Abstract
Pediatric intracranial aneurysms are rare with a reported prevalence of 0.5–4.6%. Likewise, anomalous arterial patterns are uncommon in the cerebral circulation. Recognition of these variations and knowledge of vascular territory forms the key to managing pathological conditions associated with these anomalous vessels. Ruptured dissecting aneurysm of type-3 accessory middle cerebral artery (aMCA) has not been reported in the pediatric age group. In addition to type-3 aMCA, the child in this case report had an ipsilateral type-1 aMCA with cortical supply. We describe the patterns of accessory MCA and their vascular territory, state the perplexity involved in deciding the best management strategy, and describe the technical approach we undertook to catheterize this small caliber recurrent artery (type-3 aMCA) originating at an acute angle from the anterior cerebral artery.
Keywords: Dissecting aneurysm, accessory middle cerebral artery, glue embolization, recurrent artery of Heubner
Introduction
Intracranial aneurysms are infrequent in the pediatric population and approximately 0.5–4.6% of intracranial aneurysms occur in patients 18 years of age or younger. Similarly, anomalous arterial patterns, in particular, accessory middle cerebral artery (aMCA) is a rare entity with a reported incidence of 0.3–4%.1 A systematic search of published literature in English language has revealed no reports of a ruptured dissecting aneurysm of type-3 aMCA (recurrent artery of Heubner, RAH) in the pediatric age group. In addition, the child in this case report had a type-1aMCA with cortical supply.2–5
Case report
A four-year-old girl with sudden onset severe headache was diagnosed to have Fischer grade 2 and WFNS grade 1 subarachnoid hemorrhage (SAH). Brain magnetic resonance imaging (MRI), in addition to SAH, revealed right pallidal T2 hyperintensity (Figure 1). Cerebral angiography revealed a fusiform dissecting aneurysm measuring approximately 8.8 × 2.1 mm in the right type-3 aMCA (RAH) with cortical branches to the orbitofrontal region (Figure 2). Furthermore, a right type-1 aMCA with supply to the anterior and middle temporal cortex was noted.
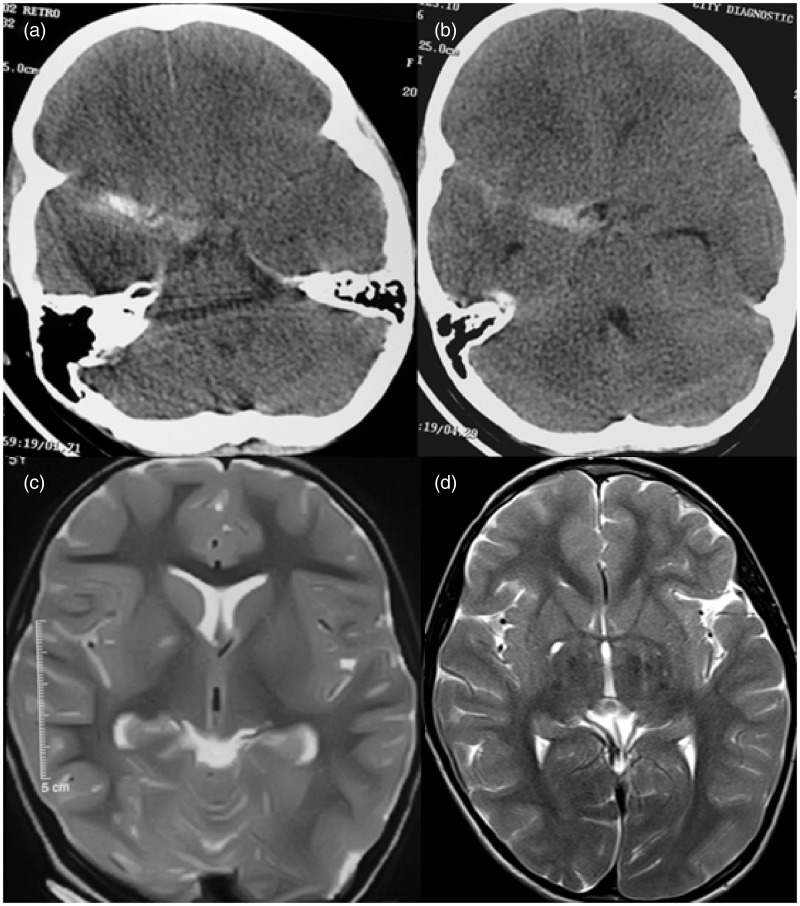
Figure 1.
Brain computed tomography (CT) and MRI prior to and post procedure. (a, b) Brain CT showing focal subarachnoid hemorrhage in the right sylvian fissure. (c) Right pallidal T2 hyperintensity (prior to procedure). (d) Right pallidal T2 hyperintensity as before on follow-up MRI at 6 months.
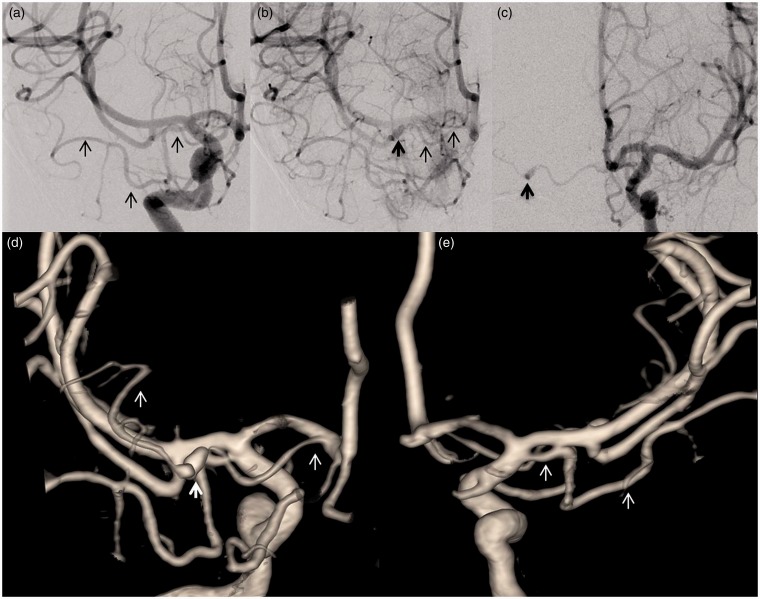
Figure 2.
Cerebral angiography demonstrating aMCA and dissecting aneurysm in type-3 aMCA. (a) Right ICA injection showing type-1 aMCA with cortical branches. (b) Late filing of dissecting aneurysm (bold dark arrow) of type-3 aMCA. (c) Early brisk filling of dissecting aneurysm of type-3 aMCA from a contralateral injection. (d) Three-dimensional (3D) surface shaded display (SSD) demonstrating right type-3 aMCA harboring a dissecting aneurysm (bold white arrow). (e) 3D SSD right type-1 aMCA (posterior view).
Both surgical clipping with bypass and endovascular parent vessel occlusion were contemplated. Morbidity related to both modalities of treatment was considered to be high with little evidence favoring one modality over the other.6 However, the probability of intra-operative rupture during dissection was considered to be high by our vascular neurosurgeon. Therefore, selective endovascular occlusion of the aneurysm with maintenance of distal arterial patency to allow for the possibility of preservation of leptomeningeal collateral flow to the cortex was undertaken.
Technical aspects
Catheterizing the type-3 aMCA from an ipsilateral approach was deemed challenging for the precise reason that navigating into a small caliber artery that arose at an acute angle from the right anterior cerebral artery (ACA) would be extremely difficult. Early aneurysmal sac filling was seen with a left as compared to right injection and the angle of the origin was obtuse and in direct line with the contralateral A1 ACA–anterior communicating artery (ACOM) complex. Therefore, a contralateral approach through the left internal carotid artery (ICA) was planned from the outset. Initial attempt to navigate the coiling microcatheter (Headway Duo1.6 F; MicroVention, Inc.) over a Traxess (MicroVention, Inc.) microguidewire, after priming with 0.5 mg of nimodipine, was unsuccessful owing to the small caliber. Consequently, a Magic 1.2 FM (Balt Extrusion, Inc.) microcatheter was navigated over a Hybrid 0.007 (Balt Extrusion, Inc.) wire across the ACOM into a position proximal to the aneurysmal sac in the right type-3 aMCA. Following that concentrated (80%) glue was injected with care to prevent proximal reflux or distal migration of the glue (Figure 3). Post procedure, the child made a good recovery without any focal deficits. No new cortical infarcts were noted and there was no significant expansion of the infarct in the lentiform nucleus on follow up MRI at 6 months (Figure 1). The child had no focal deficits on clinical follow up at 6 months.
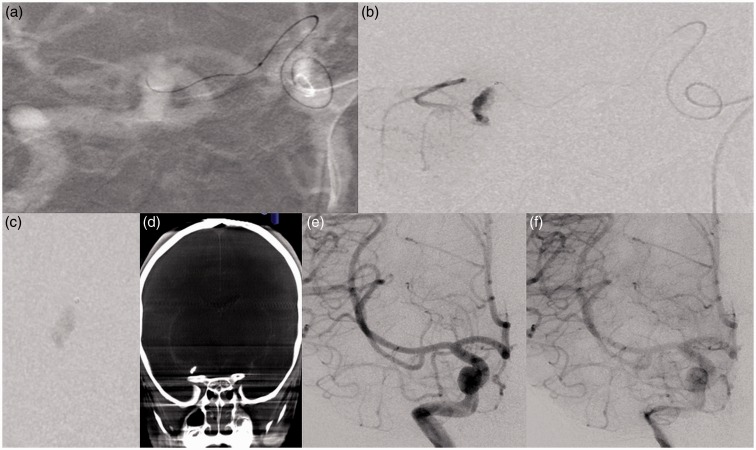
Figure 3.
Technical aspects and post procedure cerebral angiography. (a) Catheterization of type-3 aMCA from contralateral side using Magic 1.2 FM microcatheter. (b) Microcatheter injection showing filling of dissecting aneurysm and distal cortical territory. (c) Glue cast seen in the aneurysm sac. (d) Dyna CT showing glue cast within the aneurysm sac. (e, f) Early and delayed run showing obliteration of the aneurysm sac.
Discussion
Intracranial aneurysms, a rare entity in the pediatric age group, are frequently seen in boys and tend to be giant and dissecting or fusiform in morphology.1 They commonly occur at the terminal ICA followed by ACOM and MCA bifurcation, and the proposed etiology include an underlying connective tissue disorder, infection or trauma.
A fusiform aneurysm in an anomalous artery in the presence of another anomalous artery has not been reported previously. In our patient, cerebral angiography revealed both type-1 and type-3 aMCA with cortical supply. Manelfe et al. classified accessory MCA into three types based on the site of origin of the artery. Type-1 aMCA originates proximal to the ICA bifurcation, commonly supplies the orbito-frontal region and the anterior temporal cortex and is devoid of perforating arteries (duplicated MCA as per Teal et al.; main MCA trunk according to Lasjaunias et al.).7–10 Type-2 aMCA arises just distal to the bifurcation from the A1 ACA, frequently supplies the orbitofrontal, prefrontal and the pre-rolandic areas and consistently have central arteries supplying the deep structures. Type-3aMCA, a Heubner artery, usually arises at the junction of the ACA and ACOM artery; it is smaller in caliber as compared to the MCA and supplies the orbitofrontal region. Central arteries are not uncommon and when present are few in numbers. Although there is no unified consensus, initially Handa et al. and later Lasjaunias et al. based on phylogenetic analysis concluded that the type 3 aMCA represents a recurrent artery of Heubner (RAH).7,11
The RAH, a relatively constant artery that arose within 4 mm of the ACOM, has a recurrent course parallel and either superior or anterior to the A1-ACA. Inferior and posterior predispositions are rare. Extra-cerebral course ends by terminating at the anterior perforator substance and provides supply to the head of the caudate nucleus, the anterior third of the putamen, the tip of the outer segment of the globus pallidus and the anterior third/antero-inferior portion of the anterior limb of internal capsule.12 In our patient, the type-3 aMCA (RAH) arose at an acute angle from the right ACA at its junction with ACOM, took a recurrent course that was antero-inferior to the A1-ACA and harbored a fusiform aneurysm at its mid-segment along the horizontal course parallel to the M1 segment (Figure 4).
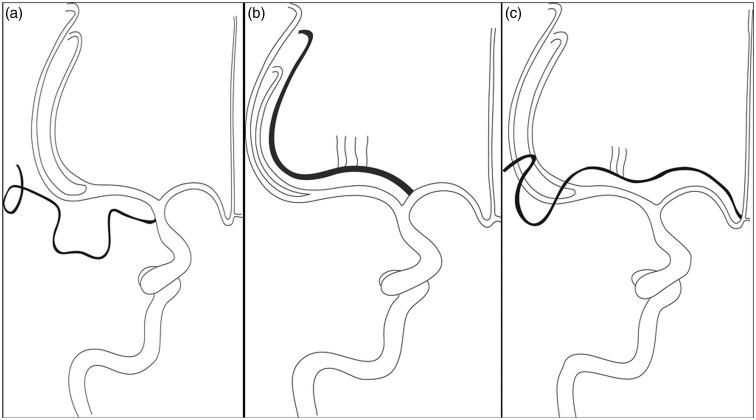
Figure 4.
Line art demonstrating aMCA anatomy (site of origin and supply). (a) Type-1 aMCA with cortical supply. (b) Type-2 aMCA with perforators and cortical supply. (c) Type-3 aMCA with perforators and cortical supply.
Surgical clipping, with or without bypass, is associated with high morbidity and mortality. Selective endovascular parent vessel occlusion also poses a significant risk of ischemia. Some authors have suggested that endovascular parent vessel occlusion can be safe in the pediatric age group owing to adequate leptomeningeal collateralization.6,13–15 Therefore, a short segment selective occlusion may allow leptomeningeal collaterals to preserve the flow in distal cortical territory. Diagnostic balloon occlusion test prior to the occlusion may predict the nature of deficit. In our case, owing to very small caliber and acute SAH this was deemed to be unnecessary.
A contralateral approach allowed us to catheterize this small artery arising at an acute angle from the ACA with relative ease. Concentrated glue (80%) rapidly polymerizes when in contact with blood. This enabled controlled delivery of glue and avoided inadvertent distal migration of the glue. Recognizing anomalous variants and their vascular territory is key to deciding on the best management strategy.
Conclusion
Ruptured dissecting aneurysm of type-3 accessory MCA (RAH) has not been reported in the pediatric population. Knowledge of anatomy and vascular supply of these anomalous vessels are crucial to obtaining good outcomes. Controlled glue embolization may prevent ischemia in the cortical territory of the occluded artery as leptomeningeal collaterals may subserve this territory, particularly in the pediatric age group.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Liang J, Bao Y, Zhang H, et al. The clinical features and treatment of pediatric intracranial aneurysm. Childs Nerv Syst 2009; 25: 317–324. [DOI] [PubMed] [Google Scholar]
- 2.LaBorde DV, Mason AM, Riley J, et al. Aneurysm of a duplicate middle cerebral artery. World Neurosurg 2012; 77: 201–e1. [DOI] [PubMed] [Google Scholar]
- 3.Bechan RS, van Rooij WJ. Endovascular treatment of a ruptured flow aneurysm of the Heubner artery as part of a moyamoya collateral network in a young patient with an occluded middle cerebral artery. Interv Neuroradiol 2014; 20: 791–795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Otawara Y, Suzuki M, Abe M, et al. Dissecting aneurysms of the anterior cerebral artery and accessory middle cerebral artery. Case report. Neurosurg Rev 1997; 20: 145–148. [DOI] [PubMed] [Google Scholar]
- 5.Lee IH, Jeon P, Kim KH, et al. Endovascular treatment of a ruptured accessory middle cerebral artery aneurysm. J Clin Neurosci 2010; 17: 383–384. [DOI] [PubMed] [Google Scholar]
- 6.Takemoto K, Tateshima S, Golshan A, et al. Endovascular treatment of pediatric intracranial aneurysms: a retrospective study of 35 aneurysms. J Neurointerv Surg 2014; 6: 432–438. [DOI] [PubMed] [Google Scholar]
- 7.Lasjaunias P, Berenstein A and ter Brugge KG. Surgical Neuroangiography - Clinical vascular anatomy and variations. 2nd ed. Berlin, Germany: Springer-Verlag, Chapter 6, Intradural arteries; pp. 590–596.
- 8.Komiyama M, Nakajima H, Nishikawa M and Yasui T. Middle cerebral artery variations: duplicated and accessory arteries. AJNR Am J Neuroradiol. 1998; 19: 45–49. [PMC free article] [PubMed]
- 9. Abanou A, Lasjaunias P, Manelfe C and Lopez-Ibor L. The accessory middle cerebral artery (AMCA). Diagnostic and therapeutic consequences. AnatClin. 1984; 6: 305–309. [DOI] [PubMed]
- 10.Teal JS, Rumbaugh CL, Bergeron RT and Segall HD. Anomalies of the middle cerebral artery: accessory artery, duplication, and early bifurcation. AJR Am J Roentgenol 1973; 118: 567–575. [DOI] [PubMed]
- 11.Handa J, Shimizu Y, Matsuda M and Handa H. The accessory middle cerebral artery: Report of further two cases. Clin Radiol. 1970; 21: 415–416. [DOI] [PubMed]
- 12.Maga P, Tomaszewski KA, Krzyżewski RM, Golec J, Depukat P, Gregorczyk-Maga I, et al. Branches and arterial supply of the recurrent artery of Heubner. Anat Sci Int. 2013; 88: 223–239. [DOI] [PubMed]
- 13.Lv X, Jiang C, Li Y, et al. Endovascular treatment for pediatric intracranial aneurysms. Neuroradiology 2009; 51: 749–754. [DOI] [PubMed]
- 14.Hetts SW, Narvid J, Sanai N, et al. Intracranial aneurysms in childhood: 27-year singleinstitution experience. AJNR Am J Neuroradiol 2009; 30: 1315–1324. [DOI] [PMC free article] [PubMed]
- 15.Andreou A, Ioannidis I and Mitsos A. Endovascular treatment of peripheral intracranial aneurysms. AJNR Am J Neuroradiol 2007; 28: 355–361. [PMC free article] [PubMed]