Abstract
Unfused basilar arteries, frequently but erroneously referred to as ‘fenestrations’, are not uncommonly associated with aneurysms. The difficulty in treating these aneurysms lie in the fact that they are often wide necked and frequently incorporate both channels of the unfused segment, with varying calibres of the channels, necessitating technically challenging treatment strategies. It is important to preserve both channels because of the potential presence of perforating arteries originating from these segments. There are numerous case reports of such aneurysms being treated by coils alone, coiling with balloon assistance and stent-assisted coiling in configurations such as ‘X’, ‘double barrel’ or waffle cone. We present an exemplary case, in which an aneurysm on a partial unfused basilar segment was treated with parallel flow diverters with an excellent result on follow-up imaging.
Keywords: Unfused basilar artery, fenestration, aneurysm, flow diverter, posterior fossa
Background
Unfused segments of the basilar artery are situated either at its cranial or caudal end. It is evident from embryology that the caudal unfused segment occurs when there is incomplete fusion of the longitudinal vertebral arteries.1 In unfused segments, normal vessel wall layers are deficient and not robust enough to counter the haemodynamic variability, thus predisposing them to aneurysmal development.2 These aneurysms could be pointed either anteriorly or posteriorly and the two channels could be of varying calibres. It is well described that there are numerous respective brainstem perforators arising from the unfused channels, which are difficult to visualise on angiograms, such as the pontomedullary arteries and the caudal and middle perforators.3 Thus it is imperative to preserve both channels during treatment. There are numerous case series and reports of varied treatment strategies of unfused basilar artery aneurysms in the literature. Some of them use coils alone. Some have used balloon assistance, some others have utilised coils with stents, with different configuration of the stents, such as double barrel, ‘X’, double microcatheter or a waffle cone technique. Whilst most of these are successful, there have been a few instances of procedural complications such as ruptures and issues with long-term durability such as aneurysmal recurrence, thrombosis of an unfused channel and strokes.4–8 We present a novel method of treating a partially unfused basilar artery aneurysm with dual flow diverters alone, with excellent long-term effect. We present a second case of an unfused basilar segment aneurysm, which was not ideal for a similar approach, thus signifying the importance of tailoring the treatment strategy to individual patients and aneurysms.
Case 1
A 55-year-old lady with history of a fall was presented to our hospital. Magnetic resonance imaging, performed to exclude a causative stroke, revealed an incidental unfused segment of the lower basilar artery. An anteriorly pointing 9 × 7 × 6 mm3 saccular aneurysm, arising from the caudal end of the unfused segment, incorporating both proximal limbs of the unfused segment, was identified. Only distal to the aneurysm the channels fuse to form the basilar artery. Digital subtraction angiography (DSA) with three-dimensional (3D) rotational angiography confirmed the above anatomy (Figure 1).
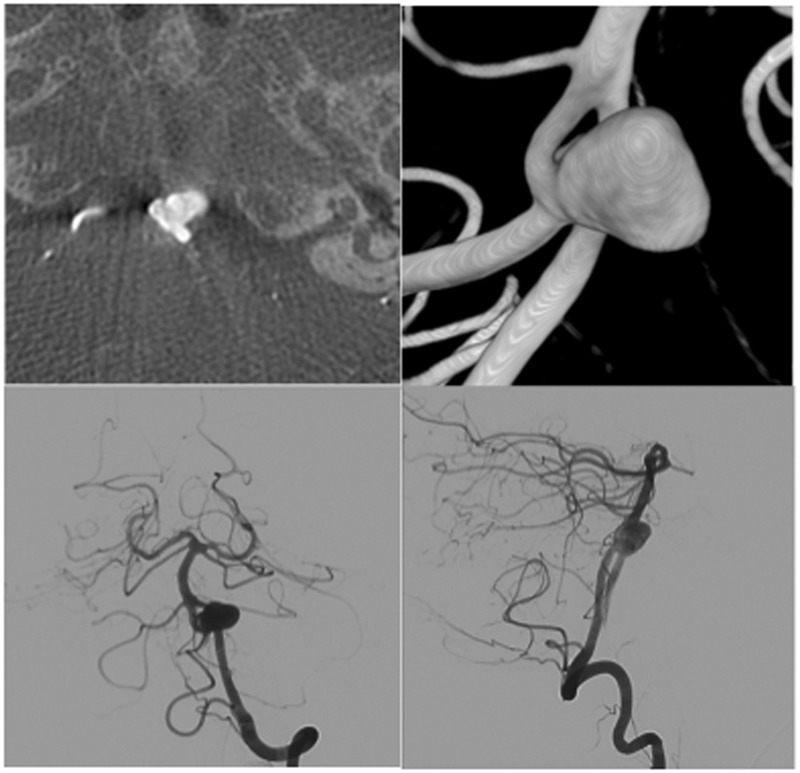
Figure 1.
Case 1 – pre-embolisation work up. Clockwise from top left: CT image demonstrating the anteriorly pointing aneurysm from the lower end of the unfused segment; 3DRA reconstruction shows equal calibre of channels; AP and lateral views of DSA confirm the above.
Following informed consent, a decision to treat the unruptured aneurysm was made based on young patient age and size of the posterior fossa aneurysm.9 Since both channels were of equal calibre, it was felt the best treatment strategy to preserve the channels, and thus the brainstem perforators, was to use double flow-diverters (FDs). We chose to use two 2.5 × 20 mm SILK-FD, (BALT Extrusion, Montmorency, France). Standard anti-aggregation therapy (aspirin and clopidogrel) was instituted 5 days prior to intervention. At endovascular treatment, both SILK-FDs were simultaneously placed with distal ends approximated in the basilar artery, thus avoiding overlap, compression and restricted flow. The proximal ends of the SILK-FDs rested in the vertebral arteries. No coils were used to fill the aneurysm. The procedure took a mere 45 minutes and was uneventful. Follow-up catheter angiography at 7 months revealed complete occlusion of the aneurysm with remodelling of the unfused channels (Figure 2). The patient did not have any adverse sequelae.
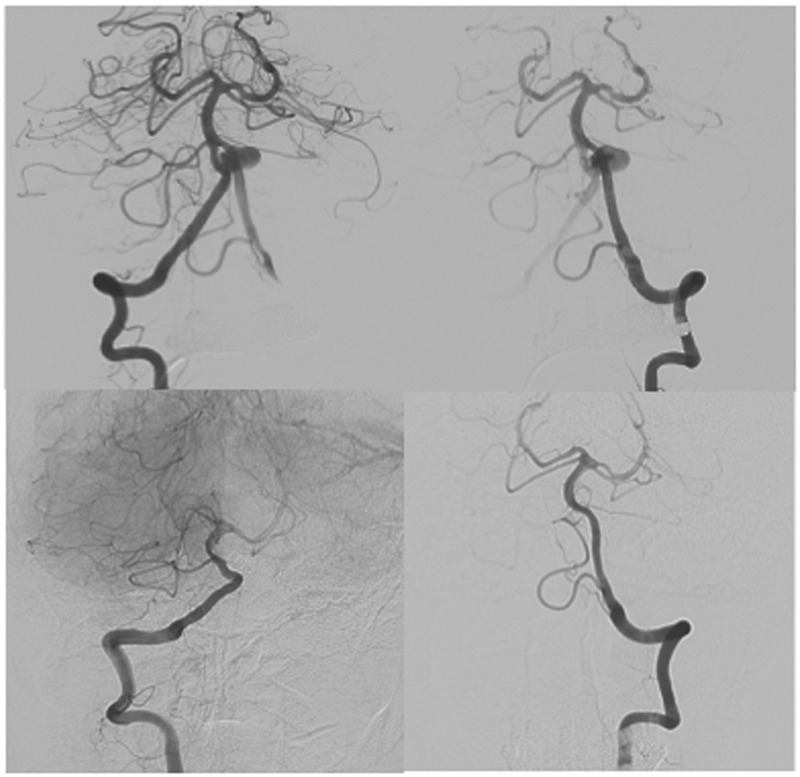
Figure 2.
Case 1 – DSA immediately after embolisation and 6 month follow-up. Top row: Dual SILK FDs in both unfused channels immediately post op. Bottom row: Excellent remodelling of the unfused channels, with complete occlusion of the aneurysm.
Case 2
A 71-year-old lady admitted for momentary apraxia, had a computed tomography (CT) brain scan, which revealed an incidental aneurysm arising from the proximal basilar artery. CT angiography (CTA) and DSA demonstrated a partial basilar artery non-fusion with a bi-lobed aneurysm from the proximal segment (Figure 3). The unfused channels were positioned antero-posteriorly across the aneurysm. The anterior channel was larger than the narrow posterior channel. Small brainstem perforators and antero-inferior cerebellar artery (AICA) were seen to arise from both channels. Both vertebral arteries were hypoplastic and did not contribute to the intracranial circulation. The basilar artery was fed by a persistent hypoglossal artery, arising from the C2 segment of the right internal carotid artery. A decision to follow the aneurysm conservatively was taken in the multidisciplinary meeting. The various factors contributing to the decision were the asymptomatic nature of the aneurysm, the unequal calibre and position of the unfused antero-posterior channels and the presence of critical brainstem perforators including the AICA.
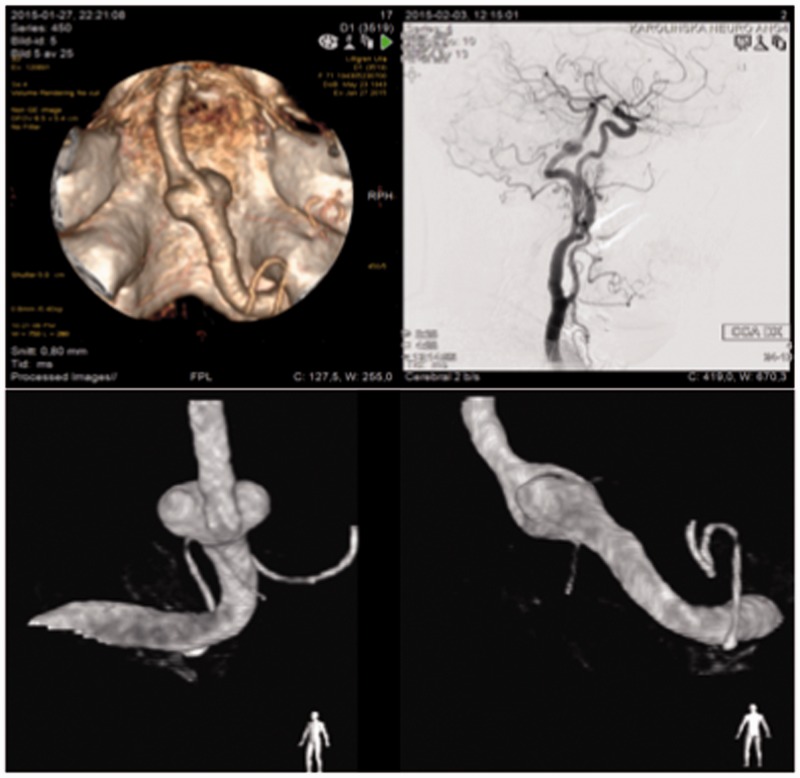
Figure 3.
Case 2 – persistent hypoglossal artery supplying an unfused BA with aneurysm. Clockwise from top left: CTA demonstrating basilar artery supply from hypoglossal artery arising from the right hypoglossal canal. Note right AICA reaching up to the right internal acoustic meatus; R CCA angiogram shows C2 origin of PHA feeding the basilar artery; oblique views demonstrating the unequal calibre of unfused channels and critical branches arising from them.
Discussion
The use of FDs in the treatment of posterior fossa aneurysms (saccular, fusiform, dissecting) is evolving, with many operators underlying the importance of careful patient selection and management in order to avoid complications.10–12 To the knowledge of the authors, this is an exemplary case of a saccular aneurysm of an unfused basilar artery treated with parallel dual FDs.
Unfused segments of the basilar artery are rare (6% post-mortem studies, <1% angiographic studies). They are associated with aneurysms in 7% of the cases due to inherent vessel wall weaknesses.13 In unruptured aneurysms of the unfused basilar artery, care must be taken to balance the risk of future rupture with peri- and post-procedural complications of challenging interventions. The technical issues lie in accurate measurement of the unfused channels, selection of FD (size, flexibility, mesh density, retrievability), accurate placement without overlap of FD ends and post procedural care to keep the FDs open. Potential complications with use of posterior fossa flow diverters are embolic, thrombotic due to perforator occlusion, haemorrhagic due to antiplatelet use and longer term sequelae of stent thrombosis, migration and aneurysm recanalisation.
In the case of a partially unfused basilar artery, where the FDs will be placed in parallel, a technical consideration should be that both devices are to be opened at the same time. Once the first FD takes its maximum diameter, it will be hard for the second device to open up to its full diameter. In these cases it is therefore advocated to perform simultaneous deployment by having two experienced operators or perform stepwise deployment in an alternate fashion. Moreover, care has to be taken to avoid the potential overlap of one of the devices on the distal portion of the second, thus hampering distal flow.
The unruptured aneurysm in Case 1 was in a young patient. The anatomy of the aneurysm with equal calibre unfused channels in a right-left orientation was conducive for double SILK-FD treatment, without the use of coils. The alternative option would have been double barrel stenting with additional coiling, at a probable downside of a longer procedural time in a critical location. The unfused channels house numerous brainstem perforators ranging 300–400 µm in diameter, which should not be occluded.3 The low profile design of the nitinol microwires (approx. 35 µm in diameter) in SILK-FDs and constant demand across the perforators are deemed adequate to maintain the patency of the perforators.3,11 In the second case, we opted to manage conservatively, balancing the risks of age, asymptomatic nature of the aneurysm, small posterior unfused channel and important vessels coming off the channels. The procedural complication risks were considered to be high, due to the above factors and in the presence of a persistent hypoglossal artery access.
Conclusion
Dual SILK-FD placement in the channels of an unfused basilar artery aneurysm is a feasible option, as demonstrated in our patient. The procedure is relatively quick and with no adverse events at 7 months follow up. However, considerable care must be taken in patient selection and availability of skilled operators to ensure optimal results. Our experience in a single patient is novel and we acknowledge this limitation and the lack of longer term follow up at this stage. Further worldwide experiences would help consolidate knowledge in this relatively uncommon topic.
Declaration of conflicting interests
The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: Patrick A Brouwer is a proctor for BALT Extrusion, Montmorency, France.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Lasjaunias PL and ter Brugge KG. Surgical neuroangiography. Springer Science & Business Media, 2001.
- 2.Finlay HM, Canham PB. The layered fabric of cerebral artery fenestrations. Stroke 1994; 25: 1799–1806. [DOI] [PubMed] [Google Scholar]
- 3.Marinković SV, Gibo H. The surgical anatomy of the perforating branches of the basilar artery. Neurosurgery 1993; 33: 80–87. [DOI] [PubMed] [Google Scholar]
- 4.Trivelato FP, Abud DG, Nakiri GS, et al. Basilar artery fenestration aneurysms: endovascular treatment strategies based on 3D morphology. Clin Neuroradiol 2014; epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 5.Kan P, Abla AA, Dumont TM, et al. Double-barrel stent-assisted coiling of a basilar artery fenestration aneurysm. J Neuroimaging 2013; 23: 496–499. [DOI] [PubMed] [Google Scholar]
- 6.Menendez JY, Harrigan MR. X-configuration stent-assisted coiling. World Neurosurg 2010; 74: 143–144. [DOI] [PubMed] [Google Scholar]
- 7.Vajpeyee A, Goyal G, Kant R, et al. Double microcatheter-assisted coiling of a basilar artery fenestration aneurysm. Neurointervention 2013; 8: 125–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gruber TJ, Ogilvy CS, Hauck EF, et al. Endovascular treatment of a large aneurysm arising from a basilar trunk fenestration using the waffle-cone technique. Neurosurgery 2010; 67: ons140–ons144. [DOI] [PubMed] [Google Scholar]
- 9.Wiebers D, Whisnant J, Forbes G, et al. Unruptured intracranial aneurysms – risk of rupture and risks of surgical intervention. N Engl J Med 1998; 339: 1725–1733. [DOI] [PubMed] [Google Scholar]
- 10.Toth G, Bain M, Hussain MS, et al. Posterior circulation flow diversion: a single-center experience and literature review. J Neurointerv Surg 2014; epub ahead of print. [DOI] [PubMed] [Google Scholar]
- 11.Kulcsár Z, Ernemann U, Wetzel SG, et al. High-profile flow diverter (SILK) implantation in the basilar artery efficacy in the treatment of aneurysms and the role of the perforators. Stroke 2010; 41: 1690–1696. [DOI] [PubMed] [Google Scholar]
- 12.Phillips TJ, Wenderoth JD, Phatouros CC, et al. Safety of the pipeline embolization device in treatment of posterior circulation aneurysms. Am J Neuroradiol 2012; 33: 1225–1231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Krings T, Baccin CE, Alvarez H, et al. Segmental unfused basilar artery with kissing aneurysms: report of three cases and literature review. Acta Neurochir 2007; 149: 567–574. [DOI] [PubMed] [Google Scholar]