Abstract
Background
Early arterial recanalisation with stent retrievers (SR) has been recently demonstrated to improve clinical outcome of patients with large-vessel occlusion of the anterior circulation. However, the benefit of SR thrombectomy in the setting of acute basilar artery occlusion (BAO) has not been proven yet. This study evaluated a series of consecutive patients with BAO treated with SR, focusing on the efficacy, safety and clinical results.
Methods
We analysed 24 consecutive patients with acute BAO who were treated with SR mechanical thrombectomy. Good clinical outcome at three months was defined as mRS ≤ 2. Data from patients with good outcome were compared to that from patients with poor outcome.
Results
Sufficient recanalisation (TICI 2 b or 3) was achieved in 63% (15/24) of patients. At three months, 33% (8/24) of patients had died; good clinical outcome was obtained in 21% (5/24). Age (46 vs. 60 years old, p = 0.05) and time from symptoms onset to recanalisation (370 vs. 521 minutes, p = 0.048) was significantly lower in patients with good outcome as compared to patients with poor outcome. There were three cases (12.5%) of periprocedural complications, all of them related to arterial wall dissection/perforation.
Conclusions
SR thrombectomy might be an efficient and safe treatment for patients with acute BAO occlusion and might help improve outcome.
Keywords: Basilar artery occlusion, thrombectomy, stent retrievers
Introduction
Acute basilar artery occlusion (BAO) is a devastating subtype of stroke, with high rates of morbidity and mortality.1 Early administration of intravenous tissue plaminogen activator (iv tPA) is still the first-line treatment modality;2 however, its results are hampered by low recanalisation rates.3,4
Recently, mechanical thrombectomy techniques have proved their value in strokes caused by large-vessel occlusion of the anterior circulation. Several studies have demonstrated that early recanalization with stent retrievers (SRs) improves functional outcome as compared to iv tPA alone.5–9 Regarding BAO, small series have suggested that thrombectomy with SRs might also contribute towards a better outcome. A recent meta-analysis and a large prospective German and Austrian registry provided further support for this hypothesis.10,11 Despite these promising results, the real benefit of SR thrombectomy for acute BAO has not been proven yet.
The aim of this study was to analyse our series of consecutive patients with BAO treated with SRs, focusing on the efficacy, safety and clinical results.
Materials and methods
Patients
Since 2012, data from all patients with acute ischaemic stroke treated with mechanical thrombectomy at our institution have been collected in a prospectively maintained database. From 2012 to 2014, 97 patients with large-vessel occlusion were treated with mechanical thrombectomy with the use of SRs. Among these, we collected data (clinical and angiographic) from all 24 patients who presented with acute BAO. The institutional ethics committee approved this analysis.
Patients were either admitted directly to our institution or secondarily referred from other hospitals. On admission, all patients were evaluated by a neurologist and their neurological condition was quantified using the National Institutes of Health Stroke Scale International (NIHSS). Imaging evaluation consisted of non-enhanced computed tomography (CT) and CT angiography (from the aortic arch to the vertex). The decision to undergo mechanical thrombectomy was made in agreement by the neurologist and the interventional neuroradiologist. The main inclusion criteria for thrombectomy were the following: baseline NIHSS ≥ 10; BAO confirmed with CT angiography; exclusion of intracranial haemorrhage; exclusion of ‘extensive established brainstem infarction’ as evaluated by the neuroradiologist (no posterior circulation Alberta Stroke Program Early CT Score (pc-ASPECTS) was used). Whenever patients arrived at the hospital within 4.5 h of symptom onset (and there were no contraindications) full-dose (0.9 mg/kg) iv-tPA was administered as bridging therapy.
Endovascular treatment
All procedures were performed by an interventional neuroradiologist with at least two years of experience. Informed consent was obtained from relatives whenever possible; for the remainder, consent was presumed. All patients were treated under general anaesthesia. The most typical setup was as follows: A 6 F sheath was introduced in the right femoral artery and a guide-catheter (usually a 6 F) was navigated to the V2 segment of the dominant vertebral artery, as high as safely possible. A diagnostic angiogram was performed to confirm the occlusion and to assess collateral circulation. Afterward, under roadmap, a 0.021-inch microcatheter and a 0.014-inch guidewire were used to cross the clot and a super-selective angiogram was performed to confirm a safe distal position. A 4 × 20 mm retrievable stent was then advanced and deployed across the occluded segment; Trevo Provue® was used in 21 patients and Solitaire FR® in three patients. The SR was left in place for 5 minutes, after which both the microcatheter and the SR were retrieved under manual aspiration at the guide catheter. If recanalisation was not successful, further attempts were performed. Whenever there was suspicion of an underlying high-grade stenosis, balloon angioplasty ± intracranial stent (Wingspan®) was considered. In cases of stent deployment, patients were loaded with abciximab (intra-arterial (ia) + iv) and, after 12 h, double antiplatelet therapy with aspirin and clopidogrel was administered. All patients were admitted to a dedicated stroke unit. Control CT was performed in all cases 24 h after the procedure. After three months, all patients were seen by a neurologist.
Outcome measurements
We analysed age, gender, baseline NIHSS score, iv tPA administration, recanalisation, time from symptoms onset to recanalisation, use of angioplasty ± stenting, periprocedural complications and clinical outcome. Recanalisation status was assessed according to the Thrombolysis in Cerebral Infarction (TICI) scale.12 TICI 2 b and 3 were rated as sufficient recanalisation; TICI ≤ 2 a was rated as insufficient. Clinical outcome three months after the procedure was quantified using the modified Rankin scale (mRS); good outcome was defined as an mRS ≤ 2.
Statistical analysis
A bivariate analysis was undertaken to determine the relationship between clinical outcome and some of the other variables. Mann-Whitney U test was used for continuous variables and Fisher’s exact test for categoric variables. A value of p < 0.05 was considered significant. All statistical analyses were performed with SPSS® (version 19.0; IBM, Armonk, NY, USA).
Results
During the study period there were 24 patients with acute BAO. Table 1 shows baseline patient characteristics according to clinical outcome.
Table 1.
Baseline characteristics of the study population.
| Good outcome (n = 5) | Bad outcome (n = 19) | Total (n = 24) | P | |
|---|---|---|---|---|
| Age, y (mean ± SD) | 46 ± 19 | 60 ± 10 | 57 ± 14 | .05 |
| Sex, male, n (%) | 3 (60%) | 14 (74%) | 17 (71%) | NS |
| Baseline NIHSS | 21 ± 9 | 23 ± 8 | 23 ± 8 | NS |
| ivtPA | 3 (60%) | 8 (42%) | 11 (46%) | NS |
| Sufficient recanalization | 4 (80%) | 11(58%) | 15 (63%) | NS |
| Time to recanalization, min | 370 ± 66 | 521 ± 145 | 480 ± 144 | .048 |
NS: non-significant.
Most patients were male (71%) and their mean age was 57 (range, 27–80 years old). Median NIHSS score at presentation was 20 and ranged from 12 to 36. It should be noted, however, that at arrival at our hospital many patients were already sedated and ventilated to allow for inter-hospital transfer. Iv tPA was administered to 11 (46%) patients prior to the endovascular procedure.
After the use of SR alone, sufficient recanalisation (TICI 2b and 3) was achieved in 54% of patients. In two cases, additional recanalization manoeuvres were adopted: In one patient, simple balloon angioplasty was performed; in the other patient, balloon angioplasty failed to reopen the artery and a stent was deployed (as described above). In both cases, final recanalisation status was improved. Combining the results of all procedures, sufficient recanalisation was achieved in 63% of patients. On average, recanalisation was achieved only 8 h after symptoms onset (ranging from 5 to 12 h).
According to the European Cooperative Acute Stroke Study (ECASS) criteria,13 there was only one case of haemorrhagic transformation, in which petechial infarction without space-occupying effect occurred (haemorrhagic infarction (HI) 1); there were no cases of parenchymal haematomas.
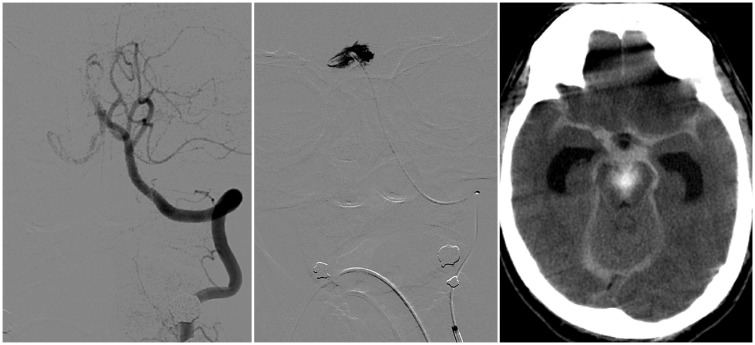
Regarding periprocedural complications, there were three cases of arterial dissection or perforation, all of them involving a lesion of the right posterior cerebral artery caused by microwire and microcatheter manipulation (i.e. unrelated to the SR itself). All patients had received iv tPA prior to the endovascular treatment. In one case the consequences were minimal and the patient evolved with good clinical outcome at three months; however, in the remaining cases the associated subarachnoid haemorrhage was severe and the patients subsequently died (for both cases the cause of death was attributed both to unresolved BAO and to haemorrhage). One of these cases is illustrated in Figure 1.
Figure 1.
Left: Left vertebral artery injection through the guide catheter demonstrated complete occlusion of the basilar artery. Centre: The microcatheter and the microwire were navigated to the P1 segment of the right posterior cerebral artery; however, selective injection through the microcatheter showed contrast extravasation due to perforation/dissection of the arterial wall. Right: As a consequence, an important subarachnoid haemorrhage developed, with hydrocephalus.
At three months, eight patients (33%) had died; most of them died during the first week after the stroke. Only five patients (21%) had an mRS score ≤ 2; there were also five patients with a score of 3.
In bivariate analysis, patients with good outcome were significantly younger (46 vs. 60 years old) and recanalised earlier (370 vs. 521 minutes) as compared to those with poor outcome. Gender, NIHSS score at presentation and prior administration of iv tPA were not found to be related to clinical outcome. Despite the fact that recanalisation results were better (80% vs. 58% of sufficient recanalisation) for the group with good clinical outcome, this variable failed to show statistical significance.
Discussion
This single-centre retrospective study analyses the use of thrombectomy with SRs in acute BAO.
The benefit of thrombectomy for cases of acute BAO has not been proven in a randomised clinical trial yet. There are, however, a few case series suggesting that, in the setting of acute BAO, thrombectomy is feasible, efficient, safe and may improve clinical outcome. The largest observational study so far published in this field was the International Multicenter Registry for Mechanical Recanalization Procedures in Acute Stroke (ENDOSTROKE),11 which included 148 consecutive patients. In this study, TICI 2b or 3 was achieved in 79% of patients; three-month mortality rate was 35% and the percentage of patients with mRS ≤ 2 was 34%. Recently, Gory et al.10 performed a meta-analysis of 15 previously published studies (case series) and included their own data, involving a total of 312 individuals. The estimated recanalisation rate reached 81%, three-month mortality was 30% and a favourable outcome was achieved in 42%.
In our series, recanalisation rate was 63%, between 16% and 18% inferior as compared to the rates published in the two largest studies mentioned above.10,11 This might be explained, on the one hand, by the conservative approach our team adopts. All thrombectomies were performed with SRs (aspiration techniques were not available) and only one device was used in each patient; the average number of passages was 2.2 and the maximum was five. Using a second device (either subsequently or simultaneously) or changing to a different thrombectomy technique (like primary aspiration or the A Direct Aspiration, First Pass Technique (ADAPT) technique) might help solve some cases of refractory arterial occlusions.14–16 On the other hand, our moderate number of thrombectomy cases per year might also have contributed to these results. By comparing the recanalization rates among recently published trials of thrombectomy for anterior circulation strokes,5–9 it is possible to notice that lower-volume centres (as those involved in the Multicenter Randomized Clinical Trial of Endovascular Treatment for Acute Ischemic Stroke in the Netherlands (MR CLEAN) trial) have lower recanalisation rates as compared to higher-volume centres.
The three-month mortality in this series was 33%, in line with the most recent studies.10,11 Acute BAO carries a very poor outcome and has the highest mortality rate among large-vessel occlusions.1,17 For anterior circulation strokes, a recent meta-analysis demonstrated that thrombectomy reduces the risk of mortality (as compared to iv tPA);18 hence, it might have a similar effect in acute BAO.
At 90 days, the proportion of patients in this series who had an mRS ≤ 2 was 21% (5/24). However, since acute BAO is such a hazardous condition, some authors have considered an mRS of 3 as a moderate result11 and a significant improvement as compared to the natural history. If we had adopted such a classification, the proportion of patients with good and moderate outcome (mRS ≤ 3) would have been 42% (10/24).
We had three periprocedural complications (12.5%), all of them consisting of inadvertent perforation or dissection of the right posterior cerebral artery. We believe that most were due to microwire penetration inside the small thalamoperforating arteries, as illustrated in a recent paper by Eom et al.19 As a consequence, most recently we have tried to keep the microwire proximal to the clot, using only the microcatheter to cross it.
We found that age and time from symptoms onset to recanalisation were associated with clinical outcome. The association between older age and poorer outcomes for patients with acute BAO treated with thrombectomy has been (retrospectively) reported by some other authors.10,11,20 Moreover, an index (SPAN index) combining age and NIHSS score has been suggested as an independent predictor of outcome both for patients treated with iv tPA and for patients treated with thrombectomy.21,22 The importance of faster recanalisation cannot be overemphasised. Two recent independent analyses demonstrated that, for anterior circulation strokes, onset-to-reperfusion time affects both mortality and outcome and should be kept in mind as the main target in acute stroke patient management.23,24 Most likely, this also applies to acute BAO. However, since the pathophysiology of the anterior and posterior circulation infarctions might not be the same, one should be aware that the temporal evolution might also differ between these two territories.25,26 This different pathophysiology might also explain why, in this series of acute BAO, recanalisation was not found to be related to good clinical outcome, unlike anterior circulation strokes, in which recanalisation has been repeatedly shown to improve the outcome.
Others have found different factors affecting the outcome of patients with acute BAO treated with SRs. Blood pressure,11,27 blood sugar levels,28 severity of symptoms at presentation,11,27,28 thrombus localisation,10 volume of the established infarction prior to treatment,20,29 collateral circulation11,28 and recanalisation status29 have all been suggested to influence the final outcome in this group of patients.
The main limitations of this study are its small population, its retrospective nature and its single-centre analysis.
Conclusions
Our results suggest that thrombectomy with the use of SRs might be an efficient and safe treatment for patients with acute BAO occlusion and might help improve outcome; however, even in the era of SRs, acute BAO is still a devastating condition. Moreover, age and time from symptoms onset to recanalisation have an influence on final outcome. Finally, arterial dissection or perforation is one of the main complications of this treatment.
Funding
The author(s) received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Schonewille WJ, Wijman CA, Michel P, et al. Treatment and outcomes of acute basilar artery occlusion in the Basilar Artery International Cooperation Study (BASICS): A prospective registry study. Lancet Neurol 2009; 8: 724–730. [DOI] [PubMed] [Google Scholar]
- 2.European Stroke Organisation (ESO) Executive Committee and ESO Writing Committee. Guidelines for management of ischaemic stroke and transient ischaemic attack 2008. Cerebrovasc Dis 2008; 25: 457–507. [DOI] [PubMed] [Google Scholar]
- 3.Lindsberg PJ, Mattle HP. Therapy of basilar artery occlusion: A systematic analysis comparing intra-arterial and intravenous thrombolysis. Stroke 2006; 37: 922–928. [DOI] [PubMed] [Google Scholar]
- 4.Saqqur M, Uchino K, Demchuk AM, et al. Site of arterial occlusion identified by transcranial Doppler predicts the response to intravenous thrombolysis for stroke. Stroke 2007; 38: 948–954. [DOI] [PubMed] [Google Scholar]
- 5.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 6.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 7.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 8.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015; 372: 2296–2306. [DOI] [PubMed] [Google Scholar]
- 9.Saver JL, Goyal M, Bonafé A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015; 372: 2285–2295. [DOI] [PubMed] [Google Scholar]
- 10.Gory B, Eldesouky I, Sivan-Hoffmann R, et al. Outcomes of stent retriever thrombectomy in basilar artery occlusion: An observational study and systematic review. J Neurol Neurosurg Psychiatry. Epub ahead of print 18 May 2015. DOI: 10.1136/jnnp-2014-310250. [DOI] [PubMed]
- 11.Singer OC, Berkefeld J, Nolte CH, et al. Mechanical recanalization in basilar artery occlusion: The ENDOSTROKE study. Ann Neurol 2015; 77: 415–424. [DOI] [PubMed] [Google Scholar]
- 12.Higashida RT, Furlan AJ, Roberts H, et al. Trial design and reporting standards for intraarterial cerebral thrombolysis for acute ischemic stroke. Stroke 2003; 34: e109–e137. [DOI] [PubMed] [Google Scholar]
- 13.Fiorelli M, Bastianello S, von Kummer R, et al. Hemorrhagic transformation within 36 hours of a cerebral infarct relationships with early clinical deterioration and 3-month outcome in the European Cooperative Acute Stroke Study I (ECASS I) cohort. Stroke 1999; 30: 2280–2284. [DOI] [PubMed] [Google Scholar]
- 14.Kim SK, Yoon W, Moon SM, et al. Outcomes of manual aspiration thrombectomy for acute ischemic stroke refractory to stent-based thrombectomy. J Neurointerv Surg 2014; 7: 473–477. [DOI] [PubMed] [Google Scholar]
- 15.Klisch J, Sychra V, Strasilla C, et al. Double Solitaire mechanical thrombectomy in acute stroke: Effective rescue strategy for refractory artery occlusions? Am J Neuroradiol 2015; 36: 552–556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Turk AS, Frei D, Fiorella D, et al. ADAPT FAST study: A direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2014; 6: 260–264. [DOI] [PubMed] [Google Scholar]
- 17.Hacke W, Zeumer H, Ferbert A, et al. Intra-arterial thrombolytic therapy improves outcome in patients with acute vertebrobasilar occlusive disease. Stroke 1988; 19: 1216–1222. [DOI] [PubMed] [Google Scholar]
- 18.Falk-Delgado A, Kuntze Söderqvist Å, Fransén J, et al. Improved clinical outcome 3 months after endovascular treatment, including thrombectomy, in patients with acute ischemic stroke: A meta-analysis. J Neurointerv Surg. Epub ahead of print 2 July 2015. DOI: 10.1136/neurintsurg-2015-011835. [DOI] [PMC free article] [PubMed]
- 19.Eom YI, Hwang YH, Hong J, et al. Forced arterial suction thrombectomy with the penumbra reperfusion catheter in acute basilar artery occlusion: A retrospective comparison study in 2 Korean university hospitals. Am J Neuroradiol 2014; 35: 2354–2359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Andersson T, Kuntze Söderqvist Å, Söderman M, et al. Mechanical thrombectomy as the primary treatment for acute basilar artery occlusion: Experience from 5 years of practice. J Neurointerv Surg 2012; 5: 221–225. [DOI] [PubMed] [Google Scholar]
- 21.Almekhlafi M, Davalos A, Bonafé A, et al. Impact of age and baseline NIHSS scores on clinical outcomes in the mechanical thrombectomy using Solitaire FR in acute ischemic stroke study. Am J Neuroradiol 2014; 35: 1337–1340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Weimar C, König I, Kraywinkel K, et al. Age and National Institutes of Health Stroke Scale score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: Development and external validation of prognostic models. Stroke 2004; 35: 158–162. [DOI] [PubMed] [Google Scholar]
- 23.Khatri P, Yeatts SD, Mazighi M, et al. Time to angiographic reperfusion and clinical outcome after acute ischaemic stroke: An analysis of data from the Interventional Management of Stroke (IMS III) phase 3 trial. Lancet Neurol 2014; 13: 567–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mazighi M, Chaudhry SA, Ribo M, et al. Impact of onset-to-reperfusion time on stroke mortality: A collaborative pooled analysis. Circulation 2013; 127: 1980–1985. [DOI] [PubMed] [Google Scholar]
- 25.Jones HR, Millikan CH, Sandok BA. Temporal profile (clinical course) of acute vertebrobasilar system cerebral infarction. Stroke 1980; 11: 173–177. [DOI] [PubMed] [Google Scholar]
- 26.Libman R, Kwiatkowski T, Hansen M, et al. Differences between anterior and posterior circulation stroke in TOAST. Cerebrovasc Dis 2001; 11: 311–316. [DOI] [PubMed] [Google Scholar]
- 27.Baek J, Yoon W, Kim S, et al. Acute basilar artery occlusion: Outcome of mechanical thrombectomy with Solitaire stent within 8 hours of stroke onset. Am J Neuroradiol 2014; 35: 989–993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mourand I, Machi P, Milhaud D, et al. Mechanical thrombectomy with the Solitaire device in acute basilar artery occlusion. J Neurointerv Surg 2013; 6: 200–204. [DOI] [PubMed] [Google Scholar]
- 29.Möhlenbruch M, Stampfl S, Behrens L, et al. Mechanical thrombectomy with stent retrievers in acute basilar artery occlusion. Am J Neuroradiol 2014; 35: 959–964. [DOI] [PMC free article] [PubMed] [Google Scholar]