Abstract
Objective
Preoperative embolization of meningioma is commonly performed; however, there is no consensus on the best embolic material to reduce intraoperative blood loss and surgery time.
Method
We retrospectively assessed the safety and efficacy of 56 cases of preoperative embolization of the middle meningeal artery with N-butyl cyanoacrylate (NBCA) in 105 cases of surgery for meningioma. We also defined a blood loss to tumor volume ratio to compensate for bias caused by tumor volume, and analyzed limited cases (the embolized group n = 52, the non-embolized group n = 21) of the convexity, the parasagittal region, the falx, and the sphenoidal ridge.
Result
The blood loss to tumor volume ratio was significantly less in the embolized group (p < 0.007). Preoperative embolization could be useful for cases with the external carotid artery as the dominant feeder vessel (p < 0.02); however, the efficacy decreased for cases with an internal carotid artery feeder. Transient complications occurred in four cases (hemiparesis secondary to edema: two cases; intratumoral bleeding: one case; trigeminal nerve disorder: one case). The cases that showed a postoperative increase in edema or intratumoral bleeding were large tumors with the early filling of veins. For such cases, surgeons should pay close attention to slow injection speed and higher NBCA viscosity, not to cause the occlusion of draining vessels.
Conclusion
Tumor embolization with NBCA can be safely performed, and the procedure significantly reduces intraoperative blood loss.
Keywords: Meningioma embolization, N-butyl cyanoacrylate (NBCA), Endovascular treatment
Introduction
Preoperative embolization of meningioma is commonly performed to facilitate surgical removal of the tumor and to reduce intraoperative blood loss and operative times. However, complications following this adjuvant preoperative procedure are not few.1–3 Despite some studies on solid embolic materials, such as polyvinyl alcohol (PVA) and microspheres, there is minimal information available on the efficacy of different solid and liquid embolic materials, the ideal interval between embolization and surgery, and surgical outcomes.4,5 As the procedure tends be performed for highly vascular tumors in particular, there is still controversy regarding patient selection and overall indication for preoperative embolization.3 We usually use N-butyl cyanoacrylate (NBCA), and have assessed the safety and efficacy of preoperative embolization with NBCA.
Materials and methods
Patients
Between April 2007 and December 2013, 114 surgeries were performed in 113 patients (40 males and 73 females; mean age, 58 years) to remove meningiomas. Indications for meningioma embolization are marked tumor staining on angiography and expectation that feeders from the external carotid artery (ECA) can be safely embolized, which are evaluated by two or more endovascular experts. One patient who underwent embolization with NBCA without surgery along with patients embolized with particles and coils only were excluded from the analysis. In total, 56 patients who underwent preoperative embolization with NBCA were assigned to the embolization group and were compared with the non-embolized group (n = 49).
The ethics committee of our hospital approved this retrospective study (No. 14–010).
Surgical procedure
A 5 F guiding catheter is inserted into the ECA at the level of the cranial base and a flow-guide microcatheter (Marathon; ev3 Neurovascular, Irvine, CA, USA, or Baltacci; Balt, Montmorency, France) is advanced into the feeder vessel as close to the dural attachment of the tumor as possible. In most cases, the meningioma is embolized with approximately 20–33% NBCA (Figure 1). We prefer this concentration because the microcatheter hardly sticks to vessels but infiltrates into the depth of the tumor. Where necessary, coils were used for feeder vessel occlusion to prevent migration of NBCA into dangerous anastomoses.
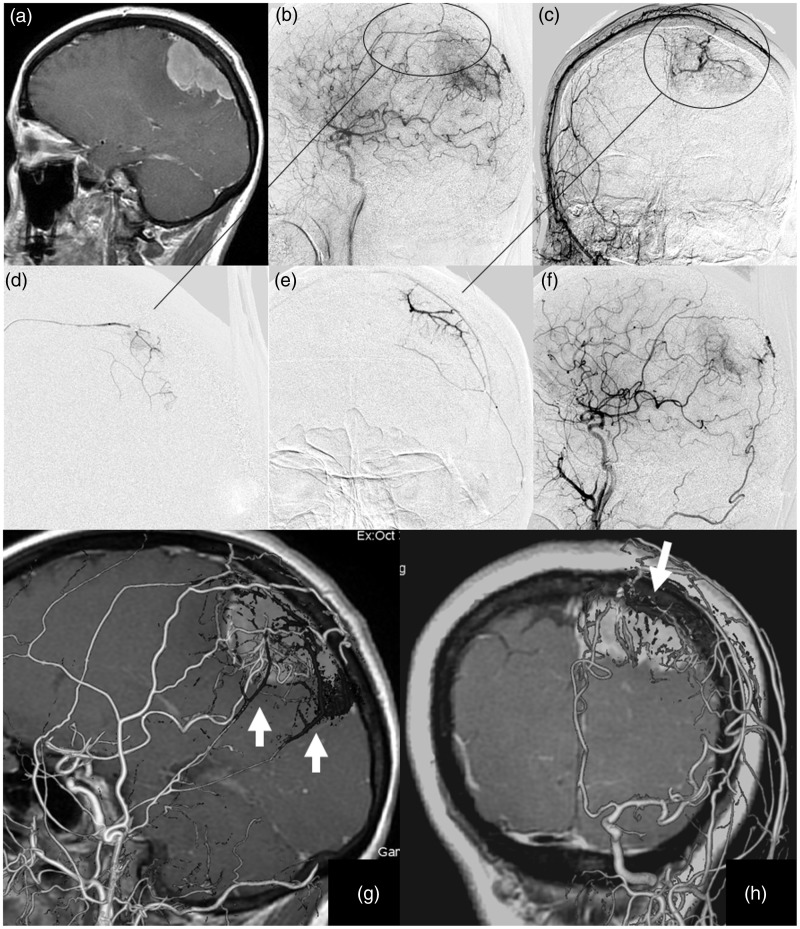
Figure 1.
(a) Gd-enhanced T1-weighted MRI shows a left parietal parasagittal meningioma. (b, c) The tumor is fed by the bilateral MMA, the left superficial temporal artery, the occipital artery, and a parietal branch from the ICA. (d, e)The anterior and posterior branches of the MMA were embolized with 33% NBCA. (f) Tumor staining from the ECA almost disappeared. (g, h) Preoperative 3D-DSA images of the ICA and ECA fused with the NBCA cast abstracted from pre- and post-Innova CT (Innova IGS630, AW 4.6, GE Healthcare, BUC, France) clarifies the distribution of NBCA within the tumor staining. Cone Beam CT was used with detector flat panel pixels of 1536 × 1536, detector size of 20 × 20 cm, voxel size of 160 micrometers, spin degree of 200, a maximum scan time of 12.5 s. MRI: magnetic resonance imaging; MMA: middle meningeal artery; ICA: internal carotid artery; NBCA: N-butyl cyanoacrylate; ECA: external carotid artery; 3D-DSA: 3D-digital subtraction angiography.
Outcome measures
We assessed the efficacy of preoperative embolization and complications following the procedure, and the relationship to associated factors: age, sex, past history, the location of the tumor, the tumor size (volume), feeder vessels, the interval between embolization and surgery, the surgery time, hardness of the tumor, blood loss, transfused blood volume, surgical result (Simpson grade), histology, regrowth rate, and prognosis. Cases were divided into four groups based on the dominant feeder vessel (internal carotid artery (ICA) or ECA) and staining (0: no staining, 1: ECA dominant, 2: ECA = ICA, 3: ICA dominant). Cases were also divided into four groups based on the result of embolization according to residual tumor staining on postembolization angiography; 0: no change 1: partial (≤50%), 2: subtotal (50–90%), 3: total (≥90%), as evaluated by two or more endovascular experts. The tumor consistency was evaluated from surgical records and divided into soft (allowing removal of >50% of the tumor volume with suction or low-power (10–30%) aspiration), moderately hard (allowing removal of >50% of the tumor volume with suction or high-power (40–100%) aspiration), or hard (requiring the use of scissors to resect >50% of the tumor volume). We performed computed tomography (CT) or magnetic resonance imaging (MRI) a few days after the embolization and surgery, and assessed the postoperative change of tumor and cerebral infarction, etc. The final outcome was assessed during a visit to the clinic after surgery (9 months–7 years).The modified Rankin Score (mRS) and the Karnofsky Performance Scale were used to evaluate the condition of the patients at follow-up.
Statistical analysis
The efficacy of preoperative embolization and the relationship with associated factors were evaluated by calculating 95% confidence intervals. Multiple regression analysis was performed with R version 3.1, using a stepwise procedure. We defined a blood loss to tumor volume ratio to compensate for bias caused by tumor volume. Regarding the blood loss to tumor volume ratio, we furthermore limited the analysis to meningiomas of the convexity, the parasagittal region, the falx, and the sphenoidal ridge to eliminate bias caused by the location of the tumor and the feeder vessels. Because the middle meningeal artery (MMA) was the predominant feeder of meningiomas in these regions, it was possible to accurately evaluate the efficacy of embolization.
Results
Of the 56 cases in the embolized group, most meningiomas were located in the convexity (35.7%), the parasagittal region (21.4%), or the falx (19.6%). Meningiomas of the tuberculum sella (16.3%), the cerebellum (12.2%), or the tentorium (12.2%) accounted for about half of the 49 cases in the non-embolized group (Table 1). In 54 of the 56 embolized cases, the embolization involved the MMA, and NBCA alone was used as the embolic material. In two cases there was another feeder, the ophthalmic artery in one case and the occipital artery in the other. In these two cases involving arteries with dangerous anastomoses, which have a possible risk of complications including skin ulceration, cerebral infarction, and neuropathy following the injection of liquid embolic material,6 the feeder arteries were initially embolized with gelfoam or a coil. NBCA was then injected into the MMA.
Table 1.
Characteristics and differences between the embolized group (n = 56) and non-embolized group (n = 49) (χ2 test, Student t-test, Mann–Whitney U test; p < 0.05).
| Embolization | P | Embolization | p | ||||
|---|---|---|---|---|---|---|---|
| Variables | + (n = 56) | − (n = 49) | Location | + (n = 56) | − (n = 49) | 0.01 | |
| Age (mean) | 60.6 | 54.9 | 0.04 | convexity | 20 | 12 | |
| Sex: male | 22 | 16 | 0.54 | parasagittal | 12 | 2 | |
| Surgery time (mean) (min) | 345 | 407 | 0.39 | falx | 11 | 1 | |
| Tumor volume (mean) (cm3) | 90.7 | 37.2 | 0.01 | sphenoidal ridge | 9 | 6 | |
| Blood loss (mean) (ml/cm3) | 8.8 | 31.6 | 0.01 | olfactory groove | 2 | 4 | |
| Feeder | 0.01 | cerebellum | 1 | 6 | |||
| ECA dominant | 27 | 12 | tentorial | 1 | 6 | ||
| ECA = ICA | 24 | 7 | tuberculum sella | 0 | 8 | ||
| ICA dominant | 5 | 13 | clival | 0 | 4 | ||
| Simpson grade | 0.15 | WHO grade | 0.53 | ||||
| 1 | 20 | 17 | 1 | 48 | 44 | ||
| 2 | 26 | 15 | 2 | 8 | 5 | ||
| 3 | 6 | 7 | Complication* | 4 | – | 0.12 | |
| 4 | 4 | 10 | Complication# | 5 | 3 | 0.53 | |
| Presurgery median mRS | 0 | 1 | 0.03 | Postsurgery mRS | 0 | 1 | 0.01 |
ECA: external carotid artery; ICA: internal carotid artery; Complication*:Complication following the embolization, Complication#: Complication following the surgery, mRS: modified Rankin scale
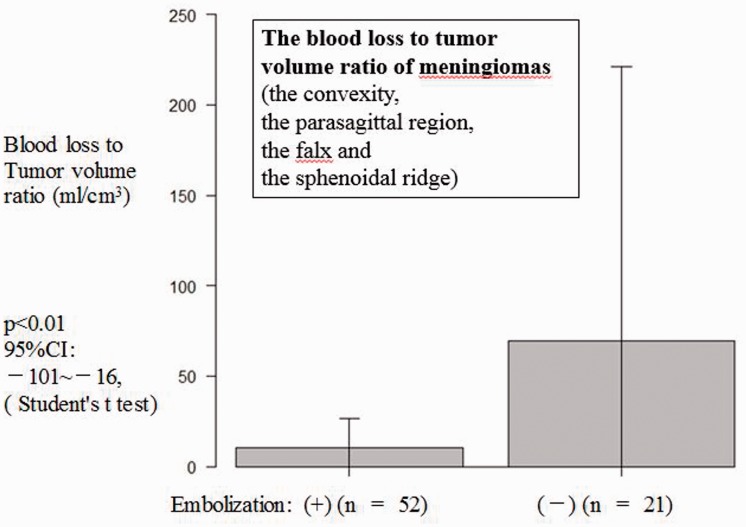
In the embolized group, mean surgery time was 345 min, mean blood loss was 516 ml, normalized blood loss 5.7 ml/cm3, and mean transfused blood volume was 439 ml. In the non-embolized group, mean surgery time was 407 min, mean blood loss was 574 ml, normalized blood loss 15.4 ml/cm3, and mean transfused blood volume was 409 ml. Tumor volume in the embolized group was significantly larger than that in the non-embolized group (90.7 vs. 37.2 cm3, p < 0.01). The ECA was significantly more dominant as the feeder in the embolized group compared with the non-embolized group (2.4 vs. 1.5, p < 0.01; Chi-squared test, Student’s t-test; Table 1). Age (60.6 years vs. 54.9 years, p < 0.04) and location (2.4 vs. 1.5, p < 0.01) were also significantly different between the embolized and non-embolized groups. The blood loss to tumor volume ratio was significantly less in the embolized group (n = 56) compared with the non-embolized group (n = 49) (Table 1, p < 0.01); it was also significantly less in the analysis of limited locations (10 ± 16 vs. 69 ± 152, p < 0.01; Figure 2).
Figure 2.
When analysis was limited to meningiomas of the convexity, the parasagittal region, the falx, and the sphenoidal ridge to eliminate bias caused by tumor location and feeder vessels, the blood loss to tumor volume ratio (ml/cm3) was significantly less in the embolized group compared with the non-embolized group (p < 0.007).
Spearman’s rank correlation analysis to determine factors associated with blood loss in the embolized group revealed a correlation with feeder vessels (p < 0.01), embolization result (p < 0.01), and Simpson grade (p < 0.01; Table 2). Simple regression analysis of associated factors for blood loss revealed a correlation with feeder (ECA dominant; p = 0.02, ICA dominant; p = 0.04), Simpson grade (p = 0.01), WHO grade (p = 0.05) and embolization result (total, p = 0.01) (Table 2). The ECA as the dominant feeder vessel (p < 0.02) was associated with significantly lower blood loss (stepwise regression analysis, Table 2).
Table 2.
Pearson’s correlation coefficient between blood loss and related variables (n = 56).
| Variables | r | p (<0.05) | Variables | r | p |
|---|---|---|---|---|---|
| Age | 0.06 | 0.66 | Feeder* | 0.36 | 0.01 |
| Histology grade | 0.26 | 0.05 | Hardness | 0.19 | 0.17 |
| Embolization result** | −0.34 | 0.01 | Simpson grade | 0.37 | 0.01 |
| *0: no staining, 1: ECA dominant, 2: ECA = 1CA, 3: ICA dominant | |||||
| **0: no change 1: partial, 2: subtotal, 3: total | |||||
| Simple regression analysis for blood loss (n = 56, p < 0.05) | |||||
| Variables | p | Location | Simpson grade | 0.01 | |
| Age | 0.66 | convexity | 0.39 | Embolization result | |
| Sex | 0.41 | parasagittal | 0.98 | subtotal | 0.62 |
| Hypertension | 0.23 | falx | 0.72 | total | 0.01 |
| Diabetes mellitus | 0.67 | sphenoidal ridge | 0.50 | Feeder | |
| Histology grade | 0.05 | tentorial | 0.62 | ECA dominant | 0.02 |
| Tumor volume (mean) (cm3) | 0.17 | Hardness | 0.17 | ICA dominant | 0.04 |
| Stepwise regression analysis for blood loss (n = 56, p < 0.05) | |||||
| Variables | Estimate | Std. error | t value | p | |
| (Intercept) | 276 | 126 | 2.19 | 0.03 | |
| Feeder ECA dominant | 444 | 184 | 2.42 | 0.02 | |
| ICA dominant | 627 | 313 | 2 | 0.051 | |
Complications following embolization occurred in four cases (hemiparesis secondary to edema (n = 2), intratumoral bleeding (n = 1), and trigeminal nerve disorder (n = 1)). However, all cases were managed by an intravenous drip infusion without the need for urgent surgery. The three cases that developed edema and intratumoral bleeding had shown prominent tumor staining with an early filling of veins in angiography. The mean interval between embolization and surgery was 3 days (range 0–11 days). Complications related to the surgery itself included hemiparesis (n = 2), meningitis (n = 2), intracerebral hemorrhage (n = 1), facial nerve palsy (n = 1), and hearing disturbance (n = 1) in the embolized group, and subcutaneous abscess (n = 1), and epidural hematoma (n = 1) in the non-embolized group. Two patients developed hemiparesis secondary to cerebral infarction caused by procedural vessel injury. Most symptoms resolved and the patients with hemiparesis recovered to a mRS of 1–3 (0: n = 2, 1: n = 6, 2: n = 2, 3: n = 1, 4: n = 0, 5: n = 0). Regrowth after surgery occurred in two cases in the embolized group and in five cases in the non-embolized group. There was no significant difference between the two groups for tumor hardness, surgical results, prognosis, regrowth rate, and other parameters (Table 1).
Discussion
The role of preoperative embolization in meningioma management remains controversial due to the lack of randomized controlled clinical trials which elucidate its potential benefit and risks.3,7 Studies of tumor embolization have indicated that infiltrating small particles (e.g. PVA: 45–150 µm) into the tumor bed is most effective for reducing blood loss, softening the tumor, and obtaining positive surgical outcomes.2,8 However, the use of small particles for embolization is associated with a risk of intratumoral bleeding secondary to capillary occlusion of the tumor bed, or cerebral infarction secondary to migration into dangerous anastomoses.7,8 To date, there have been few studies on the use of NBCA for preoperative embolization of meningioma. We frequently use this liquid material to achieve homogeneous embolization, which would reduce blood loss and surgery time compared with solid embolic materials.9
In the present study, our indications for meningioma embolization were marked tumor staining on angiography and expectation that feeders from the ECA could be safely embolized. Therefore, comparison between the embolized group and the non-embolized group was difficult because of significant differences in tumor volume, location, and feeder vessel. We defined a blood loss to tumor volume ratio to compensate for the bias caused by tumor volume, and limited analysis to meningiomas of the convexity, the parasagittal region, the falx, and the sphenoidal ridge to eliminate bias caused by location and feeder vessels. Following these efforts to reduce bias, blood loss was significantly less in the embolized group (p < 0.007). Embolization with NBCA was particularly useful for cases with the ECA as the dominant feeder vessel (p < 0.02), while the efficacy decreased for cases that involved the ICA as the dominant feeder vessel. This could be because the result of embolization of the MMA was not a significant factor to reduce blood loss. Meningiomas that were more completely removed (Simpson grade; p < 0.01) tended to show more blood loss.
Excessively forceful injection of NBCA to permeate into the tumor stain from an ICA feeder could be dangerous. Complications following meningioma embolization occur in 0–12.5% of cases1–8 with solid embolic materials. Intraoperative bleeding can result from occlusion of a drainer vessel, or intratumoral necrosis can occur and cause bleeding during the time between embolization and surgery.7,8 In the present study there was a complication rate of 7.1%, similar to the reports above. The cases of increased edema and intratumoral bleeding had an early filling of veins on the feeder, and the occlusion of the draining vessel caused by the forceful injection of NBCA was the likely cause of the complications, possibly influenced by increased necrosis or changes in the circulation in the tumor. One study reported that 41% (11 of 25 cases) of meningiomas have a large drainage vein on selective angiography, and this is related to the degree of peritumoral edema.10 However, we could not find any recent detailed studies about an early filling of veins in angiography of meningioma. In our cases, 5.8% (six of 113 cases) showed an early filling of veins on tumor staining.11 NBCA readily permeates into the tumor capillary bed and therefore, especially for tumors with an early filling of veins, surgeons should pay particular attention to the injection speed and NBCA viscosity. NBCA can be mixed with ethyl esters of iodized fatty acids from poppy seed oil (Lipiodol®; Terumo, Tokyo, Japan), providing good visibility and enabling embolization of multiple feeders. Because the present study has been completed, at this time we have only embolized the dural attachment, from which feeders radiate. We have not performed excessive embolization of veins, and have not experienced any complications. Preoperative 3D-digital subtraction angiography (DSA) images combined with NBCA casts were useful to clarify the distribution of NBCA within the area of tumor staining (Figure 1). The use of selective angiography through microcatheters, targeting other feeders, and different tumor staining methods could be useful to ensure safer NBCA injection.
With regards to the timing of surgery, meningiomas embolized with PVA are reported to soften in 7–9 days, and can be removed easily because of maximal necrosis.2,8 Tumors embolized with microspheres or cellulose porous beads are reported to soften continuously, even beyond 7 days.2,12 We could not find any reports on tumor softening following embolization with NBCA. However, a long-term effect can be expected, particularly for cases with little pial supply, because NBCA is often used for permanent embolization of vascular malformations. We generally perform surgery approximately 3 days after embolization because excessive necrosis of the tumor would make the border between normal brain and the tumor difficult to identify and increase edema. Meningiomas of 6–8 cm in size with associated necrosis or cysts tend to bleed after complete embolization.13 Large tumors with marked edema are associated with a risk of symptomatic deterioration following embolization as a result of increased edema or intratumoral bleeding. In such cases urgent surgery is indicated, and particular attention must be paid to drainer occlusion and the degree of embolization, as well as the timing of surgery.
Conclusions
Preoperative meningioma embolization with NBCA is relatively safe if physicians pay close attention to injection speed and NBCA viscosity because of the risk of drainage vein occlusion, and significantly reduces blood loss during surgery, particularly for tumors in which ECA is the dominant feeder vessel. NBCA readily permeates the tumor capillary bed, and therefore care should be taken to slow NBCA injection speed and increase viscosity of the NBCA solution more than usual, especially when treating tumors with an early filling of veins.
Author contribution
Hideaki Ishihara drafted the work and analyzed and interpreted the data. Shoichiro Ishihara revised it critically for important intellectual content. Jun Nimi accessed the data. Hiroaki Neki accessed the data. Yoshiaki Kakehi accessed the data. Nahoko Uemiya accessed the data. Fumitaka Yamane accessed the data. Shinya Kohyama proofread it critically. Hiroshi Kato accessed the data. Tomonari Suzuki accessed the data. Jun-ichi Adachi accessed the data. Kazuhiko Mishima accessed the data. Ryo Nishikawa proofread it critically.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Law-ye B, Clarençon F, Sourour NA, et al. Risks of presurgical embolization of feeding arteries in 137 intracranial meningeal tumors. Acta Neurochir 2013; 155: 707–714. [DOI] [PubMed] [Google Scholar]
- 2.Lazzaro MA, Badruddin A, Zaidat OO, et al. Endovascular embolization of head and neck tumors. Front Neurol 2011; 64: 1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Singla A, Deshaies EM, Melnyk V, et al. Controversies in the role of preoperative embolization in meningioma management. Neurosurg Focus 2013; 35: 1–6. [DOI] [PubMed] [Google Scholar]
- 4.Carli DF, Sluzewski M, Beute GN, et al. Complications of particle embolization of meningiomas: Frequency, risk factors, and outcome. Am J Neuroradiol 2010; 31: 152–154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Sluzewski M, van Rooij WJ, Lohle PN, et al. Embolization of meningiomas: Comparison of safety between calibrated microspheres and polyvinyl-alcohol particles as embolic agents. Am J Neuroradiol 2013; 34: 727–729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Borg A, Ekanayake J, Mair R, et al. Preoperative particle and glue embolization of meningiomas: Indications, results, and lessons learned from 117 consecutive patients. Neurosurgery 2013; 73: 244–251. [DOI] [PubMed] [Google Scholar]
- 7.Duffis EJ, Gandhi CD, Prestigiacomo CJ, et al. Head, neck, and brain tumor embolization guidelines. J Neurointerv Surg 2012; 4: 251–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shah AH, Patel N, Raper DM, et al. The role of preoperative embolization for intracranial meningiomas. J Neurosurg 2013; 119: 364–372. [DOI] [PubMed] [Google Scholar]
- 9.Kominami S, Watanabe A, Suzuki M, et al. Preoperative embolization of meningiomas with N-butyl cyanoacrylate. Interv Neuroradiol 2012; 18: 133–139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tanaka M, Imhof HG, Schucknecht B, et al. Correlation between the efferent venous drainage of the tumor and peritumoral edema in intracranial meningiomas: Superselective angiographic analysis of 25 cases. J Neurosurg 2006; 104: 382–388. [DOI] [PubMed] [Google Scholar]
- 11.Ishihara H, Ishihara S, Niimi J, et al. Three cases of meningioma complicated by increased edema or intratumoral bleeding following embolization with N-butyl cyanoacrylate. J Neuroendovasc Ther 2014; 8: 224–230. [Google Scholar]
- 12.Kai Y, Hamada JI, Morioka M, et al. Clinical evaluation of cellulose porous beads for the therapeutic embolization of meningiomas. Am J Neuroradiol 2006; 27: 1146–1150. [PMC free article] [PubMed] [Google Scholar]
- 13.Yu SC, Boet R, Wong GK, et al. Postembolization hemorrhage of a large and necrotic meningioma. Am J Neuroradiol 2004; 25: 506–508. [PMC free article] [PubMed] [Google Scholar]