Abstract
A retrospective review was made to assess the accuracy of four dimensional CT angiogram (4D-CTA) in diagnosis of arteriovenous malformations (AVM) and dural arteriovenous fistulas (DAVF), with catheter-based digital-subtraction angiogram (DSA) being gold standard. 33 pairs of investigations (DSA and 4D-CTA) were performed primarily for suspicion of AVM/DAVF. Based on blinded reports, sensitivity and specificity for detection of AVM/DAVF were 77% (95% CI: 46 - 95%) and 100% (95% CI: 83 - 100%) respectively. Positive predictive value was 100% (95% CI: 69 - 100%) and negative predictive value 87% (95% CI: 66-97%). 4D-CTA is a practical minimally-invasive technique for evaluating cerebrovascular pathologies. There is good agreement between the findings of 4D-CTA and DSA despite the differences in temporal and spatial resolutions. 4D-CTA may obviate the need for DSA in a subgroup of patients who would otherwise have undergone this invasive investigation, which carries a risk of important complications.
Keywords: Four-dimensional CT angiogram, digital subtraction angiography, arteriovenous malformation, dural arteriovenous fistula
Introduction
Cerebrovascular pathologies causing haemorrhage are a group of complex conditions with a debilitating and potentially life-threatening prognosis, with timely and accurate diagnosis important in obtaining the best outcome. The most common cerebrovascular pathologies are aneurysms, arteriovenous malformations (AVMs), dural arteriovenous fistulae (DAVFs) and venous/arterial occlusive diseases.
Three-dimensional computed tomography angiography (3D-CTA) of the brain has excellent sensitivity for diagnosing intracranial aneurysms and other cerebrovascular malformations.1,2 However, 3D-CTA examines only a single time point and therefore provides limited information about flow dynamics, which are important for the detection and characterisation of several cerebrovascular conditions. Technological developments of computed tomography (CT) now permit dynamic imaging techniques of the whole brain. Technical solutions include the ‘toggling table’3 and, more recently, wide area detectors capable of whole brain coverage within a single rotation.4,5
A 320 detector row CT machine has a z-axis coverage of 16 cm and can acquire volumetric scans of the brain in sub-second time. It provides the opportunity to image the flow of contrast in the cerebral vasculature by running consecutive volumetric scans over a predetermined timeframe. Such time-resolved or four-dimensional computed tomography angiography (4D-CTA) can be used to evaluate the haemodynamics, in addition to the morphology, of a variety of cerebrovascular conditions While a 3D-CTA can provide a snapshot of arteries opacified with contrast, a 4D-CTA can demonstrate the flow pattern through the different components of a cerebrovascular abnormality at different phases of contrast flow. This can be particularly useful in the assessment of AVMs and DAVFs as well flow dynamics in an aneurysm in which an isolated observation with a 3D-CTA may not reveal the components and haemodynamics of the abnormality accurately.
In this study we assess the accuracy of 4D-CTA in the detection of AVMs and DAVFs, when compared with digital subtraction angiography (DSA) the gold standard test in the assessment of such vascular conditions.1 Our assessment is based on our initial experience with this technique in a tertiary neuroscience unit, following the installation of a 320 detector row CT machine.
Materials and methods
Advice regarding the requirement of ethics approval was sought from the regional research ethics committee. Ethics approval was waived as the study involved evaluating retrospective data obtained as a part of routine clinical practice. The study was an evaluation of a service already being provided by the department.
Patient group
4D-CTA was in routine use at our institution from June 2012. The main indications were follow-up or characterisation of known AVM or DAVF, confirmation of suspected AVM or DAVF, evaluation of tumour vasculature and as part of a perfusion assessment. A summary of the clinical indications for which the investigations were performed is presented in Table 1.
Table 1.
Summary of indications for 4D-CTA and DSA and number of such studies performed.
| Indications | 4D-CTA | 4D-CTA & DSA |
|---|---|---|
| ICH | 31 | 19 |
| AVM | 18 | 10 |
| Pulsatile tinnitus | 9 | 2 |
| AV fistula | 7 | 0 |
| Vascular lesions | 8 | 2 |
| Meningioma | 5 | 1 |
| Carotico-cavernous fistula | 6 | 1 |
| Idiopathic intracranial hypertension | 1 | 1 |
4D-CTA: four-dimensional computed tomography angiography; DSA: digital subtraction angiography; ICH: intracerebral haemorrhage; AVM: arteriovenous malformation; AV: arteriovenous.
The radiology information system was searched for 4D-CTA examinations performed between the period of June 2012 and August 2013 and all were included. Eighty-five 4D-CTA examinations were performed during the study period. Of the 85 patients who underwent 4D-CTA, 36 also had DSA performed following the 4D-CTA. Of the 36 patients who had both 4D-CTA and DSA, 33 pairs of investigations were performed primarily for suspicion of AVM/DAVF and were included in our study for comparison. The suspected AVM/DAVF group included patients presenting with intracerebral haemorrhage in which an alternative diagnosis (e.g. aneurysmal subarachnoid haemorrhage or lobar haemorrhage in a patient with known cerebral amyloid angiopathy) was not apparent, and patients with imaging features suggestive of AVM/DAVF on a different imaging modality.
4D-CTA technique
The 4D-CTA examinations were performed on a CT scanner offering a 16 cm volumetric coverage with 320 detector rows of 0.5 mm each (Aquilion One; Toshiba Medical Systems, Japan). The volumetric acquisition was timed based on a time-density curve (TDC) derived from a test injection.
Time-density curve
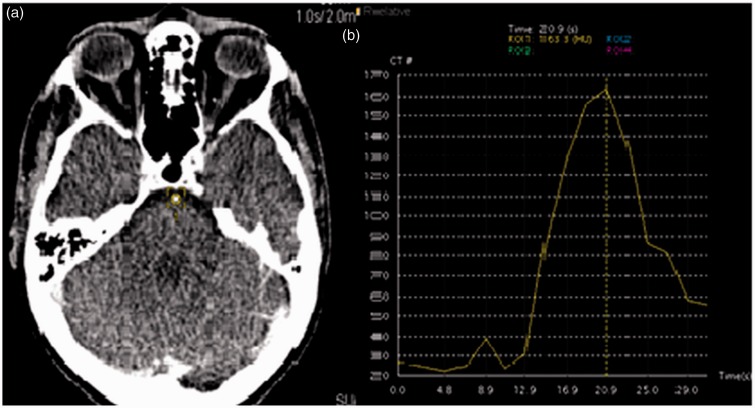
This was based on a previously described method of performing 3D-CTA.6 Briefly, a test bolus of iopromide (300 mg iodine/ml, Ultravist 300; Bayer, Leverkusen, Germany) (15 ml, at 6 ml/second followed by a 20 ml saline flush) was injected. A dynamic scan was performed at the level of the sella (80 kV, 100 mAs, 2 mm slice, 2 s repetition). From this, a TDC was constructed for a region of interest in the basilar or cavernous internal carotid artery (Figure 1(a)). Times of start of arterial enhancement and time of return to baseline were determined and used to determine the start and end times of the dynamic acquisition.
Figure 1.
(a) Region of interest placed on the basilar artery for the test dose scan. (b) Time-density curve (TDC) generated by the test dose. The time of scanning is determined from the start of the early arterial phase to the late venous phase determined by the time of contrast within the artery, in this particular example the scan was performed from 14 to 28seconds (a total scan of 14 volumes was performed).
Volumetric acquisition
A bolus injection of 35 ml of contrast was given at a rate of 6 ml/second chased with a 20 ml saline flush. The 4D-CTA scan consists of a volume scan to provide a mask-volume dataset for bone subtraction (80 kV; 338 mAs) at 4 s after the contrast injection. Using the TDC for timing, a continuous volume from the early arterial phase into the late venous phase was performed (80 kV; 113 mAs; 0.75 s rotation; 320 × 0.5 mm collimation; 0.5 s reconstruction interval) (see Figure 1(b)).
Post-processing
The mask volume was subtracted from each of the dynamic volumes using vendor software. Due to the number of images (typically 10,240 – 32 volumes of 320 images) review was primarily with whole brain maximum intensity projection (MIP) sequences in axial, coronal, sagittal and two oblique projections. These projections were generated from the subtracted data and were archived on the picture archiving and communication system. The 0.5 mm axial volume dataset was retained for viewing on dedicated workstation software (Vitrea Core; Toshiba Medical Systems, Japan) for the neuroradiologist during reporting.
DSA technique
The catheter DSA was performed in a biplane angiography system (Inova; GE Healthcare, Milwaukee, MN, USA). Femoral arterial puncture and a 5F diagnostic catheter was used to acquire an angiogram of the bilateral internal carotid artery, external carotid artery and vertebral arteries. The angiograms were performed at 2.5 frames/second. Additional magnified projections and a higher frame rate (up to 4.5 frames/second) were used if required.
Angiographic reporting and evaluation
The 4D-CTA and DSA were reported independently by experienced consultant neuroradiologists. The 4D-CTA studies were assessed by KD and MR, who have about 12 years and 6 years of experience in neuroradiology, respectively. The DSAs were reported by consultant interventional neuroradiologists – HCN and MP, who have experience of about 25 years and 8 years, respectively, in performing and interpreting DSA. To minimize bias, the original, prospective radiological report of DSA was used for determining whether a 4D-CTA study had made a diagnosis. When a vascular pathology such as AVM or DAVF was present, we also evaluated the extent to which 4D-CTA could characterise these lesions in terms of major feeders, location of shunts, nidus size and drainage.
Radiation dose calculations
For 4D-CTA, the whole body effective dose was estimated from dose length product using the conversion factor 0.0023 mSv/mGy.cm.7 For DSA, the effective dose was estimated from the dose area product and conversion factor of 0.056 mSv/Gy.cm2.8
Statistical analysis
Efficacy, sensitivity, specificity and positive and negative predictive values for 4D-CTA were calculated with reference to DSA as the gold standard. Analysis was made for the detection of both AVM and DAVF, AVM only and DAVF only. McNemar’s test was performed to check to see if the two methods are statistically equivalent in the detection of AVM or DAVF and both.
Results
The age range was between 22 and 80 years, with a male to female ratio of 41:44.
The average time gap between 4D-CTA and DSA was about 10 days (range 0–50 days), with 63.3% of the DSAs being done within 5 days of the 4D-CTA. No cerebrovascular intervention was performed in any of the cases between the 4D-CTA and DSA.
Of the 33 patients who had both 4D-CTA and DSA, 13 patients were determined to have AVM/DAVF by DSA – 10 AVMs and three DAVFs. 4D-CTA was able to detect 10 AVM/DAVF – seven AVMs and all the three DAVFs. Overall, the findings were concordant in 30 (91%) of the 33 cases. In three (9%) cases there were discrepancies between the modalities in which three AVMs were not reported on 4D-CTA. The discrepancies were two cases in which an AVM was not reported, but was just visible in retrospect on the MIP images (Figures 2 and 3), and one case of a recurrent AVM, which was visible only on the 0.5 mm axial sections, but not on the MIP images.
Figure 2.
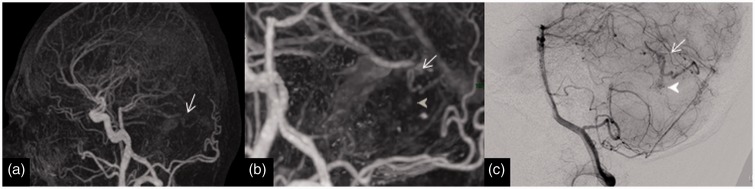
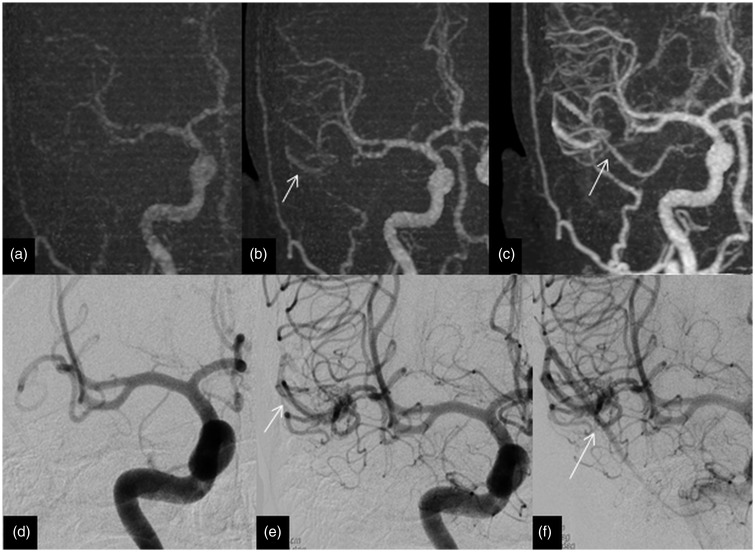
Imaging of a 32-year-old man with posterior fossa haemorrhage from an AVM (missed on 4D-CTA). (a) Lateral view of 4D-CTA MIP demonstrating a cerebellar AVM with early venous filling (arrow). (b) Magnified lateral view of 4D-CTA MIP displaying the early filling vein (arrow) and the nidus (arrow head). (c) DSA lateral view illustrating the early filling vein (arrow) and the nidus (arrow head). This is an example of a Spetzler–Martin grade 1 AVM with a nidus size of less than 3 cm with superficial venous drainage. AVM: arteriovenous malformation; 4D-CTA: four-dimensional computed tomography angiography; MIP: maximum intensity projection; DSA: digital subtraction angiography.
Figure 3.
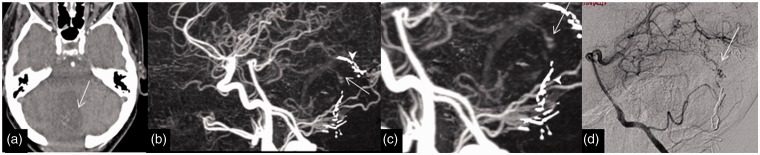
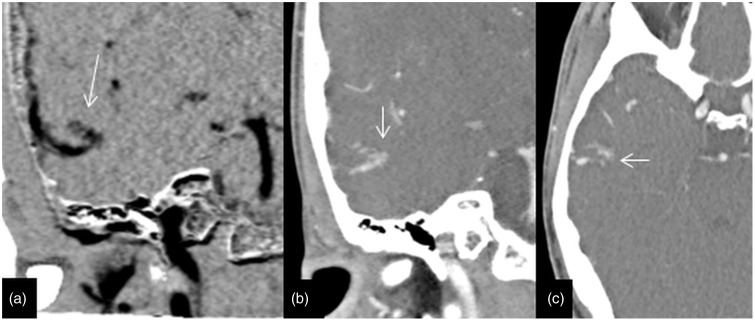
Imaging of a 62-year-old woman with a previous excision of AVM presented for follow-up and the residual early venous drainage was missed on a 4D-CTA. (a) Axial 4D-CTA base data demonstrating abnormal venous clustures (arrow) in the surgical cavity. (b) and (c) Lateral 4D-CTA MIP data displaying the early venous filling (arrow) and surronding surgical clip artefacts (arrow heads). (d) DSA lateral view highlighting the early venous filling (arrow). AVM: arteriovenous malformation; 4D-CTA: four-dimensional computed tomography angiography; MIP: maximum intensity projection; DSA: digital subtraction angiography.
Using results from the original blinded reports, the efficacy, sensitivity and specificity for the detection of both AVM and DAVF by 4D-CTA were 91%, 77% (95% confidence interval (CI) 46–95%) and 100% (95% CI 83–100%), respectively. The positive predictive value was 100% (95% CI 69–100%) and the negative predictive value was 87% (95% CI 66–97%).
The efficacy, sensitivity and specificity of 4D-CTA in the detection of AVM alone were 91%, 70% (95% CI 35–93%) and 100% (95% CI 85–100%), respectively. The positive predictive value and negative predictive value were 100% (95% CI 59–100%) and 88% (95% CI 70–98%).
Only three patients had DAVF and all were detected by 4D-CTA. Therefore, the efficiency, sensitivity and specificity for the diagnosis of DAVF by 4D-CTA were 100%, 100% (95% CI 29–100%) and 100% (95% CI 88–100%), respectively. The positive predictive value was 100% (95% CI 29–100%) and the negative predictive value was 100% (95% CI 88–100%).
None of the patients had dual pathology (AVM as well as DAVF) either on DSA or 4D-CTA.
McNemar’s test was performed and there was no statistically significant difference found in the detection of AVM or DAVF or both by the two techniques – 4D-CTA and DSA (P = 0.25).
The 4D-CTA doses had a range from 4 to 8.3 mSv with a mean of 6.6 mSv. In our series, the DSA doses ranged from 2 to 10.3 mSv, with a mean dose of 4.2 mSv.
Discussion
The high overall concordance (91% of the 33 cases) between DSA and 4D-DSA results as well as high efficacy (91–100%) demonstrate that 4D-CTA by 320 detector row CT is a practical tool with broadly comparable diagnostic accuracy to DSA for the diagnosis of AVM or DAVF. All three discrepancies in this series appeared to be interpretation errors at the time of primary reporting, as the lesions were detectable on review of thin section images. Although two lesions were visible on MIP images, they had poor visibility and were considerably more visible on thin sections. Another highlight is the 100% specificity in the detection of AVM or DAVF by 4D-CTA, indicating that it could be an effective tool in excluding these vascular conditions and thereby potentially obviating DSA.
DSA remains the gold standard for the evaluation of cerebral vasculature due to its high temporal and spatial resolution. Drawbacks include its invasive nature, time and resource requirements and small risk of significant morbidity.9–12
CT and magnetic resonance angiography (MRA) are well established techniques, and now form an essential part of the clinical management of patients.13,14 MRA avoids the use of ionising radiation; however, it has a relatively low sensitivity for the diagnosis of slow flow shunts and haemodynamic evaluation.15,16 3D-CTA is accessible and rapidly performed, and provides a comprehensive evaluation of the brain vascular pathologies.17,18 However, both modalities lack the temporal dynamic information to characterise the cerebral vascular malformations.
Time-resolved contrast enhanced MRA15,16 can also provide non-invasive imaging in cerebrovascular pathologies, but is limited in its temporal resolution by the trade-off between the signal to noise ratio and temporal resolution in comparison with 4D-CTA. Time spatial labelling inversion pulse (time-slip) magnetic resonance DSA19 provides haemodynamic information without contrast in DAVF assessment. However, the long acquisition time and the failure to identify cortical drainage are some of the shortfalls of this method.
Opportunities offered by 4D-CTA
The less invasive nature of 4D-CTA compared to DSA may play a role in cases in which there is low clinical suspicion of an underlying pathology, such as ganglionic hypertensive haemorrhage. However, as DSA is not always recommended in such groups,20 and is often omitted in our clinical practice, we cannot report positive or negative predictive values in this group.
In cases of AVM, 4D-CTA has provided adequate information for preoperative planning and intermediate follow-up in post-treatment scenarios; for example, the localisation of major feeding and draining vessels pre-operatively or detection of residual nidus post-treatment. This has led to a reduction in the number of DSAs performed as part of the treatment plan and follow-up, thus reducing the associated procedural risk for patients. Prior to the introduction of 4D-CTA in our department, all patients would have had DSA for follow-up or planning of AVM treatment, and hence a total of eight DSA examinations were avoided over the period examined.
We also found 4D-CTA useful in cases when CT or magnetic resonance imaging examination detected unusual vascularity suggesting either AVM or DAVF. In this subgroup, 4D-CTA often provided adequate information to characterise the vascular structure and assist in diagnosis. For example, a developmental venous anomaly without arteriovenous shunting could be confidently identified and further investigation avoided.
Literature reports have demonstrated the feasibility of 4D-CTA for AVM,21 DAVF,22–24 thrombus burden,25 and the assessment of collaterals in large vessel occlusion.26
In cases of DAVF, early venous filling including cortical venous drainage and major feeding arteries could be satisfactorily resolved and the appropriate Lariboisiere classification27 for dural arteriovenous fistulae (Figure 4) could be successfully determined. Several recent case series have found similar results.22–24
Figure 4.
Imaging of a 65-year-old man with an occipital haemorrhage; 4D-CTA and DSA illustrate the dural arteriovenous fistula of the falx. The upper row of images (a), (b) and (c) of lateral MIP of 4D-CTA shows the middle meningeal artery (arrow) supply to the fistula and the early draining occipital cortical vien (arrow head). The lower row of images (d, e and f) of the DSA in lateral projection display similar findings to 4D-CTA. This is an example of a Lariboisiere type III arteriovenous fistula, with direct cortical drainage. 4D-CTA: four-dimensional computed tomography angiography; MIP: maximum intensity projection; DSA: digital subtraction angiography.
In the AVM cases detected by 4D-CTA, the early venous filling and feeding arteries could be satisfactorily resolved, enabling the appropriate Spetzler–Martin classification of AVM (Figures 5 and 6). In the three cases in which subtle residual early venous filling and shunts were missed on 4D-CTA, these were considered to be interpretation errors on retrospective review. In other studies evaluating 4D-CTA in cases of AVM,21 it was concluded that small and slow shunts could lead to potential misinterpretation. There was one misclassification of venous drainage in this series (mistaking superficial for deep); the opposite error was reported in a previous series.21 A more recent case series has reported complete agreement between 4D-CTA and DSA,28 which may indicate a greater degree of experience with the technique.
Figure 5.
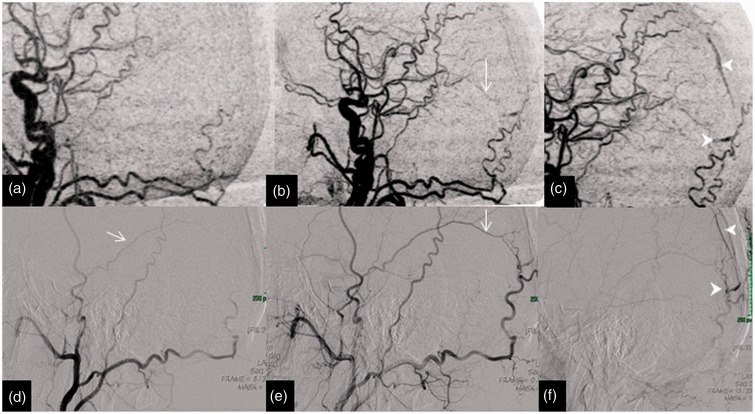
Imaging of a 54-year-old man with a right temporal haemorrhage in which 4D-CTA and DSA highlight the AVM. The upper row of images (a, b and c) of coronal MIP of 4D-CTA demonstrates the early draining superficial middle cerebral vein (arrows) from the AVM. The lower row of images (d, e and f) of the DSA in anteroposterior projection display similar findings to the 4D-CTA. This is an example of a Spetzler–Martin grade 1 AVM with a nidus size of less than 3 cm with a superficial venous drainage. AVM: arteriovenous malformation; 4D-CTA: four-dimensional computed tomography angiography; MIP: maximum intensity projection; DSA: digital subtraction angiography.
Figure 6.
This row of 4D-CTA images of the same patient in Figure 5 demonstrates the plexiform nidus (arrows) on coronal images in the right temporal lobe; (a) MIP, (b) and (c) axial data of 1 mm thickness. This demonstrates the importance of reviewing the base data to understand and delinate the anatomy of the vascular malformation. 4D-CTA: four-dimensional computed tomography angiography; MIP: maximum intensity projection.
4D-CTA as part of whole brain CT perfusion studies for evaluating intracranial tumours adjacent to large blood vessels has been described as a ‘one-stop’ examination.29 This includes a conventional non-enhanced CT image, enhanced CT images, CT perfusion images and 4D-CTA images that can be obtained simultaneously.
Problems posed by 4D-CTA
The average dose of 4D-CTA and DSA are difficult to compare; doses vary widely depending on the nature and complexity of the pathologies investigated by catheter angiogram. Contributing factors include the details/number of vessels studied, the operator and fluoroscopic exposure factors. In this study, the mean effective dose of 4D-CTA was approximately 2 mSv higher than the DSA. A large range of 4D-CTA doses may be explained by the fact that this is a relatively new technique for which doses are still being optimised. Other series have reported 4D-CTA doses lower than DSA,4,22,23 but we found the doses at best to be similar.
One potential drawback of 4D-CTA is the lower temporal resolution compared with DSA. In this protocol, we used a 360 degree reconstruction with 750 ms rotation period, effectively limiting the resolution to 750 ms. In comparison, DSA used a 400 ms interframe interval as standard.
To control the overall dose, the dose per acquired volume is significantly lower in 4D-CTA than 3D-CTA, and therefore image noise is necessarily higher due to the Poisson nature of shot noise. However, the use of a low tube potential (80 kV), resulting in photon energy more closely matched to the iodine k-edge, results in a greater contrast signal that can partially offset this increased noise.30–32 3D-CTA is recognised to offer a lower spatial resolution than DSA.33 It would follow that 4D-CTA would be similar to 3D-CTA, or slightly worse, by heavier low-pass filtering to limit noise. In the current study, DSA pixel resolution was 0.4 mm (approximately 0.25 mm after geometric magnification), and CT resolution was 0.43 × 0.43 × 0.5 mm. Modulation transfer function measurements were not performed to quantify the effect of low-pass filtering.
Non-selective enhancement of cranial vessels in a 4D-CTA gives a busy appearance that is not ideal for the detection of subtle vascular lesions, and this may be one factor behind the requirement for a review of thin section data.21
Finally, the volume of data generated by the 4D-CTA study makes review of the images time consuming, especially as the axial images must be scrutinised to avoid errors. Workflow also needs to be considered: CT image reconstruction time is prolonged, which may impact on subsequent CT studies. Requirements for workstation performance and data storage (approximately 6 GB per study) are also greater than for other examinations.
The limitations of this study are its retrospective nature and the heterogeneous groups of patients, which included both acute and elective indications. We have not performed any comparative statistical analysis for individual components of a vascular abnormality (e.g. main feeders, draining vessels and size of nidus in the case of an AVM) because of the small number. However, details of the individual components and comparative findings (4D-CTA vs. DSA) are provided in Table 2.
Table 2.
Comparison of performance of AVM and AVF classification between 4D-CTA and DSA.
| AVM and AVF characteristics | 4D-CTA | DSA |
|---|---|---|
| Brain AVM diagnosed | 7 | 10 |
| Spetzler–Martin grade | ||
| Size | ||
| <3 cm 3–6 cm >6 cm | 5 2 0 | 8 2 0 |
| Eloquent involvement | 4 | 6 |
| Deep drainage | 3a | 3 |
| Major arterial feeders identified | 7 | 10 |
| Dural supply | 0 | 0 |
| Intra-nidal aneurysm | 0b | 1 |
| Contrast stasis within lesion | 0 | 0 |
| Venous stenosis | 0 | 0 |
| Flow-related aneurysms | 0 | 0 |
| High-flow shunts | 0 | 0 |
| Dural AVF diagnosed | 3 | 3 |
| Lariboisiere (Cognard) classification | ||
| I – Antegrade sinusoidal flow | 0 | 0 |
| II – Reflux (a) sinusoidal; (b) cortical | 1 (IIb) | 1 (IIb) |
| III – Direct shunt into cortical vein | 2 | 2 |
| IV – As III with venous ectasia | 0 | 0 |
| V – Perimedullary venous drainage | 0 | 0 |
| Major ECA feeder identified | 2c | 2 |
| Venous outflow obstruction | 0 | 0 |
AVM: arteriovenous malformation; AVF: arteriovenous fistula; 4D-CTA: four-dimensional computed tomography angiography; DSA: digital subtraction angiography; ECA: external carotid artery; 4D: four dimensional; MIP: maximum intensity projection; MMA: middle meningeal artery.
4D interpreted as deep venous drainage, but DSA showed superficial cerebellar venous drainage.
Nidal aneurysm only visible on axial sections (not MIP images). Early venous filling not clearly seen on 4D-CTA.
Only occipital artery supply identified. Small calibre MMA was not seen on 4D-CTA.
Our cohort reflects a representative sample of cerebrovascular pathologies in a tertiary neuroscience unit. Our aim was to investigate the utility and sensitivity of 4D-CTA in a typical clinical case mix. Further prospective studies on each specific indication to confirm the sensitivity and specificity of the test are necessary, including statistical analysis of the detection of individual components of a vascular abnormality. However, the current study would suggest that 4D-CTA is a useful adjunct for the initial evaluation and follow-up of cerebrovascular pathologies.
Conclusion
In this study, we were able to demonstrate the usefulness and diagnostic value of 4D-CTA in the diagnosis and assessment of AVM and DAVF. We acknowledge and understand the limitations of 4D-CTA in terms of spatial and temporal resolution. Further prospective cohort trials are needed to validate the use of 4D-CTA in various indications of neurovascular pathologies. This study demonstrates that 4D-CTA is a practical diagnostic test, which in a subgroup of patients could exclude the need for invasive catheter DSA.
Acknowledgement
The authors thank Mrs Debasree Purkayastha for help provided with the statistical analysis of anonymised data.
Conflict of interest
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
References
- 1.Prestigiacomo CJ, Sabit A, He W, et al. Three dimensional CT angiography versus digital subtraction angiography in the detection of intracranial aneurysms in subarachnoid haemorrhage. J Neurointerv Surg 2010; 2: 385–389. doi: 10.1136/jnis.2010.002246. [DOI] [PubMed] [Google Scholar]
- 2.Wang H, Li W, He H, et al. 320-detector row CT angiography for detection and evaluation of intracranial aneurysms: comparison with conventional digital subtraction angiography. Clin Radiol 2013; 68: e15–e20. doi:10.1016/j.crad.2012.09.001. [DOI] [PubMed] [Google Scholar]
- 3.Roberts HC, Roberts TP, Smith WS, et al. Multisection dynamic CT perfusion for acute cerebral ischaemia: the “toggling-table” technique. AJNR Am J Neuroradiol 2001; 22: 1077–1080. [PMC free article] [PubMed] [Google Scholar]
- 4.Siebert E, Bohner G, Dewey M, et al. 320-Slice CT neuroimaging: initial clinical experience and image quality evaluation. Br J Radiol 2009; 82: 561–570. doi: 10.1259/bjr/27721218. [DOI] [PubMed] [Google Scholar]
- 5.Sorantin E, Riccabona M, Stücklschweiger G, et al. Experience with volumetric (320 rows) pediatric CT. Eur J Radiol 2013; 82: 1091–1097. doi:10.1016/j.ejrad.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 6.Das K, Biswas S, Roughley S, et al. 3D CT cerebral angiography technique using a 320-detector machine with a time density curve and low contrast medium volume: Comparison with fixed time delay technique. Clin Radiol 2014; 69: e129–e135. doi: 10.1016/j.crad.2013.10.021. [DOI] [PubMed] [Google Scholar]
- 7.European Commission. European Guidelines on Quality Criteria for Computed Tomography. Brussels, Belgium: EUR 16262, 1999.
- 8.Manninen AL, Isokangas JM, Karttunen A, et al. A comparison of radiation exposure between diagnostic CTA and DSA examinations of cerebral and cervicocerebral vessels. AJNR Am J Neuroradiol 2012; 33: 2038–2042. doi:10.3174/ajnr.A3123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kaufmann TJ, Huston J, III, Mandrekar JN, et al. Complications of diagnostic cerebral angiography: evaluation of 19,826 consecutive patients. Radiology 2007; 243: 812–819. [DOI] [PubMed] [Google Scholar]
- 10.Willinsky RA, Taylor SM, TerBrugge K, et al. Neurologic complications of cerebral angiography: prospective analysis of 2,899 procedures and review of the literature. Radiology 2003; 227: 522–528. [DOI] [PubMed] [Google Scholar]
- 11.Bendszus M, Koltzenburg M, Burger R, et al. Silent embolism in diagnostic cerebral angiography and neurointerventional procedures: a prospective study. Lancet 1999; 354: 1594–1597. [DOI] [PubMed] [Google Scholar]
- 12.Cloft HJ, Joseph GJ, Dion JE. Risk of cerebral angiography in patients with subarachnoid hemorrhage, cerebral aneurysm, and arteriovenous malformation: a meta-analysis. Stroke 1999; 30: 317–320. [DOI] [PubMed] [Google Scholar]
- 13.Agid R, Willinsky RA, Lee SK, et al. Characterization of aneurysm remnants after endovascular treatment: contrast-enhanced MR angiography versus catheter digital subtraction angiography. AJNR Am J Neuroradiol 2008; 29: 1570–1574. doi: 10.3174/ajnr.A1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Aralasmak A, Akyuz M, Ozkaynak C, et al. CT angiography and perfusion imaging in patients with subarachnoid hemorrhage: correlation of vasospasm to perfusion abnormality. Neuroradiology 2009; 51: 85–93. doi:10.1007/s00234-008-0466-7. [DOI] [PubMed] [Google Scholar]
- 15.Pekkola J, Kangasniemi M. Posterior fossa dural arteriovenous fistulas: diagnosis and follow-up with time-resolved imaging of contrast kinetics (TRICKS) at 1.5 T. Acta Radiol 2011; 52: 442–447. doi: 10.1258/ar.2011.100433. [DOI] [PubMed] [Google Scholar]
- 16.Meckel S, Maier M, Ruiz DS, et al. MR angiography of dural arteriovenous fistulas: diagnosis and follow-up after treatment using a time-resolved 3D contrast-enhanced technique. AJNR Am J Neuroradiol 2007; 28: 877–884. [PMC free article] [PubMed] [Google Scholar]
- 17.Klingebiel R, Busch M, Bohner G, et al. Multi-slice CT angiography in the evaluation of patients with acute cerebrovascular disease – a promising new diagnostic tool. J Neurol 2002; 249: 43–49. [DOI] [PubMed] [Google Scholar]
- 18.Wintermark M. Brain perfusion-CT in acute stroke patients. Eur Radiol 2005; 15: D28–D31. [DOI] [PubMed] [Google Scholar]
- 19.Hori M, Aoki S, Oishi H, et al. Utility of time-resolved three-dimensional magnetic resonance digital subtraction angiography without contrast material for assessment of intracranial dural arterio-venous fistula. Acta Radiol 2011; 52: 808–812. doi: 10.1258/ar.2011.110128. [DOI] [PubMed] [Google Scholar]
- 20.Zhu XL, Chan MS, Poon WS. Spontaneous intracranial hemorrhage: which patients need diagnostic cerebral angiography? A prospective study of 206 cases and review of the literature. Stroke 1997; 28: 1406–1409. [DOI] [PubMed] [Google Scholar]
- 21.Willems PWA, Taeshineetanakul P, Schenk B, et al. The use of 4D-CTA in the diagnostic work-up of brain arteriovenous malformations. Neuroradiology 2012; 54: 123–131. doi: 10.1007/s00234-011-0864-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Willems PW, Brouwer PA, Barfett JJ, et al. Detection and classification of cranial dural arteriovenous fistuals using 4D-CD angiography: Initial experience. AJNR Am J Neuroradiol 2011; 32: 49–53. doi:10.3174/ajnr.A2248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brouwer PA, Bosman T, van Walderveen MAA, et al. Dynamic 320-section CT angiography in cranial arteriovenous shunting lesions. AJNR Am J Neuroradiol 2010; 31: 767–770. doi: 10.3174/ajnr.A1747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Beijer TR, van Dijk EJ, de Vries J, et al. 4D-CT angiography differentiating arteriovenous fistula subtypes. Clin Neurol Neurosurg 2013; 115: 1313–1316. doi: 10.1016/j.clineuro.2012.12.015. [DOI] [PubMed] [Google Scholar]
- 25.Frölich AM, Wolff SL, Psychogios MN, et al. Time-resolved assessment of collateral flow using 4D CT angiography in large-vessel occlusion stroke. Eur Radiol 2014; 24: 390–396. [DOI] [PubMed] [Google Scholar]
- 26.Frölich AMJ, Schrader D, Klotz E, et al. 4D CT angiography more closely defines intracranial thrombus burden than single-phase CT angiography. AJNR Am J Neuroradiol 2013; 34: 1908–1913. doi:10.3174/ajnr.A3533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995; 194: 671–680. [DOI] [PubMed] [Google Scholar]
- 28.Wang H, Ye X, Gao X, et al. The diagnosis of arteriovenous malformations by 4D-CTA: a clinical study. J Neuroradiol 2014; 41: 117–123. doi: 10.1016/j.neurad.2013.04.004. [DOI] [PubMed] [Google Scholar]
- 29.Chen T, Guo D, Fang Z, et al. Preliminary study of whole-brain CT perfusion imaging in patients with intracranial tumours adjacent to large blood vessels. Clin Radiol 2014; 69: e25–e32. doi: 10.1016/j.crad.2013.08.010. [DOI] [PubMed] [Google Scholar]
- 30.Faggioni L, Neri E, Sbragia P, et al. 80-kV Pulmonary CT angiography with 40 mL of iodinated contrast material in lean patients: comparison of vascular enhancement with iodixanol (320 mg I/mL) and iomeprol (400 mg I/mL). AJR Am J Roentgenol 2012; 199: 1220–1225. doi: 10.2214/AJR.11.8122. [DOI] [PubMed] [Google Scholar]
- 31.Sun G, Ding J, Lu Y, et al. Comparison of standard- and low-tube voltage 320-detector row volume CT angiography in detection of intracranial aneurysms with digital subtraction angiography as gold standard. Acad Radiol 2012; 19: 281–288. doi: 10.1016/j.acra.2011.11.004. [DOI] [PubMed] [Google Scholar]
- 32.Tang K, Li R, Lin J, et al. The value of cerebral CT angiography with low tube voltage in detection of intracranial aneurysms. Biomed Res Int 2015. (published online first). doi:10.1155/2015/876796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Teksam M, McKinney A, Casey S, et al. Multi-section CT angiography for detection of cerebral aneurysms. AJNR Am J Neuroradiol 2004; 25: 1485–1492. [PMC free article] [PubMed] [Google Scholar]