Abstract
Desmoplastic infantile ganglioglioma is a paediatric brain tumor that is commonly seen in the infantile age group. Literature on the non-infantile variant of this low-grade supratentorial neoplasm is very scarce, except for a few case reports. Herein, we report a case of desmoplastic non-infantile ganglioglioma occurring at the age of 6 years and describe its conventional and advanced magnetic resonance imaging characteristics.
Keywords: MRI, desmoplastic ganglioglioma, spectroscopy, diffusion, perfusion
Introduction
Desmoplastic ganglioglioma (DG) is a paediatric brain tumour that is often seen in the infantile age group (desmoplastic infantile ganglioglioma, DIG), although rare cases that occur in the non-infantile age group (desmoplastic non-infantile ganglioglioma, DNIG) have been observed.1,2 The tumour usually presents as a large, peripherally located cystic mass, including a mural nodule attached to the dural side.3 The prognosis is good even if the tumours are huge and have aggressive imaging appearances. Herein, we report an unusual case of DNIG that occurred at the age of 6 years. We aim to describe the conventional and advanced magnetic resonance imaging (MRI) characteristics including proton magnetic resonance spectroscopy (MRS), MR perfusion and diffusion-weighted imaging (DWI) findings.
Case report
A 6-year-old girl was taken to her paediatrician because of gaze palsy. With the suspicion of an intracranial pathology, brain MRI was performed on a 3-Tesla scanner (Verio, Siemens Medical Solutions, Erlangen, Germany). In addition to conventional sequences, DWI, dynamic susceptibility-weighted contrast-enhanced (DSC) perfusion, and proton MRS were included.
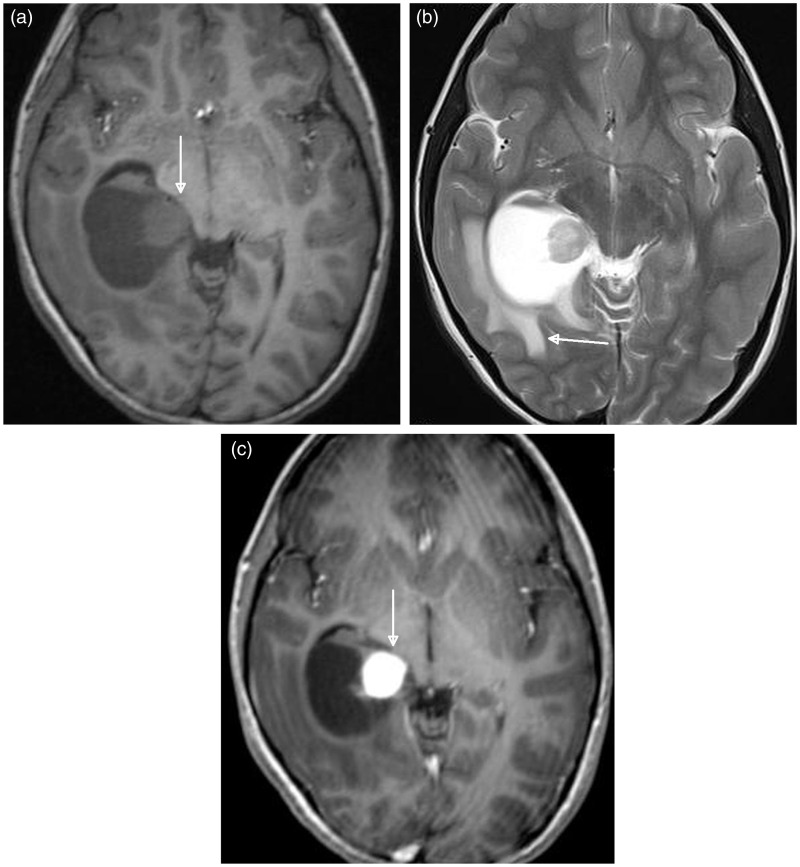
Images revealed a large, well-defined cystic mass filling the right hippocampus and parahippocampal gyrus, with obstruction in the temporal horn of the right lateral ventricle, resulting in transependymal flow of cerebrospinal fluid to the surrounding periventricular white matter (Figure 1(a) and (b)). The mass had a solid mural nodule that enhanced intensely after gadolinium administration (Figure 1(c)). The mural nodule was atypically located in the medial portion of the cystic mass and was slightly more hyperintense than the grey matter on T2-weighted image (T2WI) (Figure 1(b)). In addition, the mass had no distinctive dural attachment.
Figure 1.
Axial plane brain MRI findings of a 6-year-old female with desmoplastic non-infantile ganglioglioma. (a) T1-weighted image shows a large mass lesion in the right parahippocampal region adjacent to the ambient cistern. The cystic mass lesion has a mural nodule medially (arrow). MRI: magnetic resonance imaging. (b) T2-weighted image shows that the mural nodule is slightly more hyperintense than the grey matter. Note the transependymal flow of cerebrospinal fluid to the surrounding periventricular white matter (arrow). (c) Contrast-enhanced T1-weighted image demonstrates avid enhancement of the mural nodule (arrow).
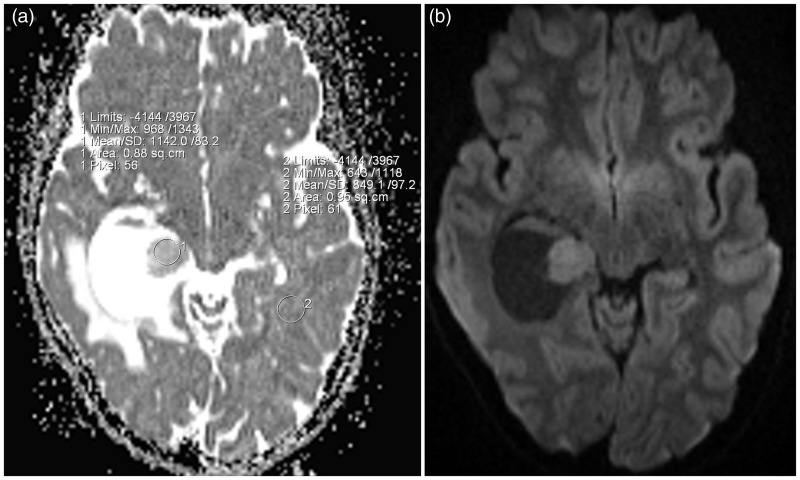
DWI (repetition time (TR)/echo time (TE) 15,000/90 ms, number of excitations (NEX 2), bandwidth 1014, slice thickness 3 mm, intersection gap 0, matrix size 128 × 128, field of view (FOV) 220 × 220 mm) was performed by using a spin-echo sequence with b of 0 and 1000 s/mm2. On DWI, the nodule was slightly hyperintense compared with the white matter, with mean (SD) apparent diffusion coefficient (ADC) values of 1.142 × 10−3 mm2/s from the solid portion of the tumoural region and 0.849 × 10−3 mm2/s from the normal white matter (Figure 2(a) and (b)).
Figure 2.
(a) The nodule shows high ADC values compared with those of the white matter of the contralateral side. (b) Axial plane DWI of the brain demonstrates a slightly hyperintense signal of the mural nodule compatible with T2 shine affect. ADC: apparent diffusion coefficient; DWI: diffusion-weighted imaging.
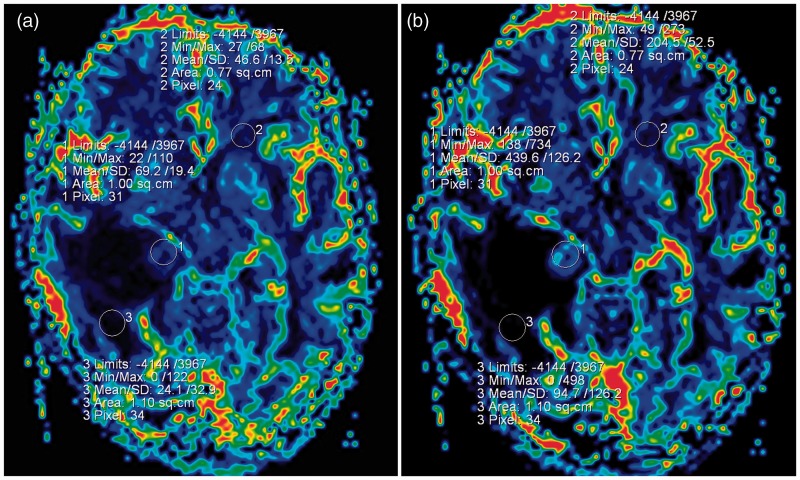
On the DSC perfusion images (TR/TE 1500/30 ms, NEX 1, bandwidth 1446, slice thickness 5 mm, intersection gap 1.5, matrix size 128 × 128, FOV 230 × 230 mm), subsequent calculation of cerebral blood flow (CBF), cerebral blood volume (CBV) and mean transit time (MTT) maps, the regional CBV and the CBF of the mural nodule were increased with respect to the peritumoural region and the opposite side of the cerebral white matter (Figure 3(a) and (b)). The CBV of the solid component, normal contralateral side and the peritumoral region were 439, 204 and 69.2, respectively. The CBV ratio was 2.15 from the solid portion to the contralateral side and 4.63 from the solid portion to the peritumoural region.
Figure 3.
(a) and (b) On CBF and CBV maps of the brain, the mural nodule shows increased values relative to the peritumoural region and the contralateral cerebral white matter. CBF: cerebral blood flow; CBV: cerebral blood volume.
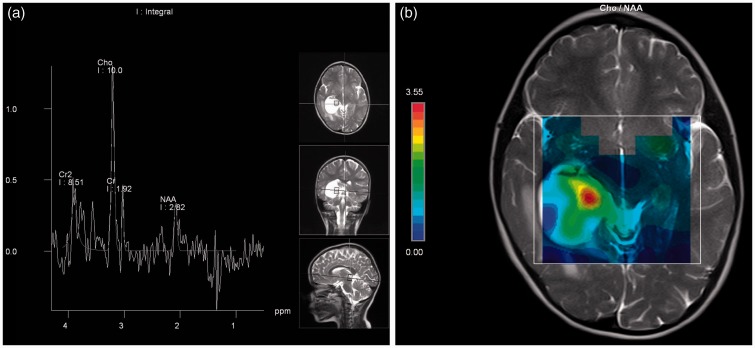
The multi-voxel proton MRS (TR/TE 1700/30–135 ms, matrix size 16 × 16, FOV 16 × 16 mm, NEX 3, bandwidth 1200) placed over the lesion and the solid component of the cystic mass showed an increase in choline (Cho) value, but the N-acetyl aspartate (NAA) value decreased compared with the contralateral normal side (Figure 4(a) and (b)). Comparison of respective metabolite ratios between the normal contralateral posterior periventricular white matter and the solid tumour region indicated decreased NAA/creatine (Cr) (2.09 vs. 1.47), increased Cho/Cr (1.24 vs. 5.22) and increased Cho/NAA (0.59 vs. 3.55). Moreover, in the Cho/NAA mapping, the mural nodule of the tumour had the highest region (Figure 4(c)).
Figure 4.
(a) Proton MRS of the mural nodule shows high choline and low NAA values. (b) Choline/NAA map demonstrates that the mural nodule of the tumour is the highest region. MRS: magnetic resonance spectroscopy; NAA: N-acetyl aspartate.
Based on conventional MRI findings, the primary differential diagnoses of a cystic tumour with an enhancing mural nodule are low-grade glial neoplasms, particularly pilocytic astrocytoma, ganglioglioma, pleomorphic xanthoastrocytoma and desmoplastic infantile gangliogliomas. However, the advanced MRI findings may have caused some confusion. After total surgical excision of the mass under neuro-navigation, histopathologic examination was compatible with DG, which was composed of ganglion cells and fibrillary astrocytes with an extensive hyaline matrix. Immunohistochemical evaluation demonstrated glial fibrillary acidic protein (GFAP) in the glial component and synaptophysin and chromogranin in the neuronal component. The solid component had intense vimentine expression and the Ki-67 proliferating index was less than 1%. During the 18-month follow-up, the control brain MRI was normal, except for postoperative changes without any recurrence or residue.
Discussion
Desmoplastic ganglioglioma is a tumour of infancy and the term ‘DIG’ was introduced in literature in 1987.3 In the past, there were few published cases of DG that occurred in the older age group.2 In 2011, DG was classified into two groups: desmoplastic infantile (<5 years) and non-infantile ganglioglioma (>5 years). Both groups had similar radiologic and pathologic appearances and clinical characteristics, except for age.1 DG appears as a huge hemispheric cystic mass with a broad dural base. In addition, the cystic mass has a peripheral enhancing plaque or nodule-like solid component. A mural nodule is frequently located in the lateral portion of the cystic mass and adjacent to the dura. Enhancement of the leptomeninges and dura adjacent to the solid component is typical.1,3 Radiologically, our case had both typical and atypical appearances. The typical findings, similar to previous reports, were well-defined and large cystic appearance with a solid mural nodule that enhanced markedly because of high vascularization. The atypical findings of our case were central, rather than peripheral, location and the lack of an apparent dural attachment. Moreover, the mural nodule was located medial to the cystic mass. Radiologically in DG, a mural nodule is isointense with grey matter on T1-weighted imagine (T1WI) and T2WI.1 In our case, the mural nodule was slightly hyperintense compared with the grey matter on T2WI, likely because of a prominent stroma.
Taking into account the age of the patient and the conventional imaging findings, low-grade glial neoplasms, especially pilocytic astrocytoma and ganglioglioma, were primarily considered in the differential diagnoses, instead of DIG. Similar to DIG, pilocytic astrocytomas are generally cystic neoplasms with an avidly enhancing mural nodule, but are usually located in the cerebellum, optic pathway, hypothalamus and brainstem and they typically arise in the first two decades of life. On the other hand, the gangliogliomas are typically located in the temporal lobe, mostly in the mesial portions and commonly present with seizures clinically. Most gangliogliomas enhance heterogeneously and have solid-cystic mixed configuration on radiologic imaging.
Besides conventional MRI, advanced MRI, including proton MRS, MR perfusion and DWI findings, may help to differentiate the grades of the glial neoplasms. According to the literature, the grade of the tumour is inversely correlated with its ADC value. Lee et al. reported that the median minimum ADC of the high-grade astrocytomas was significantly lower than that of the low-grade astrocytomas.4 Lower ADC values reflected increased tumour cellularity and nucleus-cytoplasm ratio, which lowered diffusion capability. Higher ADC values were attributed to low tumour cellularity, necrosis, or cysts. Higher ADC values in low-grade gliomas may also have reflected an increase in the water content of the interstitial spaces.4 The ADC values also suggest the presence of a large extracellular hyaline matrix, which has high diffusion capability. In our case, the mean ADC value of the tumour was higher than that of normal parenchyma at 1.142 × 10−3 mm2/s, indicating a low-grade tumour.
MRS is commonly used to evaluate cellular metabolism non-invasively. In this case the metabolite ratios caused some difficulties in differentiating the DG from high-grade glial tumours. The increased Cho/Cr ratio of 5.22, the increased Cho/NAA ratio of 3.55, and the decreased NAA/Cr ratio of 1.47 were different from the other low-grade glial tumours, likely suggesting a high-grade glioma. NAA is synthesized in neurons. Thus, the presence of NAA signifies a neural origin of the tumour.5 However, the decreased NAA value in this patient with DG may be explained by the histological findings of immature neuronal cells, which do not produce sufficient NAA. In corollary, these low values of NAA did not necessarily indicate neuronal loss in infancy and early childhood, because of neuronal immaturity. In general, NAA values increase as the brain matures and peak at the age of 10–15 years.5 Choline peak is directly associated with increased cellular proliferation and is involved in the synthesis of lecithin, a phospholipid that is essential for membrane turnover. Several reports pointed out a correlation between Cho peak and the degree of the tumour, but in most of the gangliogliomas reduced NAA and elevated Cho levels are seen. Balaji and Ramachandran also reported increased Cho and decreased NAA values in DIG.6
In the brain, perfusion imaging is important to understand the cerebral vasculature and to measure the haemodynamic parameters in a various disorder. For tumoural lesions, the findings of increased perfusion commonly indicate increased neovascularity and tumoural angiogenesis of a high-grade neoplasm. However, Law et al. described increased perfusion in gangliogliomas, which are considered to be low-grade neoplasms.7 Lev at al. described that oligodendrogliomas, without respect to tumour grade, tend to have high blood volumes.8 These authors asserted that diversity in perfusion may be due to the unusual combination of glial and neuronal elements within the same tumour and that the higher perfusion and enhancement in low-grade neoplasms may reflect disruption of the blood-brain barrier.8 In our case, the relative (r)CBV and the rCBF values of the mural nodule were increased relative to the peritumoural region and the contralateral cerebral white matter. Our case had similarities with both ganglioglioma and oligodendroglioma in this respect. Therefore, we should keep in mind that low-grade tumours may show increased perfusion. Low-grade gliomas can present with high rCBV ratios that are not reflective of the malignant status and the accuracy of rCBV mapping may be confusing in glial tumour grading.
In conclusion, DG is a low-grade tumour with unusual and specific advanced MRI findings. As exhibited in our case, perfusion and Cho levels were high and NAA values were low. To the best of our knowledge, this is the first report that explains the proton MRS, MR perfusion and DWI findings in DG. In addition, although generally accepted as a tumour of infancy, DG may also be seen in older age groups. Therefore, it may be helpful for paediatric radiologists or neuroradiologists to include DG in the differential diagnoses of a peripherally located cystic mass with a dural-based mural nodule, regardless of a patient’s age.
Funding
This research received no specific grant from any funding agency in the public, commercial or not-for-profit sectors.
Conflict of interest
None declared.
References
- 1.Gelabert-Gonzalez M, Serramito-García R, Arcos-Algaba A. Desmoplastic infantile and non-infantile ganglioglioma. Review of the literature. Neurosurg Rev 2010; 34: 151–158. [DOI] [PubMed] [Google Scholar]
- 2.Ganesan K, Desai S, Udwadia-Hegde A. Non-infantile variant of desmoplastic ganglioglioma: A report of 2 cases. Pediatr Radiol 2006; 36: 541–545. [DOI] [PubMed] [Google Scholar]
- 3.Trehan G, Bruge H, Vinchon M, et al. MR imaging in the diagnosis of desmoplastic infantile tumor: Retrospective study of six cases. AJNR Am J Neuroradiol 2004; 25: 1028–1033. [PMC free article] [PubMed] [Google Scholar]
- 4.Lee EJ, Lee SK, Agid R, et al. Preoperative grading of presumptive low-grade astrocytomas on MR imaging: Diagnostic value of minimum apparent diffusion coefficient. AJNR Am J Neuroradiol 2008; 29: 1872–1877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Panigrahy A, Kim S, Bluml S. Magnetic resonance spectroscopy and positron emission tomography. In: Coley BD. (eds). Caffey’s pediatric diagnostic imaging, 12th ed Philadelphia, PA: Elsevier Saunders, 2013, pp. 251–260. [Google Scholar]
- 6.Balaji R, Ramachandran K. Imaging of desmoplastic infantile ganglioglioma: A spectroscopic viewpoint. Childs Nerv Syst 2009; 25: 497–501. [DOI] [PubMed] [Google Scholar]
- 7.Law M, Meltzer DE, Wetzel SG, et al. Conventional MR imaging with simultaneous measurements of cerebral blood volume and vascular permeability in ganglioglioma. Magn Reson Imaging 2004; 22: 599–606. [DOI] [PubMed] [Google Scholar]
- 8.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: Confounding effect of elevated rCBV of oligodendroglimoas. AJNR Am J Neuroradiol 2004; 25: 214–221. [PMC free article] [PubMed] [Google Scholar]