 2 years. Chiari anomaly was symptomatic in only two children, but neither had cervical surgical decompression at the time of the first MRI. Three out of seven children showed ventriculomegaly but no child developed hypertensive hydrocephalus.
Table 2.
Volumetric and morphometric analysis of the posterior cranial fossa in children with Costello syndrome.
| Patients | Control | ||
|---|---|---|---|
| Mean value | Mean value | p-value | |
| Age (months) | 31.14 | 31.75 | 0.9 |
| Volumetric analysis of the posterior cranial fossa | |||
| PCFV (mm3) | 146347.85 | 169483.87 | 0.04 |
| PCFBV (mm3) | 132623.8 | 146413.3 | 0.2 |
| PCFV/PCFBV | 1.105 | 1.193 | 0.004 |
| CV (mm3) | 108387.6 | 122693.75 | 0.09 |
| Brainstem | 24236.2 | 22623.3 | 0.3 |
| V. CSF spaces | 13724.05 | 27769.5 | 0.001 |
| Morphometric analysis of the posterior cranial fossa | |||
| Basiocciput (mm) | 15.2857 | 19.4875 | 0.007 |
| Basisphenoid (mm) | 10.6428 | 13.075 | 0.03 |
| Supraocciput (mm) | 35.4914 | 38.515 | 0.2 |
| Tentorial angle (°) | 49.1 | 36.6837 | 0.0001 |
| Foramen magnum AP (mm) | 28.6714 | 34.655 | 0.0001 |
| Foramen magnum LL (mm) | 26.4714 | 30.471 | 0.003 |
| Right exocciput (mm) | 11.114 | 15.45 | 0.0007 |
| Left exocciput (mm) | 11.114 | 15.475 | 0.0005 |
| Right condyle (mm) | 3.857 | 5.775 | 0.0005 |
| Left condyle (mm) | 3.857 | 5.775 | 0.0005 |
Compared with the control group, PCFV, volume of the CSF spaces, basiocciput, basisphenoid and exocciput lengths, and latero-lateral (LL) and antero-posterior (AP) diameters of the foramen magnum were significantly reduced, whereas supraocciput length, PCFBV, CeV and hindbrain volume changes were not statistically significant (Tables 1 and 2).
Discussion
Chiari malformation (CM) is a recognized feature of CS and may be considered a possible cause of neurological abnormalities.11 Chiari anomaly is defined as herniation of the cerebellar tonsils through the foramen magnum usually by more than 3–5 mm.14,15 Tonsillar herniation is considered definitely abnormal when it exceeds 5 mm, and borderline between 3 and 5 mm.16 The anomaly is due to a disproportion between cerebellar size and available space in the PCF resulting in a crowded PCF. This disproportion is usually secondary to a reduced size of the PCF in relation to the cerebellum.17–19 Undergrowth of the occipital bone from a supposed para-axial mesodermal insufficiency and resulting CM type1 has been reported in a few children with CS.11 On the other hand, others have defined PCF overcrowding in children with CS as an enlarged cerebellum with respect to the PCF.6 In both cases, PCF crowding may decrease CSF flow and absorption, resulting in the associated ventricular enlargement and, less commonly, hydrocephalus.
Compared with a control group, our patients showed PCF crowding (ratio PCFV/PCFBV < 0.05) due to a significant reduction of PCFV and CSF spaces, whereas they did not show abnormalities of CeV, PCFBV or brain stem volume. Three out of seven children showed ventricular enlargement but no child had hypertensive hydrocephalus.
Our data also demonstrated that the PCF volumetric reduction was related to a significant reduction of bony PCF due to a significant shortening of basiocciput, basisphenoid and exocciput lengths. The finding of normal supraocciput length and normal volume of PCF neural structures suggests the “key” role of supraocciput length in cerebellum growth. Normal cerebellar growth in a hypoplastic PCF may lead to cerebellar herniation, resulting in cervico-medullary compression, syrinx formation and associated neurological symptoms. Chiari anomaly in CS is reported to represent a postnatal process rather than a static congenital malformation.6 Our data cannot show the time course of cerebellar herniation from the beginning because the first MRI in our case series was performed after 2 years of age and we do not have MRI studies at the time of the diagnosis. However, all children in our cohort developed cerebellar herniation within the 4th year of life.
In agreement with previous studies reporting that CM type 1 often remains asymptomatic,11 Chiari anomaly was symptomatic in only two children in our case series. Moreover, our data, demonstrating a significant reduction of AP and LL diameters of the foramen magnum and a significant increase in the tentorial angle, suggest that foramen magnum size and tentorial slope play an important role in the mechanism of development of cerebellar herniation. The significant increase in the tentorial angle due to vertical orientation of the cerebellar tentorium supports the view that the tentorium was pushed forward and became steeper to allow normal growth of the cerebellum. On the other hand, the significant reduction of AP and LL diameters of the foramen magnum helps to explain cerebellar growth in a cranial direction with increasing tentorial slope. Our findings suggest that cerebellar herniation occurs when cerebellar expansion along the caudo-cranial axis fails, even if a concomitant role of intracranial pressure, venous hypertension and hydrocephalus cannot be ruled out.
Conclusion
The volumetric reduction of the PCF due to bony posterior fossa hypoplasia in children with CS is a predisposing factor for the development of cerebellar tonsillar herniation through the foramen magnum. The altered anatomy of the foramen magnum and upward expansion of the PCF secondary to increased tentorial slope helps to explain the possible mechanism of cerebellar herniation in patients with CS. Future prospective studies may shed more light on the time course of tonsillar herniation development and the predisposing factors contributing to cerebellar herniation, such as pressure changes in CSF flow.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lorenz S, Lissewski C, Simsek-Kiper PO, et al. Functional analysis of a duplication (p.E63_D69dup) in the switch II region of HRAS: New aspects of the molecular pathogenesis underlying Costello syndrome. Hum Mol Genet 2013; 22: 1643–1653. [DOI] [PubMed] [Google Scholar]
- 2.Gripp KW, Lin AE, Stabley DL, et al. HRAS mutation analysis in Costello syndrome: Genotype and phenotype correlation. Am J Med Genet A 2006; 140: 1–7. [DOI] [PubMed] [Google Scholar]
- 3.Aoki Y, Niihori T, Kawame H, et al. Germline mutations in HRAS proto-oncogene cause Costello syndrome. Nat Genet 2005; 37: 1038–1040. [DOI] [PubMed] [Google Scholar]
- 4.Rauen KA, Hefner E, Carrillo K, et al. Molecular aspects, clinical aspects and possible treatment modalities for Costello syndrome: Proceedings from the 1st International Costello Syndrome Research Symposium 2007. Am J Med Genet A 2008; 146A: 1205–1217. [DOI] [PubMed] [Google Scholar]
- 5.Rauen KA. HRAS and the Costello syndrome. Clin Genet 2007; 71: 101–108. [DOI] [PubMed] [Google Scholar]
- 6.Gripp KW, Hopkins E, Doyle D, et al. High incidence of progressive postnatal cerebellar enlargement in Costello syndrome: Brain overgrowth associated with HRAS mutations as the likely cause of structural brain and spinal cord abnormalities. Am J Med Genet A 2010; 152A: 1161–1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Costello JM. A new syndrome: Mental subnormality and nasal papillomata. Aust Paediatr J 1977; 13: 114–118. [DOI] [PubMed] [Google Scholar]
- 8.Delrue MA, Chateil JF, Arveiler B, et al. Costello syndrome and neurological abnormalities. Am J Med Genet A 2003; 123A: 301–305. [DOI] [PubMed] [Google Scholar]
- 9.Gripp KW, Scott CI, Jr., Nicholson L, et al. Five additional Costello syndrome patients with rhabdomyosarcoma: Proposal for a tumor screening protocol. Am J Med Genet 2002; 108: 80–87. [DOI] [PubMed] [Google Scholar]
- 10.Say B, Gucsavas M, Morgan H, et al. The Costello syndrome. Am J Med Genet 1993; 47: 163–165. [DOI] [PubMed] [Google Scholar]
- 11.Tubbs RS, Oakes WJ. Costello syndrome and Chiari I malformation: Apropos of a case with a review of the literature regarding a potential association. J Child Neurol 2003; 18: 496–498. [DOI] [PubMed] [Google Scholar]
- 12.Vatansever D, Kyriakopoulou V, Allsop JM, et al. Multidimensional analysis of fetal posterior fossa in health and disease. Cerebellum 2013; 12: 632–644. [DOI] [PubMed] [Google Scholar]
- 13.Yushkevich PA, Piven J, Hazlett HC, et al. User-guided 3D active contour segmentation of anatomical structures: Significantly improved efficiency and reliability. Neuroimage 2006; 31: 1116–1128. [DOI] [PubMed] [Google Scholar]
- 14.Novegno F, Caldarelli M, Massa A, et al. The natural history of the Chiari Type I anomaly. J Neurosurg Pediatr 2008; 2: 179–187. [DOI] [PubMed] [Google Scholar]
- 15.Barkovich AJ, Wippold FJ, Sherman JL, et al. Significance of cerebellar tonsillar position on MR. AJNR Am J Neuroradiol 1986; 7: 795–799. [PMC free article] [PubMed] [Google Scholar]
- 16.Aboulezz AO, Sartor K, Geyer CA, et al. Position of cerebellar tonsils in the normal population and in patients with Chiari malformation: A quantitative approach with MR imaging. J Comput Assist Tomogr 1985; 9: 1033–1036. [DOI] [PubMed] [Google Scholar]
- 17.Milhorat TH, Chou MW, Trinidad EM, et al. Chiari I malformation redefined: Clinical and radiographic findings for 364 symptomatic patients. Neurosurgery 1999; 44: 1005–1017. [DOI] [PubMed] [Google Scholar]
- 18.Nishikawa M, Sakamoto H, Hakuba A, et al. Pathogenesis of Chiari malformation: A morphometric study of the posterior cranial fossa. J Neurosurg 1997; 86: 40–47. [DOI] [PubMed] [Google Scholar]
- 19.Trigylidas T, Baronia B, Vassilyadi M, et al. Posterior fossa dimension and volume estimates in pediatric patients with Chiari I malformations. Childs Nerv Syst 2008; 24: 329–336. [DOI] [PubMed] [Google Scholar]