Abstract
We report a patient with a petrosal arterio-venous dural fistula draining into the ponto-mesencephalic and medullary venous systems presenting with edema of the brain stem and complete reversal of magnetic resonance imaging (MRI) abnormalities after combined endovascular and surgical treatments. The venous anatomy of the posterior fossa and the significance of the venous involvement as the cause of clinical symptoms and imaging abnormalities in cerebro-medullary vascular lesions are discussed.
Keywords: Dural arteriovenous fistula, posterior fossa bridging veins, superior petrosal vein, cerebral venous infarction
Introduction
Neurological symptoms from cerebral or medullary arterio-venous shunts are commonly induced by changes in compliance of the venous drainage of these lesions and by their interference with the surrounding cerebral venous system. Only on rare occasions, are arterial steal and/or arterial ischemia responsible for neurological manifestations of cerebral or medullary arterio-venous shunts. Carotid-cavernous fistulas illustrate this point as they can result in a complete hemispheric arterial steal with clinical symptoms generally related to the venous drainage pattern of the fistula and more precisely to the presence or absence of intra-cerebral venous reflux into the middle cerebral or posterior fossa venous systems. Clinical and imaging recovery from venous ischemia has been demonstrated in treated spinal cord dural malformations and less commonly in cerebral dural malformations.1,2 Unlike arterial ischemia, venous ischemia requires chronicity before attaining irreversible neuronal damage.3 Misdiagnosis or delayed treatment may lead to poor neurologic outcome. We report a patient with a tentorial dural vascular malformation who presented with brainstem edema due to presumed venous congestion with complete resolution of clinical symptoms and imaging abnormalities after combined endovascular and surgical treatments.
Case report
Clinical presentation
A 69-year-old man with a negative past medical history for trauma or previous inflammatory or infectious diseases presented with intractable nausea and vomiting and progressive lower extremity weakness. Physical examination showed diminished strength in both lower extremities with hyperreflexia and positive Babinski sign suggestive of underlying myelopathy. His initial MRI study (Figure 1) demonstrated high T2 and fluid attenuated inversion recovery (FLAIR) signals in the brainstem with no restriction of diffusion on the apparent diffusion coefficient (ADC) map which was felt to be secondary to inflammation or infection. The patient was diagnosed with probable rhomboencephalitis and started on broad spectrum antibiotics. Cerebrospinal fluid (CSF) analysis failed to demonstrate an underlying etiology and, upon further review of the imaging studies, a vascular lesion was suspected as the primary cause for the brainstem abnormalities. Diagnostic cerebral and spinal arteriograms were obtained and demonstrated an arterio-venous shunt at the ridge of the right petrous bone supplied by petrous branches from the C4 segment of the internal carotid artery and from the petrous branch of the right middle meningeal artery (Figure 2). The shunt drained into a petrous or bridging vein and subsequently into a vein of the cerebello-pontine angle connecting to a lateral medullary vein and then finally to the anterior medullary vein. There were also connections with the anterior ponto-mesencephalic vein, peduncular vein and basal vein of Rosenthal (Figure 2). The venous phase of the vertebral artery injection showed a pseudo phlebitic pattern of the cerebellar hemispheres and the brainstem.
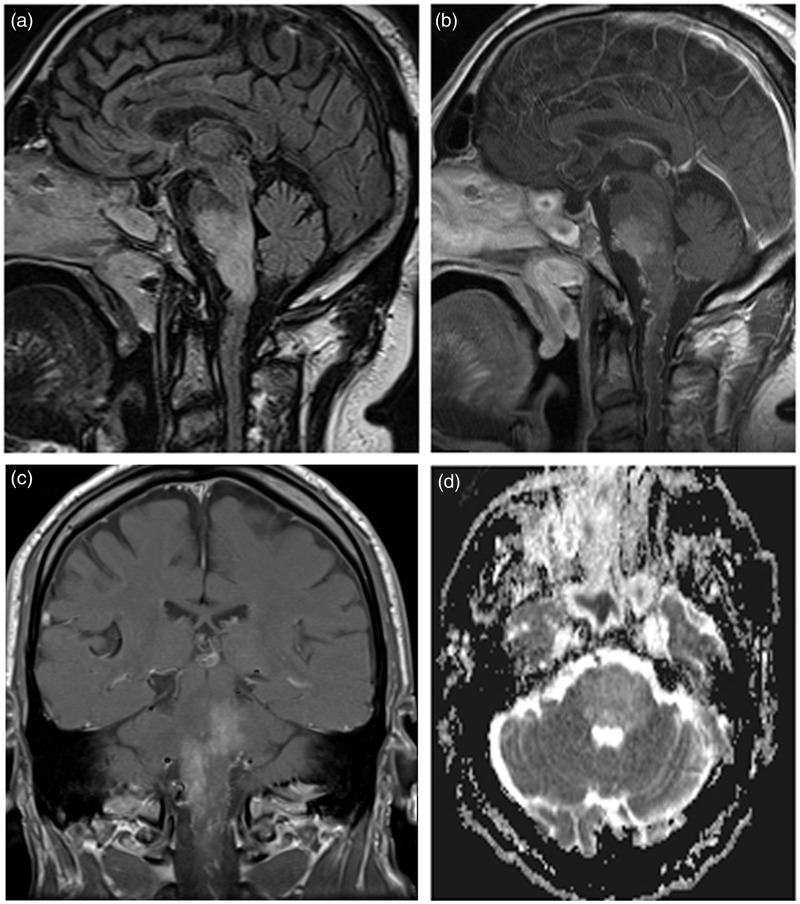
Figure 1.
Sagittal FLAIR image (a) shows high signal extending from the ponto-mesencephalic region to the ponto-medullary junction. Post-contrast T1 weighted images ((b) sagittal, (c) coronal) show enhancement of the pons and presence of prominent blood vessels on the surface of the medulla and upper cervical spinal cord. Axial apparent diffusion coefficient (ADC) map (d) shows high pontine signal and no restriction of diffusion.
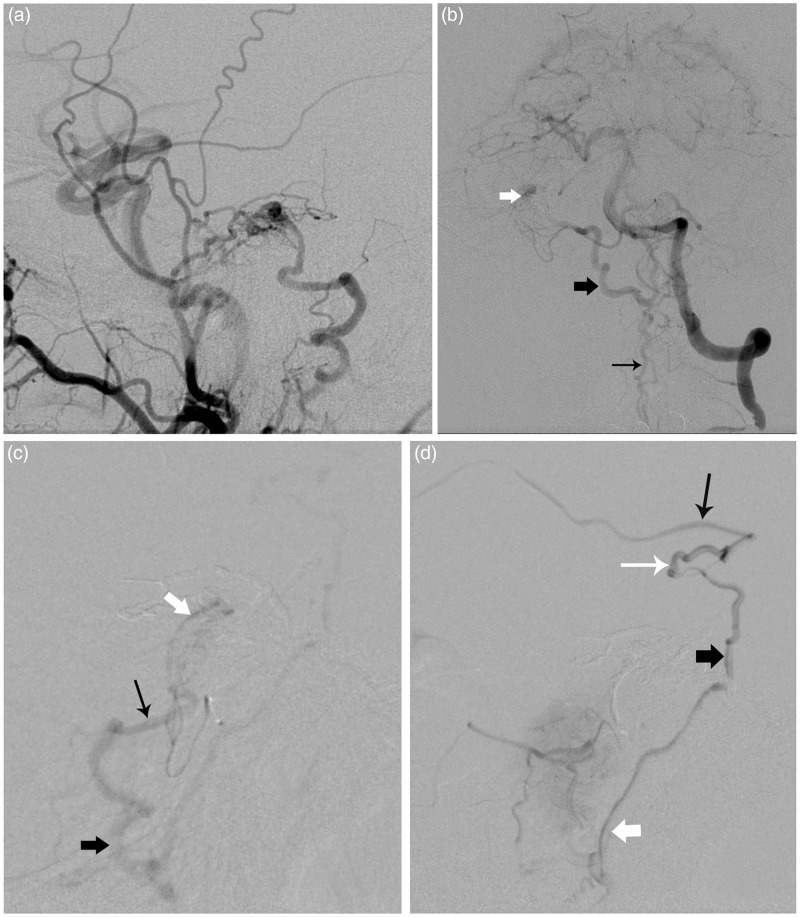
Figure 2.
Right carotid external injection in lateral view; (a) demonstrating a dural shunt draining into the petrosal vein with reflux into the anterior medullary system. Left vertebral artery in frontal view; (b) obtained after embolization of the petrosal branch of the middle meningeal artery showing persistence of the dural shunt vascularized by a dural branch (subarcuate artery) of the anterior inferior cerebellar artery (AICA) and draining in the same venous system (thick white arrow = petrosal vein, thick black arrow = lateral medullary vein, thin black arrow: anterior medullary vein). (c) Selective injection of the distal right AICA in lateral view showing the residual shunt and venous reflux into the petrosal vein (thick white arrow), vein of the cerebellopontine angle (thin black arrow) and lateral medullary vein (thick black arrow). (d) Additional venous reflux is seen into the basal vein (thin black arrow), peduncular vein (thin white arrow), ponto-mesencephalic vein (thick black arrow), and the anterior medullary vein (thick white arrow).
Endovascular intervention
A first trans-arterial approach was made through the petrosal branch of the middle meningeal artery where 1.2 ml of Onyx was injected allowing disconnection from the arterial feeder without reaching the venous drainage. Residual supply to the fistula was identified stemming from the right anterior inferior cerebellar artery, likely from its arcuate dural branch with persistent drainage into the ponto-medullary and peri-medullary venous systems (Figure 2(b), (c) and (d)). The morbidity of embolization of the cerebellolabrynthine trunk was thought to be excessive and no further arterial embolization was performed. The patient was brought back for another attempt at transvenous embolization but the fistula could not be reached and no embolization was performed.
Surgical intervention
The patient’s symptoms persisted despite significant post-embolization reduction of the arterial supply to the fistula and thus an open surgical approach was performed. He was taken to the operating room for a retrosigmoid craniotomy. The tentorium was identified and working along the superior aspect of the opening along the petrous ridge several Onyx-treated vessels were appreciated coursing in the dura. Several arterialized veins in the subarachnoid space likely corresponding to bridging veins near the 7th and 8th cranial nerve complex were identified. Aneurysm clips were placed across these veins, which were then coagulated and sharply cut. When no further abnormal veins were noted in the subarachnoid space, the wound was closed. Post-operative arteriogram demonstrated no residual shunting. The patient was discharged after a course of inpatient rehabilitation. During his most recent follow-up, he was living at home independently and able to ambulate with a cane. His nausea and vomiting resolved completely. MRI, three-months post operatively demonstrated complete resolution of the signal abnormality in the brainstem (Figure 3)
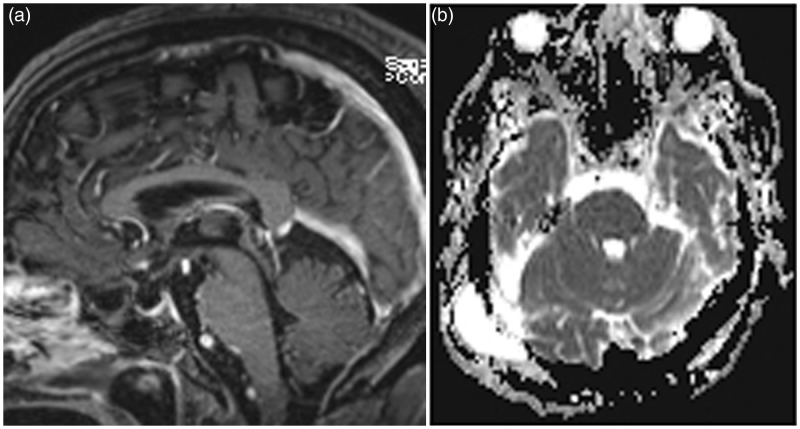
Figure 3.
Sagittal post contrast T1 reconstructed image; (a) shows resolution of the contrast enhancement and disappearance of the prominent surface blood vessels. Axial apparent diffusion coefficient (ADC) map; (b) shows normalization of the previous abnormal signal intensity. Post-operative changes are seen in the right temporo-occipital superficial soft tissues.
Discussion
Current classifications of dural arteriovenous malformation (DAVM), specifically the Borden et al.4 and Cognard et al.5 classifications, are based on the pattern of venous drainage and its relationship with the surrounding cerebral venous drainage. In either classification, the lack of cortical or sinus reflux characterize the benign nature of the shunt and such patients are likely to have uneventful clinical courses. Conversely, cortical or sinus reflux reflects an aggressive nature and anticipates a symptomatic clinical course. Therefore, neurological symptoms (from either hemorrhagic or non-hemorrhagic origins) correlate with changes in the local venous drainage and their interference with focal and/or remote cerebral venous drainage and ultimately with a lack of balance between intracellular, extracellular, and intravascular spaces leading to cerebral edema and even hemorrhage. Focal venous edema leading to brain injury results in neurological symptoms related to the affected region. Chronic diffuse supratentorial venous reflux involving large areas can lead to severe cognitive dysfunction and dementia.6–8 Our patient presented with clinical signs of a progressive myelopathy and imaging evidence of ponto-medullary and brainstem venous ischemia (Figure 1). Imaging obtained three months after anatomical cure of the lesion resulted in resolution of the venous edema (Figure 3). Contrary to arterial ischemic lesions, cerebral or medullary venous ischemic changes can be reversible as more severe and prolonged venous dysfunction is needed for cells reach a point of permanent, irreversible damage. Pathophysiology of venous infarctions is unclear and whether cytotoxic, vasogenic, or both mechanisms are involved and in which order has not yet been determined.9 It is beyond the scope of this review to discuss in detail the mechanisms of venous infarctions. Briefly, cytotoxic edema involves movement of osmotically active particles from the extracellular to the intracellular space leading to cellular swelling and death as energy-dependent processes fail.10 Cytotoxic edema occurs early with arterial occlusion and is believed to be irreversible although cases of reversibility in animal models and in humans after thrombolysis have been described.2 Vasogenic edema refers to an endothelial dysfunction leading to leakage of plasma proteins into the extravascular space.10 Vasogenic edema is a potentially reversible process and has been considered as the primary mechanism involved in the genesis of venous ischemia.11 However, clinical and animal studies show that both mechanisms, cytotoxic and vasogenic, may occur at different periods in the evolution of infarctions,12–14 and that the type of edema closely correlates with the severity and duration of venous occlusion or reflux. The venous capacity for rapid collateral establishment can result in conditions that lead to recover of functional and not irreversibly damage cells.3,10 Thus, swollen cells may return to normal function once the venous drainage is re-established by collaterals and regional hemodynamics changes allow the return of a normal arterial flow.
Cytotoxic and vasogenic edema may be evaluated by MRI using diffusion weighted images (DWIs) and ADC maps.15 DWIs and ADC values measure water mobility in tissues. Cytotoxic edema is associated with increased intracellular fluid reflected as low ADC values while vasogenic edema is associated with an increase of extracellular fluid reflected by high ADC values.16 Ischemic arterial stroke as evaluated by ADC reveals restriction of water movement in the infarcted zone believed to represent irreversibly damaged cerebral tissues (core) due to cytotoxic edema. Data on venous ischemia DWIs are limited with most reports showing heterogeneous signal tissue changes over time. In our patient, the initial MRI study (Figure 1) showed high T2/FLAIR signal intensity throughout most of the brainstem with no evidence of restricted diffusion on ADC maps implying no cytotoxic edema. Contrast enhancement in similar regions was seen as well as dilated blood vessels on the surface of the brainstem and cervical cord. After embolization, all of these abnormalities resolved.
Cavernous sinus dural arterio-venous malformations with dominant or exclusive drainage through the superior petrosal sinuses and posterior fossa veins have been associated to brainstem congestion.17–21 Reports of tentorial, sigmoid and transverse sinus dural fistulas causing brainstem edema mimicking brainstem tumors or arterial infarctions have also been described.22–25 All of these lesions are vascularized by a network of dural arteries and become symptomatic when they interfere with the venous drainage of the ponto-mesencephalic and medullary regions.26 The petrous vein or superior petrous complex vein (SPCV) is a subarachnoid structure receiving blood from the brainstem and cerebellar hemispheres (through transverse pontine veins, ponto-trigeminal veins, veins of cerebellopontine fissure and middle cerebral peduncle veins) and joins the superior petrous sinus (SPS) along the petrous ridge. As described by Matsushima et al.27 in their anatomic study of posterior fossa veins, the tributaries of the SPCV enter the SPS as terminal segments of single veins (bridging veins) or by via a common stem formed by the union of several veins. The opening site into the SPS is located medial, lateral, or above the internal auditory meatus. Kiyosue et al.28 using MRI, identified and analyzed the normal anatomy of the bridging veins in the posterior fossa. Bridging veins connect the longitudinal ponto-mesencephalic and anterior medullary systems (which normally drain the brain stem and cerebellum) with the cavernous sinus, inferior petrosal sinus, suboccipital, cavernous sinus, marginal sinus and jugular bulb. Dural arterio-venous shunts that develop at the site where the bridging veins penetrate the dura and then reflux into the ponto-mesencephalic and anterior medullary systems can potentially result in brainstem or cerebellar venous ischemia. Additionally, the rupture of one posterior fossa subarachnoid bridging vein may result in a localized subarachnoid hemorrhage. Kai et al.29 reported four patients with DAVM and two with pial arterio-venous fistulas at the cervico-medullary junction draining into the superior, inferior or cavernous sinuses who presented with subarachnoid hemorrhages; most patients reported presented with venous congestion as was seen in our patient.
Treatment of a posterior fossa DAVM draining in the superior petrous vein or other bridging veins is challenging. In most patients these malformations consist of a dense network of dural vessels supplied by branches of arteries also supplying cranial nerves (the ascending pharyngeal, middle meningeal, occipital arteries), arising from cerebellar arteries (dural branches of the superior cerebellar artery, antero-inferior artery or postero-inferior cerebellar arteries), or from the internal carotid artery (tentorial branches of the internal carotid artery). In our patient the fistula was vascularized by the petrous branch of the middle meningeal artery which along with the slylomastoid artery are the main source of blood supply to the facial nerve. Although the distal catheterization of the petrous branch likely minimized the risk of facial nerve injury, attention should paid to avoid significant reflux or to open collateral anastomotic vessels when using Onyx. Thus, trans-arterial embolization is not always feasible although it should always be considered as the first treatment option. Trans-venous approaches often require catheterization of a thrombosed or partially thrombosed sinus or catheterization of pial veins. Selective disconnection of the cortical venous reflux has also been described as a therapeutic option in patients with fistulas draining directly into cortical veins or into dural sinus combined with cortical reflux.30 Subtemporal trans-tentorial or retro-sigmoidal suprameatal approaches with direct clipping of the bridging vein is considered the best option when the architecture of the dural arteriovenous malformation prevents arterial or transvenous embolization.31 As shown in our patient, both treatments result in a cure of the lesion.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflicts of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Dabus G, Bernstein RA, Hurley MC, et al. Reversal of diffusion restriction after embolization of dural arteriovenous fistula: Case report. Neurosurgery 2010; 67: E1147–E1151. discussion E1151. [DOI] [PubMed] [Google Scholar]
- 2.Sarma D, Farb RI, Mikulis DJ, et al. Reversal of restricted diffusion in cerebral venous thrombosis: Case report. Neuroradiology 2004; 46: 118–121. [DOI] [PubMed] [Google Scholar]
- 3.Ducreux D, Oppenheim C, Vandamme X, et al. Diffusion-weighted imaging patterns of brain damage associated with cerebral venous thrombosis. AJNR Am J Neuroradiol 2001; 22: 261–268. [PMC free article] [PubMed] [Google Scholar]
- 4.Borden JA, Wu JK, Shucart WA. A proposed classification for spinal and cranial dural arteriovenous fistulous malformations and implications for treatment. J Neurosurg 1995; 82: 166–179. [DOI] [PubMed] [Google Scholar]
- 5.Cognard C, Gobin YP, Pierot L, et al. Cerebral dural arteriovenous fistulas: Clinical and angiographic correlation with a revised classification of venous drainage. Radiology 1995; 194: 671–680. [DOI] [PubMed] [Google Scholar]
- 6.Fujii H, Nagano Y, Hosomi N, et al. Dural arteriovenous fistula presenting with progressive dementia and parkinsonism. Br Med J Case Rep. Epub ahead of print 2 June 2014. DOI: 10.1136/bcr-2014-203921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hurst RW, Bagley LJ, Galetta S, et al. Dementia resulting from dural arteriovenous fistulas: he pathologic findings of venous hypertensive encephalopathy. AJNR Am J Neuroradiol 1998; 19: 1267–1273. [PMC free article] [PubMed] [Google Scholar]
- 8.Kai Y, Ito K, Kinjo T, et al. Reversibility of cognitive disorder after treatment of dural arteriovenous fistulae. Neuroradiology 2009; 51: 731–739. [DOI] [PubMed] [Google Scholar]
- 9.Filippidis A, Kapsalaki E, Patramani G, et al. Cerebral venous sinus thrombosis: Review of the demographics, pathophysiology, current diagnosis, and treatment. Neurosurg Focus 2009; 27: E3. [DOI] [PubMed] [Google Scholar]
- 10.Simard JM, Kent TA, Chen M, et al. Brain oedema in focal ischaemia: Molecular pathophysiology and theoretical implications. Lancet Neurol 2007; 6: 258–268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Makkat S, Stadnik T, Peeters E, et al. Pathogenesis of venous stroke: Evaluation with diffusion- and perfusion-weighted MRI. J Stroke Cerebrovasc Dis 2003; 12: 132–136. [DOI] [PubMed] [Google Scholar]
- 12.Forbes KP, Pipe JG, Heiserman JE. Evidence for cytotoxic edema in the pathogenesis of cerebral venous infarction. AJNR Am J Neuroradiol 2001; 22: 450–455. [PMC free article] [PubMed] [Google Scholar]
- 13.Frerichs KU, Deckert M, Kempski O, et al. Cerebral sinus and venous thrombosis in rats induces long-term deficits in brain function and morphology: Evidence for a cytotoxic genesis. J Cereb Blood Flow Metab 1994; 14: 289–300. [DOI] [PubMed] [Google Scholar]
- 14.Sakamoto S, Ohba S, Shibukawa M, et al. Course of apparent diffusion coefficient values in cerebral edema of dural arteriovenous fistula before and after treatment. Clin Neurol Neurosurg 2008; 110: 400–403. [DOI] [PubMed] [Google Scholar]
- 15.Sato K, Shimizu H, Fujimura M, et al. Compromise of brain tissue caused by cortical venous reflux of intracranial dural arteriovenous fistulas: Assessment with diffusion-weighted magnetic resonance imaging. Stroke 2011; 42: 998–1003. [DOI] [PubMed] [Google Scholar]
- 16.Hudak AM, Peng L, Marquez de la Plata C, et al. Cytotoxic and vasogenic cerebral oedema in traumatic brain injury: Assessment with FLAIR and DWI imaging. Brain Inj 2014; 28: 1602–1609. [DOI] [PubMed] [Google Scholar]
- 17.Kai Y, Hamada JI, Morioka M, et al. Brain stem venous congestion due to dural arteriovenous fistulas of the cavernous sinus. Acta Neurochir (Wien) 2004; 146: 1107–1111. discussion 1111–1112. [DOI] [PubMed] [Google Scholar]
- 18.Miyagishima T, Hara T, Inoue M, et al. Pontine venous congestion due to dural arteriovenous fistula of the cavernous sinus: Case report and review of the literature. Surg Neurol Int 2012; 3: 53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Stiebel-Kalish H, Setton A, Nimii Y, et al. Cavernous sinus dural arteriovenous malformations: Patterns of venous drainage are related to clinical signs and symptoms. Ophthalmology 2002; 109: 1685–1691. [DOI] [PubMed] [Google Scholar]
- 20.Takahashi S, Tomura N, Watarai J, et al. Dural arteriovenous fistula of the cavernous sinus with venous congestion of the brain stem: Report of two cases. AJNR Am J Neuroradiol 1999; 20: 886–888. [PMC free article] [PubMed] [Google Scholar]
- 21.Uchino A, Kato A, Kuroda Y, et al. Pontine venous congestion caused by dural carotid-cavernous fistula: Report of two cases. Eur Radiol 1997; 7: 405–408. [DOI] [PubMed] [Google Scholar]
- 22.Ishihara H, Ishihara S, Okawara M, et al. Two cases of a dural arteriovenous fistula mimicking a brain tumor. Interv Neuroradiol 2009; 15: 77–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Li J, Ezura M, Takahashi A, et al. Intracranial dural arteriovenous fistula with venous reflux to the brainstem and spinal cord mimicking brainstem infarction–case report. Neurol Med Chir (Tokyo) 2004; 44: 24–28. [DOI] [PubMed] [Google Scholar]
- 24.Mathon B, Gallas S, Tuillier T, et al. Intracranial dural arteriovenous fistula with perimedullary venous drainage: Anatomical, clinical and therapeutic considerations about one case, and review of the literature. Neurochirurgie 2013; 59: 133–137. [DOI] [PubMed] [Google Scholar]
- 25.Ricolfi F, Manelfe C, Meder JF, et al. Intracranial dural arteriovenous fistulae with perimedullary venous drainage. Anatomical, clinical and therapeutic considerations. Neuroradiology 1999; 41: 803–812. [DOI] [PubMed] [Google Scholar]
- 26.Baltsavias G, Parthasarathi V, Aydin E, et al. Cranial dural arteriovenous shunts. Part 1. Anatomy and embryology of the bridging and emissary veins. Neurosurg Rev 2015; 38: 253–264. [DOI] [PubMed] [Google Scholar]
- 27.Matsushima T, Rhoton AL, Jr, de Oliveira E, et al. Microsurgical anatomy of the veins of the posterior fossa. J Neurosurg 1983; 59: 63–105. [DOI] [PubMed] [Google Scholar]
- 28.Kiyosue H, Tanoue S, Sagara Y, et al. The anterior medullary-anterior pontomesencephalic venous system and its bridging veins communicating to the dural sinuses: Normal anatomy. Neuroradiology 2008; 50: 1013–1023. [DOI] [PubMed] [Google Scholar]
- 29.Kai Y, Hamada J, Morioka M, et al. Arteriovenous fistulas at the cervicomedullary junction presenting with subarachnoid hemorrhage: Six case reports with special reference to the angiographic pattern of venous drainage. AJNR Am J Neuroradiol 2005; 26: 1949–1954. [PMC free article] [PubMed] [Google Scholar]
- 30.Van Dijk JM, TerBrugge KG, Willinsky RA, et al. Selective disconnection of cortical venous reflux as treatment for cranial dural arteriovenous fistulas. J Neurosurg 2004; 101: 31–35. [DOI] [PubMed] [Google Scholar]
- 31.Mitsuhashi Y, Aurboonyawat T, Pereira VM, et al. Dural arteriovenous fistulas draining into the petrosal vein or bridging vein of the medulla: possible homologs of spinal dural arteriovenous fistulas. Clinical article. J Neurosurg 2009; 111: 889–899. [DOI] [PubMed] [Google Scholar]