Abstract
Congenital aortic arch and vertebral artery anomalies are a relatively rare finding discovered on imaging either incidentally or for evaluation of entities like dysphagia or subclavian steal. Right aortic arch is an uncommon anatomical anomaly that occurs in less than 0.1% of the population, and in half of these cases the left subclavian artery is also aberrant.1 Unilateral vertebral artery (VA) duplication is rare with an observed prevalence of 0.72% in cadavers.2 Fenestration of the VA is more common than duplication, with a prevalence of approximately 0.23%–1.95%.3,4 We describe the case of a 25-year-old female who was found to have a right aortic arch with aberrant left subclavian artery, duplicated left vertebral artery and a fenestrated right vertebral artery on CT angiography performed for evaluation of dysphagia. This combination of findings has not been reported before, to the best of our knowledge. We review the embryologic mechanism for the development of the normal aortic arch, right aortic arch, vertebral artery duplication and vertebral artery fenestration. The incidence of these entities, resultant symptoms and clinical implications are also reviewed. The increased associated incidence of aneurysm formation, dissection, arteriovenous malformations and thromboembolic events with fenestration is also discussed.
Keywords: Right aortic arch, Fenestrated vertebral artery, Duplicated vertebral artery, CT angiography
Introduction
Right aortic arch, vertebral artery (VA) duplications and VA fenestrations are rare congenital vascular anomalies. Identifying them is important, as they can have diagnostic and therapeutic implications. We describe the case of a 25-year-old female who was found to have a right aortic arch with aberrant left subclavian artery (SCA), duplicated left VA and a fenestrated right vertebral artery on computed tomography (CT) angiography performed for evaluation of dysphagia. Duplication and fenestration are two different entities and should not be used interchangeably. Knowledge of the embryologic development of the aortic arch is paramount to understanding the anomalies of the aortic arch and the supra-aortic branches.
Case report
A 25-year-old female presented with dysphagia. Her past medical history was significant for cerebral palsy, partial agenesis of corpus callosum, as well as visual and speech deficits. During her initial presentation, she underwent esophagoscopy to remove impacted food. A proximal esophageal stenosis with an extrinsic pulsating bulging mass was discovered. Further workup with barium swallow esophagram demonstrated indention on the posterior esophageal wall. An aberrant vessel coursing behind the esophagus was suspected. The symptoms of dysphagia had progressed over the years, compounded with notable weight loss and episodes of choking. Therefore, the patient was referred for surgical correction. CT angiogram (CTA) of the chest, head and neck was obtained for anatomic mapping of the thoracic aorta and supra-aortic vessels in preparation for a vascular repair with left carotid-subclavian bypass.
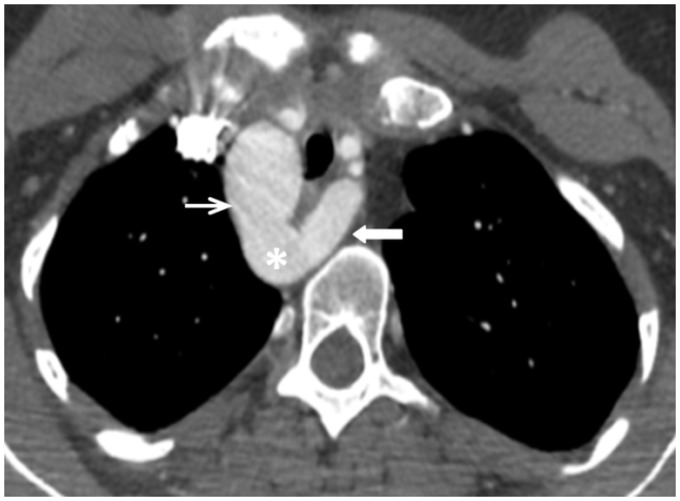
The CTA demonstrated a right-sided aortic arch with aberrant left SCA arising from a diverticulum of Kommerell (Figure 1) and coursing behind the trachea/esophagus. There was narrowing of the mid-trachea and significant compression of the posterior esophagus caused by the left SCA at the thoracic inlet (Figure 2).
Figure 1.
Axial computed tomography (CT) of the chest. Right-sided aortic arch (thin white arrow) with an aberrant left subclavian artery arising (thick white arrow) from a diverticulum of Kommerell (asterisk).
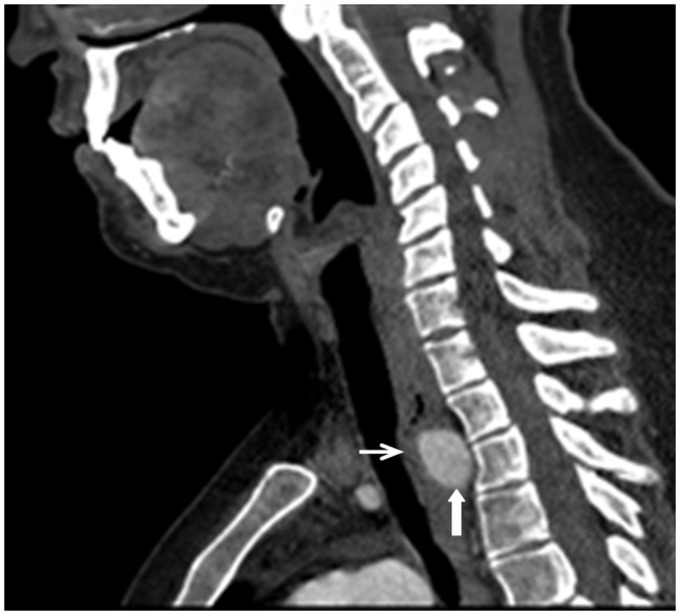
Figure 2.
Sagittal computed tomography (CT) of the neck. Posterior esophagus (thin white arrow) compressed by the aberrant left subclavian artery (thick white arrow) at the thoracic inlet. Incidental note of an elongated body of C2. The posterior arch of C1 is out of the field of view.
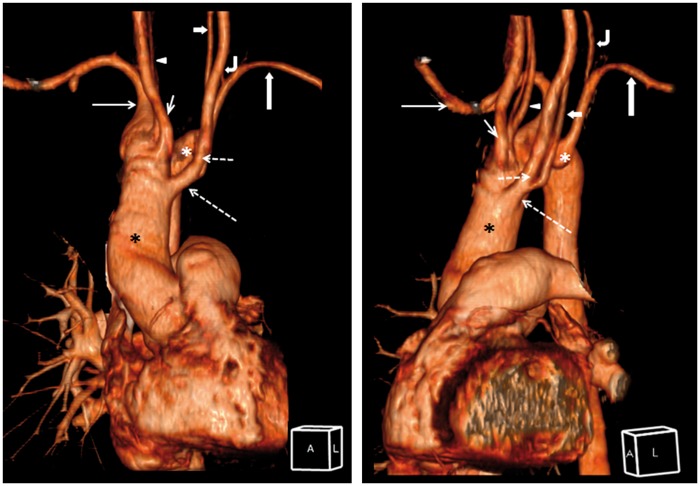
There were five supra-aortic branches originating directly from the right arch (Figure 3). The first branch was a common trunk giving off the left common carotid artery and one of two proximal left VAs. The second branch was the right common carotid artery. The third branch was a fenestrated right VA. The fourth branch was the right SCA. The fifth branch was the aberrant left SCA with a diverticulum of Kommerell that gave rise to the second left VA.
Figure 3.
(Left) Coronal three-dimensional computed tomography (CT) reconstruction of the vessels. Orientation illustrated by reference cube, A – anterior, L – left. (Right) Oblique-sagittal three-dimensional CT reconstruction of the vessels. Right aortic arch (black asterisk) with five supra-aortic branches. First branch (long dash arrow): common trunk giving off left common carotid artery (short dash arrow) and duplicated left vertebral artery (short thick arrow). Second branch (short thin arrow): right common carotid artery. Third branch (white arrowhead): fenestrated right vertebral artery. Fourth branch (long thin arrow): right subclavian artery. Fifth branch (long thick arrow): aberrant left subclavian arising from the diverticulum of Kommerell (white asterisk). Left vertebral artery (curve arrow) originated from the aberrant left subclavian artery.
On the left, two VAs with separate origins and distal fusion were seen, thus constituting “duplication.” The one originating from the left SCA ascended in the prevertebral space and entered the left C5 transverse foramen. The second left VA originated from the left common carotid artery, ascended in the carotid space, and entered the left C4 transverse foramen, where it fused with the intraforaminal VA.
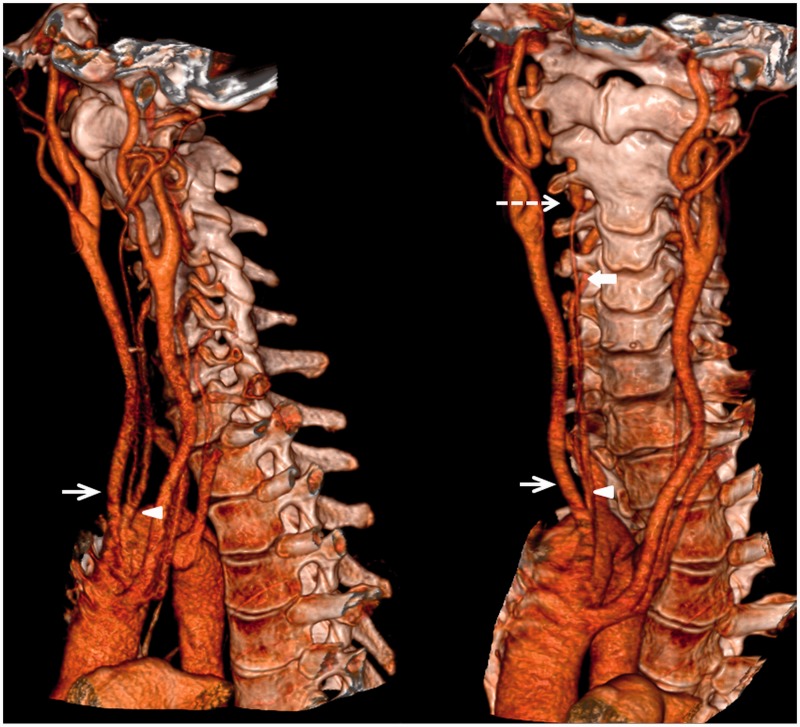
On the right, the VA arose directly from the aortic arch and divided into two trunks near the origin, thus constituting a “fenestration” (Figure 4). The dominant VA entered the right C4 transverse foramen. The fenestrated VA ascended in the retropharyngeal space, and merged with the intraforaminal VA as it entered the transverse foramen at the level of C3 (Figure 4).
Figure 4.
(Left) Sagittal and (Right) oblique coronal three-dimensional computed tomography (CT) reconstruction of the right vertebral artery fenestration. Right vertebral artery originated from the aortic arch and divided into two trunks near the origin (arrowhead). The dominant right vertebral artery entered the right C4 transverse foramen. The fenestrated vertebral arteries joined at the level of C3 (short dash arrow). The right common carotid artery (short white arrow) was the second branch off the aortic arch. There is incomplete fusion of the anterior arch of C1 and absence of posterior arch of C1.
Discussion
Terminology
“Duplication” and “fenestration” are two different entities and should not be used interchangeably. Duplication of the VA is present if there are two proximal VAs with separate origins, distinct courses up the neck, and distal fusion. “Fenestration” has a single origin and termination, but has parallel segments along its course. Parallel segments fuse before reaching their destination. Fenestrations of the VA can be subdivided into intracranial and extracranial types based on their localization.
Embryology
Knowledge of the embryologic development of the aortic arch is paramount to the understanding the anomalies of the aortic arch and the supra-aortic branches. The embryogenesis of the VA begins at approximately 32 days and is completed by 40 days. Formation of the left aortic arch and supra-aortic vessels are accomplished through a series of regression and reformation. A mis-step occurring in this well-orchestrated progress can give rise to anomalies.
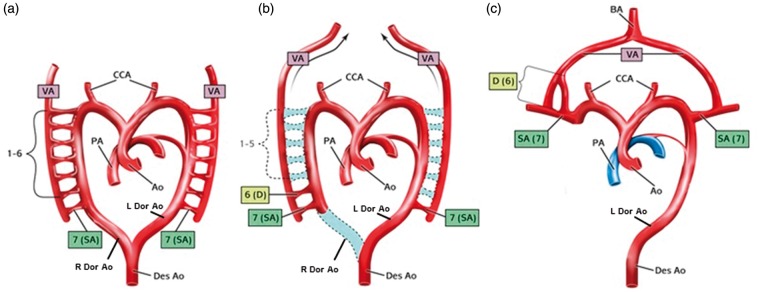
The primitive aorta consists of a ventral and a dorsal segment. The ventral aortae fuse to form the aortic sac (truncus arteriosus), while the dorsal aortae fuse to form the descending aorta. Six paired branchial arches develop between the ventral and dorsal aortae. In addition, seven intersegmental arteries branch off the dorsal aorta5 and are linked by longitudinal plexiform anastomosis (Figure 5).
Figure 5.
Embryologic development of a normal left aortic arch and single bilateral VAs. (a) Dorsal and ventral primitive aorta. Seven intersegmental arteries branch off the dorsal aorta and are linked by longitudinal plexiform anastomosis. (b) Involution of the first through sixth intersegmental arteries bilaterally. The dorsal branch of the seventh intersegment artery forms the V1 segment of the VA. The dorsal aortae fuse to form the descending aorta (Des Ao). (c) The VA extends cranially, and unites with the contralateral counterpart to form the basilar artery (BA). A: aorta; R Dor Ao: right dorsal aorta; L Dor Ao: left dorsal aorta; CCA: common carotid artery; Des Ao: descending aorta; PA: pulmonary artery; SA: segmental artery; VA: vertebral artery. (Adapted from Polguj et al.).
The left aortic arch is created by regression of the right dorsal aortic root between the right SCA and the descending aorta. Following a series of regression of the branchial arches, the subclavian arteries, carotid system, and the pulmonary arteries are formed (Figure 5).
The seventh intersegmental branch persists, as the first through sixth intersegmental branches involute (Figure 5(b)). The longitudinal anastomotic chain becomes the VA. The dorsal branch of the seventh intersegment artery forms the V1 segment of the VA (Figure 5(c)). The VA extends cranially, and unites with the contralateral counterpart to form the basilar artery (Figure 5(c)).
Anomaly
Right arch
A right aortic arch is formed when the right fourth branchial arch persists while the left dorsal aortic segment between left SCA and left common carotid artery regresses6 (Figure 6). Right aortic arch is an uncommon anatomical anomaly that occurs in less than 0.1% of the population, with a coexisting aberrant left SCA in half of those cases.1 Right aortic arch with aberrant left SCA is more common than right aortic arch with mirror image.
Figure 6.
Embryologic development of a right aortic arch and single bilateral VAs. (a) Dorsal and ventral primitive aorta. Seven intersegmental arteries branch off the dorsal aorta and are linked by longitudinal plexiform anastomosis. (b) The left dorsal aortic segment between left subclavian artery and left common carotid artery regresses (c) Right aortic arch with aberrant left subclavian artery. (Adapted from Polguj et al.). Ao: aorta; R Dor Ao: right dorsal aorta; L Dor Ao: left dorsal aorta; CCA: common carotid artery; Des Ao: descending aorta; PA: pulmonary artery; SA: segmental artery; VA: vertebral artery.
Duplication
Several mechanisms that may give rise to a separate origin of the VA have been proposed. One explanation suggests that duplication occurs when a persistent primitive dorsal aorta is connected to the true VA via an intersegmental vessel.3,7,8 Another possible explanation is the failure of the fourth, fifth or sixth intersegmental artery to regress8,9 (Figure 7).
Figure 7.
Embryologic development of VA duplication. (a) Dorsal and ventral primitive aorta. Seven intersegmental arteries branch off the dorsal aorta and are linked by longitudinal plexiform anastomosis. (b) Involution of the first through fifth intersegmental arteries on the right. The sixth intersegmental artery (6D) failed to regress. (c) Sixth and seventh intersegment arteries form a right duplicated VA, which extends cranially, and unites with the contralateral counterpart to form the basilar artery. Ao: aorta; R Dor Ao: right dorsal aorta; L Dor Ao: left dorsal aorta; CCA: common carotid artery; Des Ao: descending aorta; PA: pulmonary artery; SA: segmental artery; VA: vertebral artery. (Adapted from Polguj et al.).
When duplication occurs on the right side, the two origins usually arise from the right SCA.10 In left-sided duplications, duplicated origins both arising from the left SCA is less common.10 More often seen is an origin from the left SCA, with the duplicated origin arising directly from the aortic arch, between the left common carotid artery and the left SCA.10 Other duplicated origins arising from the thyrocervical trunk, brachiocephalic trunk, or aorta have also been reported.11 In this patient, the duplicated VA arises from the common carotid artery, which is unusual. The medial origin often enters the transverse foramen at a higher level than the normal VA, which is in keeping with the theory of intersegmental vessel regression failure.12
Fenestration
About 70% of VA fenestrations are located extracranially, 30% occur intracranially.4 The most common extracranial location is at the atlantoaxial level. Several mechanisms have been proposed as the cause of extracranial fenestrations. The first explanation is the failure of the plexiform anastomoses to involute. The reported predilection of fenestrations to occur in the upper segments of the cervical VA argues against this theory,13 as one would expect an equal probability of fenestrations in the upper and lower segments of the cervical VA.
Another explanation suggests the failure in regression of two intersegmental arteries gives rise to the fenestrated VA13 (Figure 8). An alternative mechanism is having a persistent section of the dorsal aorta in close proximity to the normal VA and connected to the normal VA by short intersegmental arteries.3
Figure 8.
Embryologic development of VA fenestration. (a) Dorsal and ventral primitive aorta. Seven intersegmental arteries branch off the dorsal aorta and are linked by longitudinal plexiform anastomosis. (b) The left second and third intersegmental arteries (F2,3) fail to regress. Involution of the remaining intersegmental arteries. (c) Formation of a left fenestrated VA, which extends cranially, and unites with the contralateral counterpart to form the basilar artery. Ao: aorta; R Dor Ao: right dorsal aorta; L Dor Ao: left dorsal aorta; CCA: common carotid artery; Des Ao: descending aorta; PA: pulmonary artery; SA: segmental artery; VA: vertebral artery. (Adapted from Polguj et al.).
Intracranial fenestrations are thought to be due to the persistence of fetal anastomotic vessels such as the trigeminal, optic or hypoglossal arteries.7,14,15 Partial fusion of the proatlantal artery with the normal VA has been suggested as a possible mechanism as well.7
Association with pathology
Duplication
The association of duplication with vascular pathology has not been conclusively proven. Some believe that since the duplicated arteries have a normal wall structure, there is no predisposition to pathological processes.16 However, there are reports of coexisting pathologies including aneurysm formation, vertebrobasilar dissection,17,18 and Ehlers-Danlos syndrome.10
Fenestration
Fenestrated arteries often have irregularities in the wall structure of the vessels, in particular in connection with the tunica media.16 The tunica media at the proximal and distal ends of the fenestrated segment can be underdeveloped and may contain irregular elastic fibers.3,19 This might increase the risk of developing conditions such as saccular aneurysms,19 arteriovenous malformations,15 dissections20,21 and thromboembolic events.22 Intracranial aneurysms were found in up to 20% of patients with VA fenestration.23 Vertebrobasilar junction aneurysms are frequently associated with fenestration.24 In patients with known arteriovenous malformations, 7% were found to have VA fenestration.15 VA fenestration increases the risk of arterial dissection, 65% of which occur at the atlantoaxial level.20,21 Although there are case reports of arterial dissection associated with fenestration, the exact association rate is unclear.25
Incidence/symptoms
Right arch
A right-sided aortic arch is a rare congenital abnormality with a prevalence of less than 0.1% in the population.3 According to the Edwards classification, there are three subtypes: (1) Mirror image aortic arch (type 1) has a prevalence of 59%; (2) aberrant left SCA (type 2) has a prevalence of 39.5%; (3) obliteration of the left SCA with collateralization via persistent ductus arteriosus Botalli (type 3) is rare with a prevalence of 1.5%.26 An association with a deletion of chromosome 22q11 and 24% prevalence was reported.1 Edwards type II is associated with congenital cardiac anomalies in 5%–10%, as opposed to a 75%–85% association observed in Edwards type 1.26
In Edwards type II, the aberrant left SCA arises from the descending aorta and has a variable course—80% courses posterior to the esophagus, 5% courses anterior to the trachea and 15% courses between the trachea and esophagus.26,27 Often patients with a right aortic arch with aberrant SCA are asymptomatic. Others have miscellaneous symptoms like dysphagia, wheezing, choking and coughing in adulthood.
Kommerell’s diverticulum is the aneurysmal dilation of the left aortic arch remnant, from which the aberrant subclavian arises. Patients are often asymptomatic. Others present with nonspecific symptoms such as dysphagia lusoria, dyspnea, asthma-like symptoms or recurrent tracheobronchial infections.27 Rarely, patients may present with syncope and left subclavian steal syndrome.28
Duplication
Unilateral duplication of the VA is a rare anomaly, with a prevalence of approximately 0.72% observed in studied cadavers2 and 26 cases reported in the literature.8,29 Bilateral VA duplication is extremely rare with three cases reported in literature.8,14,30
Presenting symptoms of vertigo or dizziness have been attributed to narrowing of the lumen, which is susceptible to kinking causing posterior circulation insufficiency.16 Some believe the altered hemodynamics secondary to the anomalous origin may predispose to aneurysm formation and dissection.18,31
Fenestration
Fenestration of the VA is more common than duplication, with a prevalence of approximately 0.23%–1.95%.3,4 Since this anomaly is most often incidentally diagnosed, the actual prevalence is probably higher.
Clinical implications
Although right aortic arch, VA duplications and VA fenestrations are rare congenital vascular anomalies, identifying them is important, as they can have diagnostic and therapeutic implications.
Diagnostic
These anomalies are often clinically silent, and are incidentally diagnosed on CTA, MR angiogram (MRA) or conventional angiography. Identifying these vascular anomalies is facilitated by improving technology, specifically by the improved resolution in CTA and MRA. Correct identification of VA fenestration and duplication is important, because they can mimic VA dissection,32 which bears a drastically different prognosis and requires urgent treatment. VA dissection on conventional angiography shows irregular stenosis with or without pseudoaneurysm, double lumen, intimal flap or occlusion.33 On magnetic resonance imaging (MRI), dissection typically shows an eccentric signal void surrounded by a semilunar hyperintensity corresponding to the mural hematoma.34 The luminal compromise, intimal flap and mural hematoma are not seen in duplication and fenestration.
Altered hemodynamics associated with VA anomalies has been hypothesized to predispose certain cerebrovascular pathologies like aneurysm formation and dissection.9,12,17,18,31 Therefore, thorough scrutiny of intracranial vessels is essential in patients with VA anomalies.
Cervical spinal trauma, sustained from rapid deceleration, flexion or subluxation as seen in motor vehicle accidents, are often associated with VA injuries that have morbid consequences. Duplication and fenestration essentially doubles the possibility of VA injuries.31
Therapeutic
Recognition of VA anomalies is important in vascular surgery or endovascular procedures. For instance, in planning intracranial interventions requiring VA catheterization, the VA with the widest lumen would be preferred over the narrower duplication.
Indications for surgical correction of a right aortic arch with aberrant SCA include dysphagia and compressive symptoms from dilated Kommerell’s diverticula. Symptomatic patients can be treated by total arch replacement (TAR) and anatomic reconstruction of the SCA with a graft to release the vascular ring. Anatomic reconstruction of the SCA is performed to prevent hand ischemia and subclavian steal syndrome. Potential complications of such surgery include rupture, dissection, hand ischemia, and subclavian steal syndrome.
Conclusion
We report a rare case of right aortic arch with aberrant left SCA, duplicated left VA and a fenestrated right vertebral artery. To the best of our knowledge, this is the first such case to be reported. Recognizing aortic arch and supra-aortic vessels anomalies is important, as they have significant clinical and surgical implications. Being cognizant of these VA anomalies can prevent misdiagnosing VA dissections. Appropriate differentiation between fenestration and dissection can mitigate confusion of the two distinct entities. Careful scrutiny for aneurysms and dissections in the presence of VA anomalies is advised owing to predisposition of VA anomalies for such pathologies.
Acknowledgments
The authors acknowledge Philip J Cohen, Illustrator, Department of Radiology and Medical Imaging, University of Virginia Health System, Charlottesville, Virginia, VA 22908, USA, for Figures 5–8, which are adapted from Polguj et al.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
None declared.
References
- 1.Cina CS, Arena GO, Bruin G, et al. Kommerell’s diverticulum and aneurysmal right-sided aortic arch: A case report and review of the literature. J Vasc Surg 2000; 32: 1208–1214. [DOI] [PubMed] [Google Scholar]
- 2.Bergman RA, Thompson SA, Afifi AK, et al. Compendium of human anatomic variation: Text, atlas, and world literature, Baltimore: Urban and Schwarzenberg, 1988. [Google Scholar]
- 3.Sim E, Vaccaro AR, Berzlanovich A, et al. Fenestration of the extracranial vertebral artery: Review of the literature. Spine (Phila Pa 1976) 2001; 26: E139–E142. [DOI] [PubMed] [Google Scholar]
- 4.Drapkin AJ. The double lumen: A pathognomonic angiographic sign of arterial dissection? Neuroradiology 2000; 42: 203–205. [DOI] [PubMed] [Google Scholar]
- 5.Padget DH. The development of cranial arteries in the human embryo. Contr Embryol 1948; 32: 205–261. [Google Scholar]
- 6.Kadir S. Regional anatomy of the thoracic aorta. In: Atlas of normal and variant angiographic anatomy. 1st ed. Philadelphia: Saunders, 1991, pp.19–54.
- 7.Vasović LP. Reevaluation of the morphological parameters according to 11 different duplications of the fetal vertebral artery at prevertebral (V1) and intracranial (V4) parts. Cells Tissues Organs 2004; 176: 195–204. [DOI] [PubMed] [Google Scholar]
- 8.Rameshbabu C, Gupta OP, Gupta KK, et al. Bilateral asymmetrical duplicated origin of vertebral arteries: Multidetector row CT angiographic study. Indian J Radiol Imaging 2014; 24: 61–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kendi AT, Brace JR. Vertebral artery duplication and aneurysms: 64-slice multidetector CT findings. Br J Radiol 2009; 82: e216–e218. [DOI] [PubMed] [Google Scholar]
- 10.Polguj M, Podgόrski M, Jedrzejewski K, et al. Fenestration and duplication of the vertebral artery: The anatomical and clinical points of view. Clin Anat 2013; 26: 933–943. [DOI] [PubMed] [Google Scholar]
- 11.Kiss J. Bifid origin of the right vertebral artery: A case report. Radiology 1968; 91: 931. [DOI] [PubMed] [Google Scholar]
- 12.Thomas AJ, Germanwala AV, Vora N, et al. Dual origin extracranial vertebral artery: Case report and embryology. J Neuroimaging 2008; 18: 173–176. [DOI] [PubMed] [Google Scholar]
- 13.Tseng YC, Hsu HL, Lee TH, et al. Fenestration of the vertebral artery at the lower cervical segment—imaging findings and literature review. Eur J Rad Extra 2004; 49: 37–40. [Google Scholar]
- 14.George B, Cornelius JF. Vertebral artery: surgical anatomy. In: Spetzler RF. (ed). Operative techniques in neurosurgery, Philadelphia: WB Saunders, 2001, pp. 168–181. [Google Scholar]
- 15.Uchino A, Sawada A, Takase Y, et al. Extreme fenestration of the right vertebral artery: Magnetic resonance angiographic demonstration. Eur Radiol 2002; 12(Suppl 3): S32–S34. [DOI] [PubMed] [Google Scholar]
- 16.Ionete C, Omojola MF. MR angiographic demonstration of bilateral duplication of the extracranial vertebral artery: Unusual course and review of the literature. Am J Neuroradiol 2006; 27: 1304–1306. [PMC free article] [PubMed] [Google Scholar]
- 17.Dare AO, Chaloupka JC, Putman CM, et al. Vertebrobasilar dissection in a duplicated cervical vertebral artery: A possible pathoetiologic association? A case report. Vasc Endovasc Surg 1997; 31: 103–109. [Google Scholar]
- 18.Melki E, Nasser G, Vandendries C, et al. Congenital vertebral duplication: A predisposing risk factor for dissection. J Neurol Sci 2012; 314: 161–162. [DOI] [PubMed] [Google Scholar]
- 19.Kubo M, Hacein-Bey L, Varelas PN, et al. Ruptured saccular aneurysm of distal vertebral artery fenestration managed with Guglielmi detachable coils and intraventricular tissue plasminogen activator. Surg Neurol 2005; 63: 244–248; discussion 248. [DOI] [PubMed] [Google Scholar]
- 20.Schievink WI. Spontaneous dissection of the carotid and vertebral arteries. N Engl J Med 2001; 344: 898–906. [DOI] [PubMed] [Google Scholar]
- 21.Tay KY, U-King-Im JM, Trivedi RA, et al. Imaging the vertebral artery. Eur Radiol 2005; 15: 1329–1343. [DOI] [PubMed] [Google Scholar]
- 22.Harnier S, Harzheim A, Limmroth V, et al. Duplication of the common carotid artery and the ipsilateral vertebral artery with a fenestration of the contralateral common carotid artery. Neurol India 2008; 56: 491–493. [DOI] [PubMed] [Google Scholar]
- 23.Miyazaki S, Kamata K, Yamaura A. Multiple aneurysms of the vertebrobasilar system associated with fenestration of the vertebral artery. Surg Neurol 1981; 15: 192–195. [DOI] [PubMed] [Google Scholar]
- 24.Yoon SM, Chun YI, Kwon Y, et al. Vertebrobasilar junction aneurysms associated with fenestration: Experience of five cases treated with Guglielmi detachable coils. Surg Neurol 2004; 61: 248–254. [DOI] [PubMed] [Google Scholar]
- 25.Zhang QJ, Kobayashi S, Gibo H, et al. Vertebrobasilar junction fenestration associated with dissecting aneurysm of intracranial vertebral artery. Stroke 1994; 25: 1273–1275. [DOI] [PubMed] [Google Scholar]
- 26.Edwards JE. Anomalies of the derivates of the aortic arch system. Med Clin North Am 1948; 32: 925–949. [DOI] [PubMed] [Google Scholar]
- 27.Ebner L, Huber A, Christe A. Right aortic arch and Kommerell’s diverticulum associated with acute aortic dissection and pericardial tamponade. Acta Radiol Short Rep 2013; 2: 2047981613476283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yang MH, Weng ZC, Weng YG, et al. A right-sided aortic arch with Kommerell’s diverticulum of the aberrant left subclavian artery presenting with syncope. J Chin Med Assoc 2009; 72: 275–277. [DOI] [PubMed] [Google Scholar]
- 29.Komiyama M, Nakajima H, Yamanaka K, et al. Dual origin of the vertebral artery—case report. Neurol Med Chir (Tokyo) 1999; 39: 932–937. [DOI] [PubMed] [Google Scholar]
- 30.Mordasini P, Schmidt F, Schroth G, et al. Asymmetrical bilateral duplication of the extracranial vertebral arteries: Report of a unique case. Eur J Rad Extra 2008; 67: e91–e94. [Google Scholar]
- 31.Satti SR, Cerniglia CA, Koenigsberg RA. Cervical vertebral artery variations: An anatomic study. Am J Neuroradiol 2007; 28: 976–980. [PMC free article] [PubMed] [Google Scholar]
- 32.Nogueira TE, Chambers AA, Brueggemeyer MT, et al. Dual origin of the vertebral artery mimicking dissection. Am J Neuroradiol 1997; 18: 382–384. [PMC free article] [PubMed] [Google Scholar]
- 33.Glauser J, Hastings O, Mervant M, et al. Dissection of the vertebral arteries: Case report and discussion. J Emerg Med 1994; 12: 307–315. [DOI] [PubMed] [Google Scholar]
- 34.Levy C, Laissy JP, Raveau V, et al. Carotid and vertebral artery dissections: Three-dimensional time-of-flight MR angiography and MR imaging versus conventional angiography. Radiology 1994; 190: 97–103. [DOI] [PubMed] [Google Scholar]