Abstract
We compared flat-detector computed tomography angiography (FD-CTA) to multislice computed tomography (MS-CTA) and digital subtracted angiography (DSA) for the visualization of experimental aneurysms treated with stents, coils or a combination of both.
In 20 rabbits, aneurysms were created using the rabbit elastase aneurysm model. Seven aneurysms were treated with coils, seven with coils and stents, and six with self-expandable stents alone. Imaging was performed by DSA, MS-CTA and FD-CTA immediately after treatment. Multiplanar reconstruction (MPR) was performed and two experienced reviewers compared aneurysm/coil package size, aneurysm occlusion, stent diameters and artifacts for each modality.
In aneurysms treated with stents alone, the visualization of the aneurysms was identical in all three imaging modalities. Residual aneurysm perfusion was present in two cases and visible in DSA and FD-CTA but not in MS-CTA. The diameter of coil-packages was overestimated in MS-CT by 56% and only by 16% in FD-CTA compared to DSA (p < 0.05). The diameter of stents was identical for DSA and FD-CTA and was significantly overestimated in MS-CTA (p < 0.05). Beam/metal hardening artifacts impaired image quality more severely in MS-CTA compared to FD-CTA.
MS-CTA is impaired by blooming and beam/metal hardening artifacts in the visualization of implanted devices. There was no significant difference between measurements made with noninvasive FD-CTA compared to gold standard of DSA after stenting and after coiling/stent-assisted coiling of aneurysms. FD-CTA may be considered as a non-invasive alternative to the gold standard 2D DSA in selected patients that require follow up imaging after stenting.
Keywords: Endovascular treatment, experimental aneurysms, flat-detector computed tomography, follow-up examination
Introduction
Specialty devices or combinations of those devices are used to treat intracranial aneurysms. In particular, stents and flow diverting devices are used in increasing numbers. Due to the potential risk of aneurysm recanalization or other complications (such as in-stent stenosis), follow-up imaging is mandatory. Currently, this is primarily performed using digital subtraction angiography (DSA). Thus, to improve patient safety and comfort, a non-invasive technique would be highly desirable.
In aneurysms treated with coils alone, treatment results can be followed by magnetic resonance imaging (MRI).1 However, MRI becomes much more challenging for the visualization of stents. The interaction of the devices with the magnetic field and radio waves may result in a significant signal-intensity loss at the lumen of a stent and also in the area immediately adjacent to the stented parent artery. This phenomenon of stent-induced radio-frequency shielding is well known. Therefore, MRI has only limited benefit for the assessment of aneurysms treated with stents.2–5
The use of multislice computed tomography angiography (MS-CTA) for the visualization of stents is impaired by blooming artifacts that result in an artificial lumen narrowing (ALN). In this situation, stent struts appear larger than they actually are and thus impair the visibility of the lumen. In-vitro studies of small vessel stents demonstrated that the minimum diameter of stents for reliable visualization of the stent lumen by MS-CTA was 4.0 mm.6–8
Additionally, the visualization of coiled aneurysms by MS-CTA is impaired due to streak-like beam hardening artifacts in the surrounding region of the implanted material, and may prevent the visualization of important anatomical structures.9 Similar to ALN, it is assumed that, due to blooming of the metallic structures, coil packages may appear larger than they really are.
In the last few years flat-detector computed tomography (FD-CT) has been introduced into clinical routine.10–16 The primary advantage of FD-CT in comparison to MS-CTA and MRI is its superior spatial resolution.8 It is well known that FD-CT provides a high spatial resolution for the visualization of implanted devices, which can provide useful information when investigating their behavior and positioning after deployment.17 In combination with an intravenous contrast injection, FD-CT can be used to perform non-invasive high spatial resolution flat-detector computed tomography angiography (FD-CTA). FD-CTA has been shown that it can be used in the follow-up imaging of intracranial stents for atherosclerotic disease.10,18 Additionally, it has been shown that FD-CTA is a promising new imaging modality for the follow-up of clipped aneurysms.19,20 Feasibility of non-invasive aneurysm visualization by FD-CTA has been shown in an animal model and in humans.14,21
The idea of using FD-CTA as a non-invasive follow-up imaging modality in aneurysms treated with coils and stents or combinations of these devices is therefore obvious. Our hypothesis was that FD-CTA may show limited artifacts in comparison to MS-CTA and thus may allow accurate evaluation of both the aneurysm as well as the implanted stent (evaluation of the lumen).
Materials and methods
The study was approved by the local Animal Protection Committee. The elastase aneurysm model in New Zealand White rabbits is well known and accepted to simulate human aneurysms and has been used for many different studies.22,23 In addition, it has been shown that FD-CTA is feasible in this model.14
Endovascular treatment
Aneurysms were successfully created in 20 rabbits. This procedure has been described in detail in another publication.22 For each subject, three weeks after aneurysm creation the femoral artery was surgically exposed, distally ligated, and a 4 F introducer sheath (Radiofocus Introducer II, Terumo Cooperation, Terumo Europe N.V., Leuven, Belgium) was placed. Using standard angiographic techniques, a microcatheter (Tracker Excel 14, Boston Scientific/Target, Fremont, California, USA) was advanced to the brachiocephalic trunk. DSA series were performed in order to visualize the aneurysm. The aneurysms were then treated by coils (n = 7), by stent and coils (n = 7) or exclusively by a stent (n = 6). We used a self-expandable stent system (Neuroform3, Microdelivery Stent System, Boston Scientific, Fremont, USA) and detachable bare platinum coils (GDC 10, Boston Scientific, Fremont, USA) exclusively. Stents sizes ranged from 3–3.5 mm in diameter and 15–20 mm in length. After treatment, DSA series were acquired via a microcatheter.
Next, the microcatheter and introducer sheat were removed. The femoral vein was exposed and a puncture was performed using a standard venous 22 G catheter (Vasofix Safety, Braun Melsungen AG, Melsungen, Germany). This venous access was used for subsequent FD-CTA and MS-CTA imaging. Immediately following the imaging, the animals were sacrificed.
Imaging
DSA
DSA was performed using a biplane flat detector angiographic system (Axiom Artis dBA, Siemens AG Healthcare Sector, Forchheim, Germany). We injected 1 ml of non-ionic contrast material manually (Imeron 300, Bracco Altana Phrama GmbH, Konstanz, Germany) via the microcatheter. A standard 2D application was used (tube voltage 73 kV, dose/frame 3 µGy, frame rate 4 frames/s).
FD-CTA
FD-CTA was performed using the flat detector angiographic system. As described by others we used 1 ml contrast material per 1 kg body weight, followed by 6 ml saline flush with an injection rate of 1 ml/s. This contrast application protocol was used both for FD-CTA and MS-CTA.10 After a delay time of 4 s, the FD-CTA acquisition was initiated.14 Power injection was performed using a dual syringe power injector (Accutron HP-D, MEDTRON, Saarbrücken, Germany).
FD-CTA data acquisition was carried out using the 20sDR-H program, which is provided as a standard acquisition program by the manufacturer. This program is characterized by the following parameters: 20 s rotation time, 30 × 40 cm detector field of view, 200° angular range, angular increment of 0.4°/image, and a dose per frame of 1.2 µGy/frame. Post-processing was performed with a commercially available dedicated workstation and software (syngo MMWP, InSpace 3D software, Siemens AG Healthcare Sector, Forchheim, Germany). The reconstruction software includes algorithmic corrections for beam hardening, scattered radiation, truncated projections, and ring artifacts. Post-processing resulted in a volume of isotropic voxel data with a batch of approximately 450 slices in a 512 × 512 matrix. Reconstruction was performed using kernel type “HU”, image impression “normal” as recommended by the manufacturer. Single slice thickness of this batch of images was 0.1 mm and the field of view was 80 mm.10,14,15
MS-CTA
After FD-CTA imaging the animals were investigated by MS-CTA. To eliminate the contrast material used for DSA and FD-CTA, the rabbits were hydrated using 15 ml saline. After a delay time of 60 min the animals were examined by MS-CTA. Scanning was performed using a 64 row CT system (Siemens Somatom Sensation 64, Siemens Healthcare, Forchheim, Germany). We used a standard acquisition program as proposed by the manufacturer with the following parameters: 160 mAs, 100 kV, a rotation time of 0.5 s, 64 × 0.6 mm collimation, pitch of 1.3, reconstruction increment 0.4 mm, slice thickness 0.6 mm, total exposure time of 4 s, matrix 512 × 512, field of view 80 mm resulting in a batch of about 450 slices. Reconstruction was done using the b40 kernel as recommended by the manufacturer as a standard kernel in MS-CTA. Bolus tracking was performed at the level of the heart. Scanning was started when maximal contrast enhancement in the ascending aorta was visible.
Post-processing
DSA images were post-processed using the standard software provided with the angiographic system. Both FD-CTA and MS-CTA images were post-processed using a dedicated workstation (syngo MMWP, Siemens AG Healthcare Sector, Forchheim, Germany). Multi-planar reconstruction (MPR) reformats were created with 1 mm slice thickness and 0.5 mm spacing in coronal orientation and an additional perpendicular (“axial”) orientation of long axis of the stent. DSA, FD-CTA and MS-CTA datasets were anonymized, randomized and transferred to a workstation for evaluation. The reviewers were allowed to perform windowing to achieve a sufficient visualization of the arteries and the implanted material. For MS-CTA, a window center of 250 ± 50 Hounsfield units (HU) with a window width of 650 ± 75 HU was used. For FD-CTA, a window center of 200 ± 30 HU with a window width of 900 ± 100 HU was chosen.
Imaging analysis
Due to the limited number of subjects, images were reviewed by two experienced neuroradiologists in consensus reading. Images were uploaded in random order on a dedicated workstation. There was no time limit for image evaluation.
First, overall image quality of the three imaging modalities was assessed using the following scales:
Perfect image quality (no movement artifacts, perfect contrast vessel opacification).
Good image quality (minimal movement artifacts, sufficient contrast vessel opacification).
Moderate image quality (obvious movement artifacts, poor contrast vessel opacification).
Poor image quality (not evaluable due to massive movement artifacts or insufficient contrast vessel opacification).
The reviewers were asked to evaluate the images for beam hardening/metal streak-like artifacts:
Massive artifacts, surrounding area not visible.
Obvious artifacts, surrounding area evaluable.
No artifacts.
To assess the occlusion of the aneurysms a modified Raymond classification was used.24,25
Complete obliteration.
Residual inflow.
No occlusion visible.
To quantify the blooming artifact of coiled aneurysms, the reviewers performed measurements (dome height, equaling the maximum aneurysm length; width, equaling the maximum diameter orthogonal to dome height) of the coil package. In the stent-only group, the aneurysm size in the three different imaging modalities was measured. Additionally, the diameter and area of the stent lumen was measured at the neck region of the aneurysms to quantify blooming of the stent struts. We assumed that DSA does not exhibit blooming issues. All measurements were done using standard tools of the syngo MMWP workstation.
Statistics
The mean value of each pair of measurements was calculated and used for further statistical analysis by Wilcoxon-Mann-Whitney-Test. Statistical significance was assumed for p values <0.05. All statistics were performed using SPSS 18.0.
Results
Aneurysms were successfully created in 20 rabbits, with sizes ranging from 5–9 mm. All treatment procedures were performed successfully. In five of the seven subjects in the coiling group, the reviewers assessed the overall image quality of FD-CTA to be of grade 1. In two cases of FD-CTA studies the image quality grading was 4. In the stent-assisted coiling group, five of seven FD-CTA imaging studies were rated as grade 1, with the other two studies receiving a grade of 4. In the stent-only group, five of six FD-CTA imaging studies received a grade of 1, and in one imaging study, the grading was 4. In all studies that received a grade of 4 (n = 5), contrast opacification of the arteries was not sufficient. All animals with grade 4 imaging were excluded from further analysis, leaving five aneurysms remaining in each of the three groups. Image quality for all DSA and MS-CTA studies were rated as grade 1.
Beam hardening/metal artifact grading
The reviewers were also asked to review the extent of the beam hardening/metal artifacts caused by the implanted coils and markers of the stent. For all MS-CTA images with stents and coils, reviewers found that these artifacts significantly impaired the image quality (grade 1). These artifacts were also visible in FD-CTA, but were not as pronounced as in MS-CTA and, therefore, all FD-CTA images received a rating of grade 2.
Coil package size
After treatment, the coil package size was measured in FD-CTA and MS-CTA and compared to the size of the coil package in DSA. Similar viewing orientations were used. The size of the coil package was calculated from the dome height and the width as area values. The size of coil package in FD-CTA and MS-CTA was set in ratio to aneurysm size in DSA and calculated as percentage values. In MS-CTA coil-package size was significantly overestimated to 56% compared to DSA (p < 0.005). In FD-CTA coil package size was overestimated only to 16% compared to DSA and was significant at Wilcoxon’s test (p < 0.047) (Figure 1 and 2).
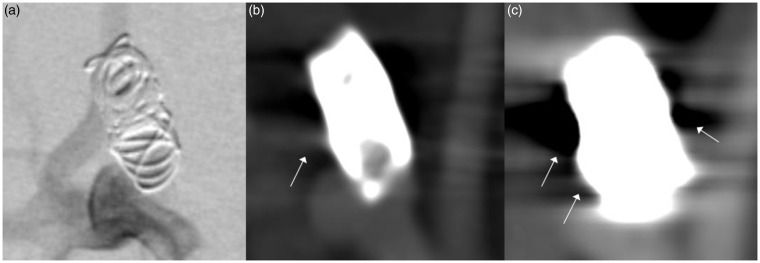
Figure 1.
Comparison of (a) digital subtracted angiography (DSA), (b) flat-detector computed tomography angiography (FD-CTA) and (c) multislice computed tomography (MS-CTA) in an aneurysm treated with coils. DSA clearly shows residual perfusion of the aneurysm neck. This could also be identified in FD-CTA, whereas in MS-CTA the aneurysm seems to be occluded completely. Beam hardening artifacts significantly impair image quality in MS-CTA, (c) (white arrows). These artifacts are also visible in FD-CTA (white arrow) but image quality is still suitable for evaluation. The size of the coil package in MC-CTA appears much larger than in DSA and FD-CTA indicating blooming artifact.
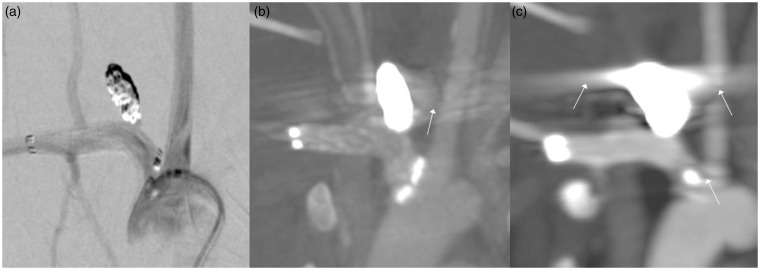
Figure 2.
In this aneurysm treated with a stent and coils ((a) digital subtracted angiography (DSA), (b) flat-detector computed tomography angiography (FD-CTA) and (c) multislice computed tomography (MS-CTA)) beam hardening artifacts severely impair the image quality of MS-CTA (white arrows). The left carotid and right vertebral artery are only partially visible. The markers of the stent appear as one white mass. In FD-CTA, beam hardening artifacts are visible (white arrow) but not as severe as in MS-CTA. The left carotid and right vertebral artery are nicely displayed. Coil package size is enlarged in MS-CTA compared to DSA and FD-CTA.
Occlusion assessment
Residual perfusion of aneurysms has been assessed by the reviewers (Grade x in n aneurysms, modified Raymond classification: 1 = complete obliteration, 2 = residual inflow, 3 = no occlusion visible). In the stent alone group, all the three different imaging modalities clearly showed no occlusion and were rated grade 3 (n = 5). The coiling group showed residual aneurysm inflow in two of the five aneurysms which could be identified by DSA and FD-CTA (Grade 2 in n = 2, grade 1 in n = 3,) but not by MS-CTA (Grade 1 in n = 5) (Figure 1). In the stent-assisted coiling group, all aneurysms were occluded (Grade 1 in n = 5) (Figure 2).
Size of aneurysms
Aneurysm size in the stent alone group was measured in all three imaging modalities. Reviewers could clearly identify aneurysms in DSA, FD-CTA and MS-CTA (Figure 3). Wilcoxon’s test showed no significant differences of the values between the different imaging modalities (p > 0.05).
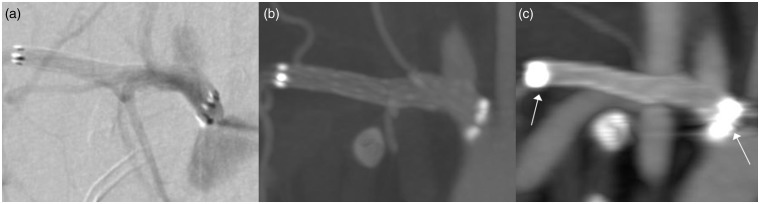
Figure 3.
Visualization of an aneurysm treated with a stent: (a) digital subtracted angiography (DSA), (b) flat-detector computed tomography angiography (FD-CTA) and (c) multislice computed tomography (MS-CTA). The aneurysm could be clearly identified in all the three imaging modalities. There is improved image quality and sharper delineation of the vascular structures and the aneurysm in FD-CTA compared to MS-CTA. Due to blooming the stents markers are severely enlarged in MS-CTA (white arrows).
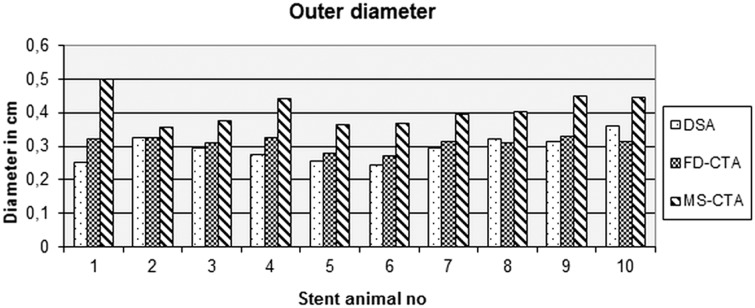
Stent diameter and lumen area
After exclusion of subjects with grade 4 image quality, stents in 10 animal subjects were used for evaluation. In FD-CTA and MS-CTA an “inner” and “outer” diameter of the stent is visible (Figure 4c/d). In DSA images a differentiation of “inner” and “outer” diameter is not possible. Therefore, measurements of the stented vessel diameter were performed on DSA images to serve as the gold standard and were compared to the “outer” diameter as measured by FD-CTA and MS-CTA.10 The reviewers were advised to measure the “inner” and “outer” diameter of the stents on axial perpendicular MPR reconstructions. Compared to DSA outer stent diameter was significantly overestimated in MS-CTA (p = 0.005) (Figure 5). In comparison of FD-CTA and DSA no significant difference in terms of outer stent diameter was found (p = 0.123).
Figure 4.
Reconstructed axial MPR-images of implanted stents of (a) flat-detector computed tomography angiography (FD-CTA) and (b) multislice computed tomography (MS-CTA). Stent lumen and struts can be identified in FD-CTA (a) but not in MS-CTA (b). Stent lumen diameter in MS-CTA is difficult to recognize and appears to be smaller than in FD-CTA. Measurements of the “inner” and “outer” diameter were performed as shown in (c) and (d).
Figure 5.
Measurements of the diameter of the stents. In comparison to digital subtracted angiography (DSA) there is no significant difference to flat-detector computed tomography angiography (FD-CTA) while in multislice computed tomography (MS-CTA) the outer diameter is significantly larger than DSA.
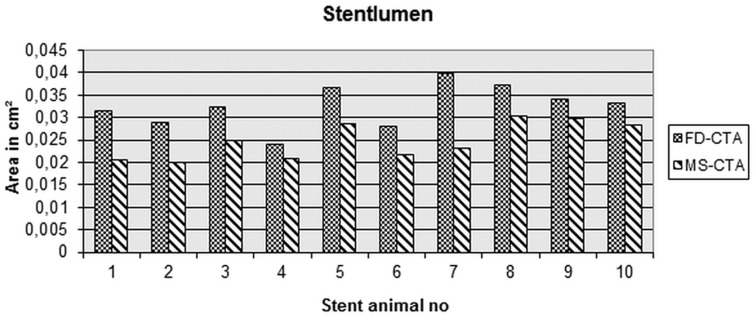
Stent struts could be identified clearly in FD-CTA (Figure 4a) while in MS-CTA the stents appeared as a ring structure (Figure 4b). The inner lumen area was significantly underestimated in MS-CTA compared to FD-CTA after intravenous contrast application (p = 0.028) (Figure 4a/b and Figure 6).
Figure 6.
The area of the stent lumen is underestimated in in multislice computed tomography (MS-CTA) in comparison to flat-detector computed tomography angiography (FD-CTA).
Discussion
Endovascular treatment of aneurysms is accepted as the standard treatment option in many centers. Due to the risk of recanalization, aneurysm re-growth, or other complications, follow-up imaging is necessary. If stents have been used, the implanted device lumen needs evaluation as well to rule out in-stent stenosis. MRI and CTA imaging can often be impaired by artifacts if stents are implanted. A minimal invasive imaging modality (as an alternative to MRI and CTA) is highly desirable to improve patient safety and comfort and avoid potential risks and expense of DSA. The aim of our pilot study was to show (using a widely accepted animal model) the feasibility that aneurysms treated by common devices may be evaluated by the new method of intravenous FD-CTA. Our hypothesis was that the superior spatial resolution provided by FD-CTA would allow the visualization of aneurysms treated with coils, stent and coils or stent alone would compare favorably to DSA with limited artifacts. Therefore, FD-CTA may serve as a minimal invasive follow-up imaging modality in these patients.
We have found that in the stent alone group, the visualization and the size of the aneurysms was identical for the three different imaging modalities. This is in line with other publications.14 Concerning the visualization of the lumen of the stents (stent with coils and stent alone group), we recognized subjectively and by measurements that the lumen cannot be evaluated by MS-CTA. It is well known that stents show a blooming artifact in MS-CTA meaning that the size of the stent struts will appear much larger than they are.6,7,10 Thus, visibility of the lumen of a stent in MS-CTA less than 4 mm diameter is not feasible. In FD-CTA the stent struts could be identified in axial MPR reconstructions, while in MS-CTA, the stent struts appeared as an enlarged ring structure. The measurements of the diameter in MS-CTA have clearly shown that there is no correlation to DSA. However, FD-CTA lumen diameter measurements compared well to the gold standard of DSA. Due to blooming artifacts, the markers of the stent system appeared to be enlarged in MS-CTA. The proximal and distal end of the stent appeared as one bright part while in FD-CTA the different markers could be distinctly recognized (Figures 2 and 3). Our findings indicate that FD-CTA can be used to evaluate the lumen of a stent and compares well to DSA.
The measurements of the size of the coil packages indicated that the size appears exaggerated in MS-CTA compared to DSA. We assume that this “enlargement” of the coil packages is also caused by blooming artifacts. This may be an explanation as to why, in two incomplete coiled aneurysms, the residual perfused parts at the neck could not be recognized. However, it has to be recognized that the appearance of the coil packages in FD-CTA was also overestimated in comparison to DSA, but not as severely as in MS-CTA. Nevertheless, FD-CTA could identify the two incomplete coiled aneurysms in our study.
The reviewers noted massive beam/metal hardening artifacts in the adjacent area due to the coils packages in MS-CTA. These artifacts were significantly less severe in FD-CTA. Though the animals and the aneurysms were positioned parallel to the scanning direction in FD-CTA as well as in MS-CTA, these artifacts were visible adjacent to the aneurysms and did not visually impair the parent vessel, but did others (Figure 1 and 2). If not parallel to the scanning direction (this may be unpredictable in patients), these artifacts may affect the parent vessel or neck region of aneurysms and may prevent evaluation by MS-CTA. These artifacts are obviously less severe but also present in FD-CTA. However, new post-processing algorithms in FD-CT are aiming to reduce these beam/metal hardening artifacts for further improvement of image quality.26–29 One limitation of this study is the size of the aneurysms, which was limited to small aneurysms. As described by others, it is possible that coiled aneurysms larger than 10 mm in size FD-CTA may have unacceptable artifacts (beam hardening, blooming) so that an evaluation may be no longer feasible.16 However, aneurysms of the size investigated here certainly represent the majority of aneurysms treated in patients.
FD-CTA has been shown to be useful for stent evaluation in order to recognize the shape, deployment, and lumen.18 Our findings confirm these results by others. Additionally, our results indicate that FD-CTA may be used as a minimal invasive follow-up imaging modality if patients are treated by a stent or a combination of coils and stents. In these two groups, the lumen of the stent and the aneurysms could be evaluated equally as well as DSA, while MS-CTA failed to visualize the lumen of the stents and, due to blooming artifacts, was inferior to FD-CTA in the evaluation of the occlusion of the aneurysm. This finding is of relevance since, in these patients, evaluation by MRI is limited due to artifacts as described before.
This pilot study is limited due to the small number of samples and lack of comparison to MRI. Histologic evaluation has not been performed, but was assumed to not be mandatory due to the comparison to DSA. However, this study is sufficient to show that MS-CTA is limited due to massive artifacts (blooming, metal/beam hardening). Currently MS-CTA is not appropriate in the follow up of coiled and stented aneurysms. Blooming and beam hardening artifacts are also present in FD-CTA, but are not as severe as in MS-CTA. Efforts are ongoing to reduce these artifacts in FD-CTA to further improve image quality. The results are valid for small aneurysms (<9 mm), as larger aneurysms could not be created with this model. Publications by others indicate that in larger aneurysms (>10 mm) treated with coils, these artifacts may be significant enough to impair FD-CT quality.16 In five out of 20 animals, an evaluation of the FD-CTA images was not possible due to insufficient opacification of the vascular structures in FD-CTA. A bolus tracking method has been described in patients and seems to be a robust method to control the contrast material application.18 This study would have benefited from this bolus tracking method, but unfortunately it was not available in our department at the time of the study. Further evaluation of FD-CTA is necessary to clearly define the role of FD-CTA relative to DSA.
Conclusions
FD-CTA is a promising new imaging modality for the visualization of aneurysms treated with coils and stents or combinations of these devices, and compared well to DSA. However, beam/metal hardening artifacts due to the coils may impair image quality, and further efforts to reduce this are ongoing. FD-CTA may be used in selected patients with aneurysms less than 10 mm in size and may be especially helpful if a stent had been used additionally. FD-CTA is feasible in the elastase aneurysm model and may be used for test and evaluation of new devices.
Funding
The authors gratefully acknowledge funding of the Medical Valley national leading edge cluster, Erlangen, Germany, diagnostic imaging network, sub-project BD 16, research grant no.: 13EX1212G.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Okahara M, Kiyosue H, Hori Y, et al. Three-dimensional time-of-flight MR angiography for evaluation of intracranial aneurysms after endosaccular packing with Guglielmi detachable coils: Comparison with 3D digital subtraction angiography. Eur Radiol 2004; 14: 1162–1168. [DOI] [PubMed] [Google Scholar]
- 2.Van Holten J, Wielopolski P, Bruck E, et al. High flip angle imaging of metallic stents: Implications for MR angiography and intraluminal signal interpretation. Magn Reson Med 2003; 50: 879–883. [DOI] [PubMed] [Google Scholar]
- 3.Lovblad KO, Yilmaz H, Chouiter A, et al. Intracranial aneurysm stenting: Follow-up with MR angiography. J Magn Reson Imaging 2006; 24: 418–422. [DOI] [PubMed] [Google Scholar]
- 4.Schaafsma JD, Velthuis BK, Majoie CB, et al. Intracranial aneurysms treated with coil placement: Test characteristics of follow-up MR angiography–multicenter study. Radiology 2010; 256: 209–218. [DOI] [PubMed] [Google Scholar]
- 5.Schaafsma JD, Velthuis BK, van den Berg R, et al. Coil-treated aneurysms: Decision making regarding additional treatment based on findings of MR angiography and intraarterial DSA. Radiology 2012; 265: 858–863. [DOI] [PubMed] [Google Scholar]
- 6.Hahnel S, Trossbach M, Braun C, et al. Small-vessel stents for intracranial angioplasty: In vitro comparison of different stent designs and sizes by using CT angiography. AJNR Am J Neuroradiol 2003; 24: 1512–1516. [PMC free article] [PubMed] [Google Scholar]
- 7.Trossbach M, Hartmann M, Braun C, et al. Small vessel stents for intracranial angioplasty: In vitro evaluation of in-stent stenoses using CT angiography. Neuroradiology 2004; 46: 459–463. [DOI] [PubMed] [Google Scholar]
- 8.Ionescu M, Metcalfe RW, Cody D, et al. Spatial resolution limits of multislice computed tomography (MS-CT), C-arm-CT, and flat panel-CT (FP-CT) compared to MicroCT for visualization of a small metallic stent. Acad Radiol 2011; 18: 866–875. [DOI] [PubMed] [Google Scholar]
- 9.Jiang L, He ZH, Zhang XD, et al. Value of noninvasive imaging in follow-up of intracranial aneurysm. Acta Neurochir Suppl 2011; 110: 227–232. [DOI] [PubMed] [Google Scholar]
- 10.Struffert T, Ott S, Adamek E, et al. Flat-detector computed tomography in the assessment of intracranial stents: Comparison with multi detector CT and conventional angiography in a new animal model. Eur Radiol 2011; 21: 1779–1787. [DOI] [PubMed] [Google Scholar]
- 11.Psychogios MN, Schramm P, Buhk JH, et al. Angiographic CT after intravenous contrast agent application: A noninvasive follow-up tool after intracranial angioplasty and stenting. AJNR Am J Neuroradiol 2011; 31: 1886–1891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Heran NS, Song JK, Namba K, et al. The utility of DynaCT in neuroendovascular procedures. AJNR Am J Neuroradiol 2006; 27: 330–332. [PMC free article] [PubMed] [Google Scholar]
- 13.Soderman M, Babic D, Holmin S, et al. Brain imaging with a flat detector C-arm: Technique and clinical interest of XperCT. Neuroradiology 2008; 50: 863–868. [DOI] [PubMed] [Google Scholar]
- 14.Struffert T, Doelken M, Adamek E, et al. Flat-detector computed tomography with intravenous contrast material application in experimental aneurysms: Comparison with multislice CT and conventional angiography. Acta Radiol 2010; 51: 431–437. [DOI] [PubMed] [Google Scholar]
- 15.Doelken M, Struffert T, Richter G, et al. Flat-panel detector volumetric CT for visualization of subarachnoid hemorrhage and ventricles: Preliminary results compared to conventional CT. Neuroradiology 2008; 50: 517–523. [DOI] [PubMed] [Google Scholar]
- 16.Richter G, Engelhorn T, Struffert T, et al. Flat panel detector angiographic CT for stent-assisted coil embolization of broad-based cerebral aneurysms. AJNR Am J Neuroradiol 2007; 28: 1902–1908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Benndorf G, Strother CM, Claus B, et al. Angiographic CT in cerebrovascular stenting. AJNR Am J Neuroradiol 2005; 26: 1813–1818. [PMC free article] [PubMed] [Google Scholar]
- 18.Struffert T, Kloska S, Engelhorn T, et al. Optimized intravenous flat detector CT for non-invasive visualization of intracranial stents: First results. Eur Radiol 2011; 21: 411–418. [DOI] [PubMed] [Google Scholar]
- 19.Psychogios MN, Wachter D, Mohr A, et al. Feasibility of Flat Panel Angiographic CT after Intravenous Contrast Agent Application in the Postoperative Evaluation of Patients with Clipped Aneurysms. AJNR Am J Neuroradiol. 2011; 32(10): 1956–1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Golitz P, Struffert T, Ganslandt O, et al. Optimized angiographic computed tomography with intravenous contrast injection: an alternative to conventional angiography in the follow-up of clipped aneurysms? J Neurosurg 2012; 117: 29–36. [DOI] [PubMed] [Google Scholar]
- 21.Golitz P, Struffert T, Knossalla F, et al. Angiographic CT with intravenous contrast injection compared with conventional rotational angiography in the diagnostic work-up of cerebral aneurysms. AJNR Am J Neuroradiol 2012; 33: 982–987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cloft HJ, Altes TA, Marx WF, Raible RJ, et al. Endovascular creation of an in vivo bifurcation aneurysm model in rabbits. Radiology 1999; 213: 223–228. [DOI] [PubMed] [Google Scholar]
- 23.Struffert T, Roth C, Romeike B, et al. Onyx in an experimental aneurysm model: Histological and angiographic results. J Neurosurg 2008; 109: 77–82. [DOI] [PubMed] [Google Scholar]
- 24.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2001; 32: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 25.Raslan AM, Oztaskin M, Thompson EM, et al. Neuroform stent-assisted embolization of incidental anterior communicating artery aneurysms: Long-term clinical and angiographic follow-up. Neurosurgery 2011; 69: 27–37; discussion 37. [DOI] [PubMed] [Google Scholar]
- 26.Prell D, Kyriakou Y, Beister M, et al. A novel forward projection-based metal artifact reduction method for flat-detector computed tomography. Phys Med Biol 2009; 54: 6575–6591. [DOI] [PubMed] [Google Scholar]
- 27.Prell D, Kyriakou Y, Struffert T, et al. Metal artifact reduction for clipping and coiling in interventional C-arm CT. AJNR Am J Neuroradiol 2010; 31: 634–639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Psychogios MN, Scholz B, Rohkohl C, et al. Impact of a new metal artefact reduction algorithm in the noninvasive follow-up of intracranial clips, coils, and stents with flat-panel angiographic CTA: Initial results. Neuroradiology 2013; 55: 813–818. [DOI] [PubMed] [Google Scholar]
- 29.Van der Bom IM, Hou SY, Puri AS, et al. Reduction of coil mass artifacts in high-resolution flat detector conebeam CT of cerebral stent-assisted coiling. AJNR Am J Neuroradiol 2013; 34: 2163–2170. [DOI] [PMC free article] [PubMed] [Google Scholar]