Abstract
Purpose
To date only a few studies have compared the effectiveness and functional outcomes of stent retrievers versus intravenous thrombolysis in acute ischaemic stoke. Our aim was to identify and collate all the available data and to assess for statistical differences in patient outcomes between the two treatments.
Materials and methods
We performed a systematic review and meta-analysis of studies with a randomised controlled design which utilised stentrievers and intravenous thrombolysis in acute ischaemic stroke.
Results
Five randomised controlled studies published in 2015 were identified. Second-generation thrombectomy devices constituted at least 80% of thrombectomy devices in the included studies, namely MR CLEAN, ESCAPE, EXTEND-IA, SWIFT PRIME and REVASCAT. No significant heterogeneity was seen in the included studies and the five trials were therefore included in the meta-analysis.
A total of 46.10% of patients treated with stentrievers achieved an independent functional outcome (mRS < 2) at 90 days compared with 26.46% of those treated with intravenous thrombolysis with an odds ratio of 2.40 (p < 0.001). The weighted recanalisation mean in the thrombectomy arms was 76.02%.
A lower mortality rate was observed with stentrievers compared to intravenous thrombolysis (15.33% vs 18.74%; OR 0.81, p = 0.15). Stentrievers were also associated with a lower risk of symptomatic intracranial haemorrhage (7.86% vs 8.64%; OR 1.02, p = 0.93). The differences in the secondary/safety outcomes were not statistically significant.
Conclusion
Stentrievers can achieve a high rate of recanalisation and functional independence in acute ischaemic stroke and have a relatively good safety profile. Our meta-analysis demonstrates a clear benefit of an intra-arterial mechanical approach vs standard treatment.
Keywords: Functional outcomes, mechanical thrombectomy, stroke
Introduction
The second generation of mechanical thrombectomy devices assumed a retrievable stent design and achieved United States Food and Drug Administration (FDA) clearance in 2012 following the publication of the SOLITAIRE with the Intention for Thrombectomy (SWIFT)1 and TREVO 22 trials. These studies showed improved recanalisation rates and functional outcomes of stent retrievers (stentrievers) compared to first-generation devices.
Intravenous recombinant tissue plasminogen activator (rt-PA) still remains the standard treatment in acute ischaemic stroke3,4 despite having stringent inclusion criteria and a relatively short time window.
To date only a few randomised controlled studies (RCTs) have compared the effectiveness and functional outcomes of stent retrievers versus intravenous thrombolysis in acute ischaemic stoke.
We systematically searched for RCTs comparing the use of stentrievers vs standard treatment in acute ischaemic stoke to compare the performance of the two treatments.
The importance of this review lies in the generation of evidence regarding mechanical thrombectomy which may subsequently influence the management of acute ischaemic stroke.
Materials and methods
Design
We followed the guidelines developed by the Cochrane Database of Systematic Reviews5 in order to conduct a systematic review. The research question was developed according to the PICO framework.6 The ‘population’ consisted of acute ischaemic stroke patients treated either with mechanical thrombectomy with a stentriever device (‘intervention’) or standard treatment (‘comparison’). The primary ‘outcome’ was 90-day functional outcome whereas secondary outcomes included three-month mortality and symptomatic intracranial haemorrhage (sICH).
Data sources and study selection
We conducted electronic searches in Embase and OVID Medline databases by utilising free text and subject headings (Medical Subject Headings (MeSH) and EMTREE) without language restrictions from 2010 up to 2015 (Appendix 1). The search was validated by hand-searching The New England Journal of Medicine (NEJM) since it was the journal which published all of the included studies. We also sought unpublished studies and studies published in the grey literature.
Selection criteria
RCTs in any language with living human participants were sought for inclusion. All studies which could potentially be included in our meta-analysis were further assessed by a quality assessment tool, namely the RTI item bank.7 Inclusion and exclusion criteria were developed beforehand (Table 1) and used throughout the selection process in order to minimise bias.
Table 1.
Inclusion and exclusion criteria.
| Inclusion criteria |
| 1. Studies which include patients presenting with acute ischaemic stroke (AIS) symptoms |
| 2. Age of participants >18 years |
| 3. Number of participants included in the study (n) > 20 |
| 4. Patients who were treated with mechanical thrombectomy using a stentriever |
| 5. Studies which specify functional outcomes utilising the modified Rankin score at 90 days |
| 6. Study design: randomised controlled trials (RCTs) |
| 7. Dates: 2010 to date |
| Exclusion criteria |
| 1. Patients who presented with transient ischaemic attack (TIA), stroke-mimics, haemorrhagic stroke or other diagnosis apart from AIS |
| 2. Age of participants <18 years |
| 3. Fewer than 20 participants in the study |
| 4. Use of pooled data from other trials |
| 5. In vitro/animal studies |
| 6. Use of thrombolysis/thrombectomy in pregnancy |
| 7. Iatrogenic stroke (as a result of cardiac, carotid or other interventional procedures) |
| 8. Author replies/editorials/comments/guidelines/reviews/case reports |
| 9. Studies in cancer, patients with hyper-coagulable states and haemodialysis patients |
| 10. Treatment of stroke other than with methods specified under inclusion criteria |
Data extraction
A primary evaluation of the studies was performed which involved screening their titles and abstracts.
Full text articles of eligible studies were then obtained and assessed with the aid of a standardised assessment form to allow for a consistent, systematic approach.
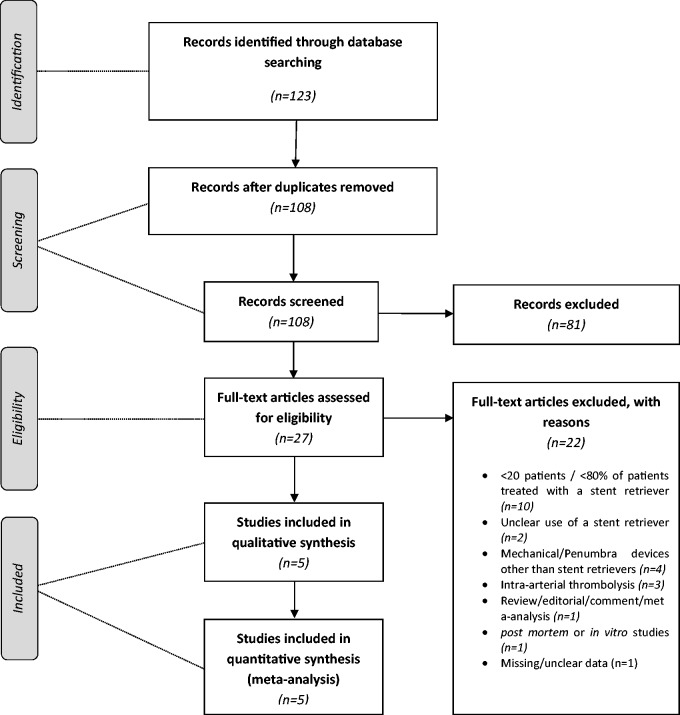
Throughout the evaluation process a Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flowchart (Figure 1) was completed including any inclusion and exclusion decisions. EndNote X7 was used to organise the included studies, and duplication bias was limited by careful review of the extracted data.
Figure 1.
Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) flow diagram.
Study and patient characteristics
The year and journal of publication, the study design and number of participants in the treatment arms of each study were recorded. The number of participating centres in multicentre trials was also extracted. Patient baseline characteristics including the mean age and range of participants, male-to-female ratio, stroke severity at presentation and time to treatment were recorded.
Treatment characteristics
We also extracted treatment-specific data such as the percentage and types of stentriever devices used and the percentage of patients who were eligible and received intravenous thrombolysis.
Outcomes
The primary outcome was functional outcome at 90 days using the modified Rankin scale score (mRS) where functional independence was defined as a score of ≤2. Secondary outcomes sought were rates of sICH and mortality at 90 days post-procedure. Thrombolysis in Cerebral Infarction (TICI) score of 2b–3 and Thrombolysis in Myocardial Infarction (TIMI) score of 2–3 defined successful recanalisation.
Statistical analysis
We assessed for publication bias by constructing a funnel plot and tested for inter-study heterogeneity with the likelihood ratio χ2 test and the I2 statistic for the primary outcome. Meta-analysis was then performed using the random effects model. The extracted data were also used to calculate weighted means of both primary and secondary outcomes, and statistical significance was assessed using the unpaired t-test.
Results
Characteristics of included studies
Five eligible studies which included a total of 1288 patients were identified. The studies were all published in NEJM in 2015 and are summarised in Table 2. All studies were prospective RCTs in which stentrievers (n = 634) (used as a sole treatment or as part of multimodal therapy) were compared against intravenous thrombolysis (n = 653). The mean number of patients included was 258 per study.
Table 2.
Study characteristics.
| Trial | Authors | Date | Journal | Study design | Stentriever | % Stentrievers | % i.v. RTPA | N | No of centres | Age (mean) | Sex (males) | Alternative Rx | Follow-up |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MR CLEAN | Berkhemer et al.8 | 2015 | NEJM | RCT | Multiple | 81.50% | 89.00% | 500 | 16 | 65 | 58.4% | Usual care | 90 days |
| ESCAPE | Goyal et al.9 | 2015 | NEJM | RCT | Multiple | 86.10% | 75.30% | 316 | 22 | 71 | 47.8% | Usual care | 90 days |
| EXTEND IA | Campbell et al.10 | 2015 | NEJM | RCT | Solitaire | 100.00% | 100.00% | 70 | 14 | 69 | 49.0% | i.v. alteplase | 90 days |
| SWIFT PRIME | Saver et al.11 | 2015 | NEJM | RCT | Solitaire | 100.00% | 100.00% | 196 | 39 | 66 | 51.0% | i.v. tPA | 90 days |
| REVASCAT | Jovin et al.12 | 2015 | NEJM | RCT | Solitaire | 100.00% | 100.00% | 206 | 4 | 66 | 53.0% | i.v. alteplase | 90 days |
NEJM: The New England Journal of Medicine; RCT: randomised controlled trial; RTPA: recombinant tissue plasminogen activator; i.v.: intravenous; tPA: tissue plasminogen activator.
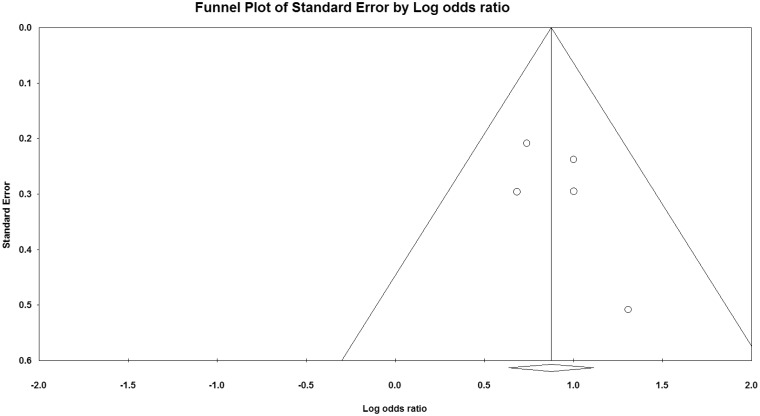
A funnel plot (Figure 2) did not show significant publication bias, and heterogeneity statistics failed to demonstrate significant heterogeneity between the included studies.
Figure 2.
Funnel plot.
The five studies all utilised the mRS to assess 90-day functional outcomes.
Participant and patient characteristics
The included studies were all multicentre participations with a mean number of 19 participating studies per trial. The mean age of participants was 67.4 years and 51.8% were males. The average stroke severity at presentation (National Institutes of Health Stroke Scale International (NIHSS)) was 16.8 and 16.4 in the mechanical thrombectomy and intravenous thrombolysis groups, respectively.
Procedural characteristics
The weighted mean symptom onset to intravenous therapy was 111.3 minutes in the mechanical thrombectomy group and 114.5 minutes within the standard treatment group. Stentrievers were utilised as part of a multimodal treatment approach whereby the majority of patients also received intravenous tissue plasminogen activator (tPA).
Three studies (EXTEND-IA10, SWIFT PRIME11 and REVASCAT12) utilised Solitaire (ev3/Covidien, Irvine, CA, USA) exclusively whilst MR CLEAN8 and ESCAPE9 used multiple stentriever devices within the mechanical thrombectomy arm.
Outcomes
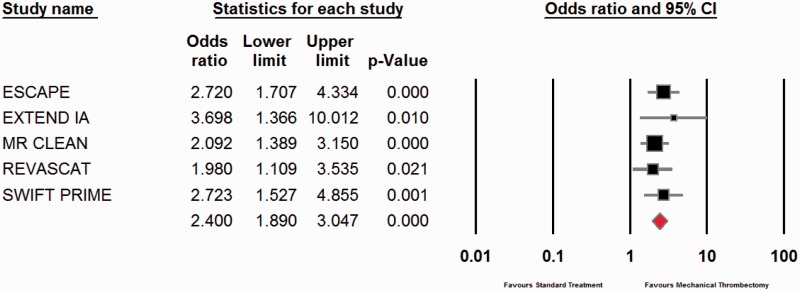
Functional independence at 90 days (defined as mRS ≤ 2) was achieved in 46.10% and 26.46% in the mechanical thrombectomy and intravenous thrombolysis groups, respectively. The odds ratio (OR) in favour of mechanical thrombectomy was 2.40 with a p value of <0.001 (Figure 3).
Figure 3.
Meta-analysis of functional outcomes.
CI: confidence interval.
No statistical difference was demonstrated between secondary outcomes namely mortality at 90 days (p = 0.15) and rates of sICH (p = 0.93).
The mortality rate within the mechanical thrombectomy arm was 15.33% compared to 18.74% within the intravenous thrombolysis group (OR 0.81, p = 0.15). A total of 7.86% and 8.64% of patients suffered from sICH within the thombectomy and thrombolysis groups, respectively (OR 1.02, p = 0.93).
Primary and secondary outcomes are summarised in Table 3.
Table 3.
Summary of patient baseline characteristics, treatments and outcomes.
| Mechanical thrombectomy |
Standard treatment |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Trial | N | NIHSS (mean) | Time window to i.v. Rx | Recanalisation | sICH | mRS = 2 | Mortality | N | NIHSS (Mean) | Time window to i.v. Rx | Recanalisation | sICH | mRS = 2 | Mortality |
| MR CLEAN | 233 | 17 | 85 | 75.40% | 18.00% | 32.60% | 21.00% | 267 | 18 | 87 | 32.90% | 17.00% | 19.10% | 22.00% |
| ESCAPE | 165 | 16 | 110 | 72.40% | 3.60% | 53.00% | 10.40% | 150 | 17 | 125 | 70.50% | 2.70% | 29.30% | 19.00% |
| EXTEND IA | 35 | 17 | 127 | 94.00% | 0.00% | 71.00% | 9.00% | 35 | 13 | 145 | 43.00% | 6.00% | 40.00% | 20.00% |
| SWIFT PRIME | 98 | 17 | 117 | 88.00% | 0.00% | 60.20% | 9.20% | 98 | 17 | 110.5 | n/a | 3.00% | 35.50% | 12.40% |
| REVASCAT | 103 | 17 | 117.5 | 65.70% | 1.90% | 43.70% | 18.40% | 103 | 17 | 105 | n/a | 1.90% | 28.20% | 15.50% |
NIHSS: National Institutes of Health Stroke Scale International; sICH: symptomatic intracranial haemorrhage; mRS: modified Rankin scale score.
Successful recanalisation (defined as TICI score of 2b–3 or TIMI score of 2–3) was achieved in 76.02% of patients treated with stentrievers.
Comparison to previous RCTs
Three RCTs published in NEJM in 2013 (MR RESCUE13, SYNTHESIS Expansion14 and IMS III15) failed to prove the superiority of endovascular therapy vs systemic rt-PA in acute ischaemic stroke. Stentrievers were utilised in only 0.6%, 0.0% and 12.0% of the studies, respectively. The OR of 90-day functional outcomes was 0.98 and there was no statistically significant difference between the two treatments (p value = 0.86). No significant difference in mortality and rate of sICH was demonstrated in the three studies (p values = 0.817 and 0.624, respectively).
Discussion
MR RESCUE13, SYNTHESIS Expansion14 and IMS III15 trials published in 2013 failed to prove the superiority of mechanical thrombectomy vs standard care in acute ischaemic stroke. Though these studies may have had potential design flaws and utilised mainly first-generation thrombectomy devices, they resulted in clinical equipoise surrounding the use of intra-arterial thrombectomy.
To date only a few RCTs comparing functional outcomes of patients treated with second-generation thrombectomy devices (stentrievers) versus intravenous thrombolysis have been published.
The importance of this meta-analysis was to aggregate all data available regarding the use of stentrievers in order to achieve a higher statistical power and provide a high level of evidence regarding their use in acute ischaemic stroke.
Five studies were eligible following an extensive search of the literature. These all followed strict protocols which were set prior to enrolment. Patient baseline characteristics were very similar in the treatment and control arms of all included studies which limited bias. Specifically no significant difference in the time to administer intravenous thrombolysis from symptom onset was registered in the two arms. Three of the studies made exclusive use of Solitaire whereas in MR CLEAN8 and ESCAPE9 trials multiple second-generation devices were utilised. Thus the extracted data are applicable to stentrievers in general.
As opposed to the SYNTHESIS Expansion,14 the patients within the thrombectomy arms also received timely intravenous thrombolysis, which reflects the practice in most interventional centres and this was likely responsible for the improved outcomes.
The mean presentation NIHSS was 16.5 (suggesting an occlusion of a large intracranial vessel16), and the trials actively searched for a large vessel occlusion before a decision to proceed with intra-arterial thrombectomy was made. Again this contrasts with the studies published in 2013 in which large vessel occlusion was not necessarily confirmed.
The mean time from symptom onset to intravenous thrombolysis was just 112.9 minutes in the included patients. This was also likely responsible for the excellent outcomes achieved in the trials with almost half (46%) of the treated patients achieving functional independence at 90 days. The fact that the number needed to treat decreases the earlier stroke treatment is administered has long been proven.17 ‘Time is Brain’ still applies to any treatment option and is likely the most important factor that influences functional outcome.
Mechanical thrombectomy is known to achieve better recanalisation rates compared to standard treatment.18 What clinicians were after, however, was a difference in functional outcomes as this has a huge impact on the patient’s quality of life. The meta-analysis confirms a huge difference in 90-day mRS between the two treatment arms, with 46.10% and 26.46% of patients obtaining an mRS <2 in the mechanical thrombectomy and intravenous thrombolysis groups, respectively. The OR of 2.40 unequivocally demonstrates the superiority of thrombectomy and the difference was statistically significant (p value < 0.001).
There were no significant differences in secondary or safety outcomes between the two treatment groups. This may also prove an essential point in the push for the introduction of mechanical thrombectomy as a first-line treatment in acute ischaemic stroke as the perceived risks of thrombectomy (possibly resulting from the use of first-generation devices) still haunts this treatment. The meta-analysis proves that thrombectomy is in fact as safe as standard treatment.
The key factors resulting in such positive outcomes as opposed to MR RESCUE,13 SYNTHESIS Expansion14 and IMS III15 were the fact patients within the thrombectomy arm also received intravenous thrombolysis in a timely fashion, and the improved efficacy and safety of second-generation thrombectomy devices.
We believe that the data available so far are enough to justify the implementation of mechanical thrombectomy as part of standard treatment in acute ischemic stroke. The meta-analysis in fact supports a multimodal approach whereby mechanical thrombectomy is administered in conjunction with intravenous thrombolysis.
The recruitment within some of the trials included in our meta-analysis was halted because of emerging data supporting the superiority of mechanical thrombectomy. This will also surely be a driving force to amend future stroke treatment guidelines. It goes without saying that patient selection19,20 including clinical evaluation and radiological confirmation of large vessel occlusion is still crucial when it comes to treatment decisions in acute ischaemic stroke.
The major limitation of our meta-analysis is the small number of studies included (n = 5). These, however, included 1288 patients in total from multiple centres worldwide. The collected data may be used in future to cumulatively add further trial data that are published.
Conclusion
Patients treated with mechanical thrombectomy as part of a multimodal approach in acute ischaemic stroke were proven to have a better functional outcome at 90 days compared to those who received standard care. This suggests that current standard care should be improved as 25% more patients treated with mechanical thrombectomy achieved long-term independent outcome.
The data pool of 1288 patients provides robust evidence supporting the use of stentriever devices in acute ischaemic stroke.
Appendix 1
Search strategy
Database: Embase <1996 to 2015 April 03>, Ovid MEDLINE(R) <1946 to March Week 5 2015>
exp cerebrovascular accident/ (184772)
exp stroke/ (184772)
exp brain ischemia/ (180708)
exp brain infarction/ (73216)
exp brain hypoxia/ (17603)
exp occlusive cerebrovascular disease/ (23955)
cerebrovasc$ accident.mp. or cerebrovasc$ event.tw. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui] (99417)
((MCA or middle cerebral artery or ACA or anterior cerebral artery or ICA or internal cerebral artery or vertebrobasilar or basilar artery) adj5 (insufficiency or ischaemi$ or ischemi$ or embol$ or thromb$)).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui] (16207)
((penumbra or core or perfusion) adj5 (brain or cerebr$)).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui] (37089)
AIS.tw. (12823)
cva.tw. (5023)
1 or 2 or 3 or 4 or 5 or 6 or 7 or 8 or 9 or 10 or 11 (396232)
exp percutaneous thrombectomy/ or exp thrombectomy/ or exp mechanical thrombectomy/ (14410)
exp embolectomy/ (3709)
exp cerebral revasculari$/ (2831)
((retriev$ or device or stent or extraction) adj5 (brain or cereb$)).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui] (11383)
(stentriever or stent retriever or retriever device).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui] (476)
((Trevo or Solitaire or Merci or Revive or ERIC or Catch or Capture or Preset) adj5 (brain or cerebr$)).mp. [mp = ti, ab, sh, hw, tn, ot, dm, mf, dv, kw, nm, kf, px, rx, an, ui] (751)
thrombectomy.tw. (10434)
embolectomy.tw. (4117)
13 or 14 or 15 or 16 or 17 or 18 or 19 or 20 (36849)
12 and 21 (9333)
limit 22 to human (8338)
limit 23 to “all adult (19 plus years)” [Limit not valid in Embase; records were retained] (7527)
limit 24 to yr = “2010 -Current” (4935)
limit 25 to randomised controlled trial (123)
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Saver JL, Jahan R, Levy EI, et al. SOLITAIRE™ with the intention for thrombectomy (SWIFT) trial: Design of a randomized, controlled, multicenter study comparing the SOLITAIRE™ Flow Restoration device and the MERCI Retriever in acute ischaemic stroke. Int J Stroke 2014; 9: 658–668. [DOI] [PubMed] [Google Scholar]
- 2.Nogueira RG, Lutsep HL, Gupta R, et al. Trevo versus Merci retrievers for thrombectomy revascularisation of large vessel occlusions in acute ischaemic stroke (TREVO 2): A randomised trial. Lancet 2012; 380: 1231–1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence. Diagnosis and initial management of acute stroke and transient ischaemic attack (TIA). CG68, London: National Institute for Health and Care Excellence, 2008. [Google Scholar]
- 4.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 5.Higgins JPT and Green S (eds) Cochrane handbook for systematic reviews of interventions. Version 5.0.2. The Cochrane Collaboration, 2009.
- 6.Schardt C, Adams MB, Owens T, et al. Utilization of the PICO framework to improve searching PubMed for clinical questions. BMC Med Inform Decis Mak 2007; 7: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Viswanathan M, Berkman N. Development of the RTI item bank on risk of bias and precision of observational studies. J Clin Epidemiol 2012; 65: 163–178. [DOI] [PubMed] [Google Scholar]
- 8.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 9.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 10.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 11.Saver JL, Goyal M, Bonafé A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 12.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 13.Kidwell CS, Jahan R, Gornbein J, et al. A trial of imaging selection and endovascular treatment for ischemic stroke. N Engl J Med 2013; 368: 914–923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ciccone A, Valvassori L, Nichelatti M, et al. Endovascular treatment for acute ischemic stroke. N Engl J Med 2013; 368: 904–913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Broderick JP, Palesch YY, Demchuk AM, et al. Endovascular therapy after intravenous t-PA versus t-PA alone for stroke. N Engl J Med 2013; 368: 893–903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fischer U, Arnold M, Nedeltchev K, et al. NIHSS score and arteriographic findings in acute ischemic stroke. Stroke 2005; 36: 2121–2125. [DOI] [PubMed] [Google Scholar]
- 17.Emberson J, Lees KR, Lyden P, et al. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: A meta-analysis of individual patient data from randomised trials. Lancet 2014; 384: 1929–1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Smith WS. Safety of mechanical thrombectomy and intravenous tissue plasminogen activator in acute ischemic stroke. Results of the multi Mechanical Embolus Removal in Cerebral Ischemia (MERCI) trial, part I. AJNR Am J Neuroradiol 2006; 27: 1177–1182. [PMC free article] [PubMed] [Google Scholar]
- 19.Grech R, Galvin PL, Power S, et al. Outcome prediction in acute stroke patients considered for endovascular treatment: A novel tool. Interv Neuroradiol 2014; 20: 312–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hallevi H, Barreto AD, Liebeskind DS, et al. Identifying patients at high risk for poor outcome after intraarterial therapy for acute ischemic stroke. Stroke 2009; 40: 1780–1785. [DOI] [PMC free article] [PubMed] [Google Scholar]