Abstract
A high cervical dural arteriovenous fistula (dAVF) is relatively rare and tends to have different features, as compared with a thoracolumbar dAVF. Here, we report a case involving a complex AVF located at the craniocervical junction that was fed by the dural and pial arteries, combined with a contralateral dAVF.
Keywords: Dural arteriovenous fistula, craniocervical junction, perimedullary arteriovenous fistula
Introduction
pinal dural arteriovenous fistulas (dAVFs) are the most frequently observed lesions among spinal arteriovenous malformations. Most dAVFs are located at the thoracolumbar region and rarely at the cervical spine, in particular, the craniocervical junction. dAVFs are usually solitary lesions,1 whereas multiple or bilateral fistulas,2 and the coexistence of a pial shunt,3,4 are rarely diagnosed. Perimedullary AVFs comprise approximately 10–20% of all spinal vascular malformations.3 Only nine cases involving a perimedullary AVF at the craniocervical junction have been reported.5 Recently, complex AVFs fed by the dural and radicular or radiculomedullary arteries at the high cervical spine have been recognized increasingly,3,4 despite their rareness. Herein, we will present a patient who had complex AVFs and was initially diagnosed with a dAVF at the craniocervical junction, which had main pial shunts and additional dural shunt, combined with a typical dAVF on the contralateral side.
Case report
A 73-year-old male was admitted for the evaluation of progressive weakness of both lower extremities. The symptom had begun 10 months prior to admission and had been accompanied by urinary retention and paresthesias of both upper extremities. Sagittal images of T2-weighted magnetic resonance imaging (MRI) showed a high signal intensity lesion from the medulla oblongata to the C3 spinal cord with multiple small flow voids around the lesion (Figure 1(a)). Bilateral dAVFs at the craniocervical junction were suspected on both vertebral angiographies (Figure 1(b) to (f)). The right-sided AVF was not a typical dAVF, but the left-sided one was a typical dAVF. As for the right-sided AVF, a long distance existed between the feeding artery from the vertebral artery (VA) to the fistula point, with the recurrent course having tortuosity. However, he was of an older age at presentation, and the fistula point was located at the lateral portion near the C1 root sleeve. Therefore, we initially considered the right-sided fistula to be a dAVF at the craniocervical junction. The left-sided AVF was fed only by the dural artery from the left VA. The right-sided fistula mainly drained into the anterior perimedullary vein, while the left-sided one drained into the posterior vein. Both perimedullary veins were connected to one another. Other arterial feeders from the ECA branches were not opacified.
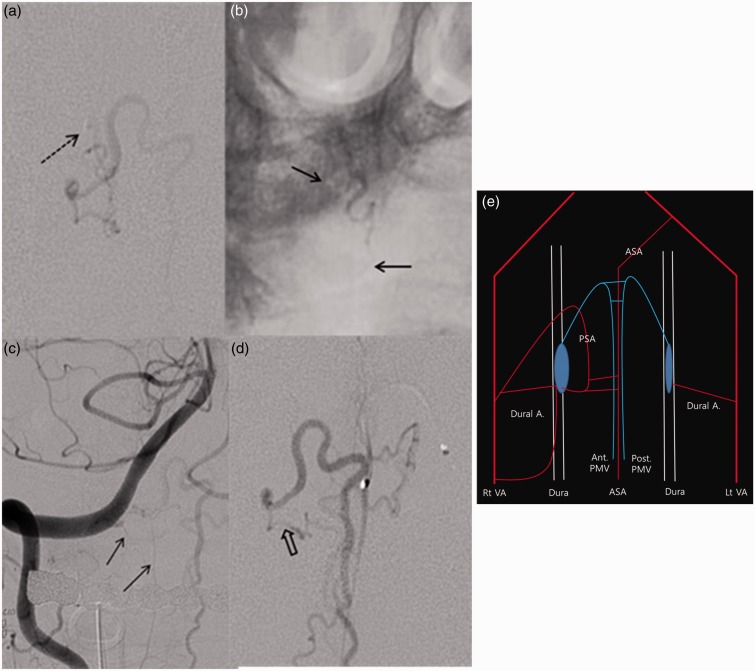
Figure 1.
Preoperative magnetic resonance imaging (MRI) and angiographic findings. (a) Sagittal images of T2-weighted MRI reveal a high signal intensity lesion from the medulla oblongata to the C3 with multiple small flow voids around the lesion. (b) A right vertebral angiogram reveals a suspected dural arteriovenous fistula (dAVF) that was fed by a tortuous feeding artery with a recurrent course (The white dotted arrow indicates a shunt point). (c) The shunt flow mainly drained into the anterior perimedullary vein. (d) The shunt flow partially into the posterior perimedullary vein. (e) A left vertebral angiogram illustrates a typical dAVF (The white dotted arrow indicates a shunt point). (f) The shunt flow mainly drained into the posterior perimedullary vein (the white arrow indicates the anterior perimedullary vein; and the black arrow indicates the posterior perimedullary vein).
Transarterial embolization was performed to obliterate the dAVFs on both sides. At first, the endovascular procedure for the right-side AVF was performed as follows: (1) a microcatheter (Echelon-10, eV3 Neurovascular, Inc., Irvine, CA) was inserted as far as possible into the main feeding artery; and (2) after confirmation of the fistula point and arterial connection by selective angiography through the microcatheter (Figure 2(a)), histoacryl glue (B. Braun, Tuttlingen, Germany) was infused to help reach the proximal venous side over the fistula point. However, the embolic material did not reach the fistula point, which resulted in proximal occlusion (Figure 2(b)). Post-embolization angiography demonstrated that the small dural branches originated from the right VA at the C1 and C2 levels and supplied the remaining fistula (Figure 2(c)). Moreover, a branch of the anterior spinal artery (ASA) originated from the left VA and was believed to supply the right-sided AVF. Superselective angiography confirmed that a branch of the ASA supplied the right-sided AVF, and a branch of the ASA was connected to the initial feeding artery on the right side (the artery was previously embolized using glue) (Figure 2(d)). We recognized that the initial feeding artery was a radiculomedullary artery which was connected to posterior spinal artery (PSA). Finally, the right-sided AVF was supplied from both the dural branches and radiculomedullary artery. A schematic illustration of these AVFs is shown in Figure 2(e). Endovascular treatment of the left-sided dural AVF failed because of technical limitations related to catheter navigation secondary to a tortuous, small-sized feeding artery. We recommended that the patient have surgery for both AVFs.
Figure 2.
Angiographic findings of complex arteriovenous fistula (AVF) at craniocervical junction. (a) Superselective angiography reveals a right-sided AVF that drained mainly into the anterior medullary vein (the black dotted arrow indicates the distal tip of the microcatheter). (b) Transarterial embolization using histoacryl glue was conducted (the black arrows refer to the proximal and distal points of the histoacryl glue). (c) A post-embolization vertebral angiogram reveals that the remaining AVF was fed by the dural branches (black arrow) of the vertebral artery (VA). (d) Superselective anterior spinal angiography revealed that the right AVF was supplied by branches of the ASA. The distal portion of the ASA branch has a common channel with the initial embolized feeding artery (The black hollow arrow indicates the common channel). (e) A schematic diagram illustrating both AVFs. (A, artery; ASA, anterior spinal artery; PSA, posterior spinal artery; PMV, perimedullary vein; VA, vertebral artery).
A suboccipital craniectomy and C1 laminectomy on both sides were conducted 2 weeks later. After dural opening, the C1 radicular artery and vein, along with the C1 root, were noted on the left side. Some feeding arteries on the dura were coagulated, and a temporary clip was applied to the draining vein under evoked potential (EP) monitoring. Intraoperative Doppler demonstrated that the arterial pulse wave on the posterior perimedullary vein had nearly disappeared. Therefore, coagulation and cutting of the feeding arteries and draining vein were conducted (Figure 3(a)). Then, the surgical removal of the right-sided complex AVFs was performed. The PSA had a dark appearance because of the embolized material that was observed. However, we could not confirm the accurate fistula point and could not see the branch of the ASA because of limitations regarding the operative field, as well as the patient’s prone position. Coagulation and cutting of the suspected feeding artery on the dura and radicular artery, and draining vein were performed using EP monitoring (Figure 3(b)). Doppler demonstrated that the arterial pulse wave of the posterior perimedullary vein had completely disappeared. Postoperative angiography revealed the complete disappearance of the right-sided complex AVFs and left-sided dAVF (Figures 3(c) and (d)). The patient was discharged after symptom improvement.
Figure 3.
Intraoperative and postoperative images. (a) Intraoperative photographs of the left (A) posterior views of the craniocervical junction (C1 DR = C1 dorsal root; C1 RA = C1 radicular artery; C1 VR = C1 ventral root; DV = draining vein; PICA = posteior inferior cerebellar artery; RMA = radiculomedullary artery SAN = spinal accessory nerve). (b) Intraoperative photographs of the right posterior views of the craniocervical junction. (c) Right vertebral angiograms immediately after surgery revealed the disappearance of complex AVF. (d) Left vertebral angiograms taken immediately after surgery showed the disappearance of AVF (the arrow indicates the ASA axis).
Discussion
Differentiation between dural and perimedullary AVFs can be conducted based on clinical and radiologic manifestations. Spinal dAVFs have been considered acquired lesions,3 and their mean age at symptom presentation is usually over 50.6,7 On the contrary, perimedullary AVFs were thought to be congenital.3 Regarding shunting point, dAVFs have an abnormal connection between the radiculomeningeal arteries and meningeal vein in the dura mater close to the spinal nerve root and spinal cord that drain into the perimedullary veins. Shunting of the anterior location can be an atypical feature.4 Perimedullary AVFs are characterized by direct shunts in the pia mater of the superficial spinal cord. They usually occur in the anterior or anterolateral aspect of the spinal cord that is fed by the ASA.4,5 For our patient, we initially thought that an old age at presentation and a fistula point near the root sleeve in a lateral spinal cord location were consistent with a right-sided dAVF, although not a typical appearance. In addition, a typical, left-sided dAVF was noted. Accordingly, endovascular treatment was conducted to obliterate both dAVFs. However, superselective angiography was performed immediately after the initial embolization demonstrated that the right-sided AVF was actually complex AVFs fed by both the dural arteries and pial arteries that originated from the ASA and assumed PSA, with medullary venous drainage.
The disease concept for AVFs with concomitant dural and pial shunts on the ipsilateral side, referred to as complex AVFs herein, has not been well understood. One explanation for complex AVFs that are fed by dural and spinal arteries is a metameric linkage. Rodesch et al. reported that multiple arteriovenous shunts in the spinal cord,8 in particular genetic nonhereditary lesions, were frequently associated with vascular malformations in the limbs. Matsumaru et al. showed that 16% of patients with spinal cord arteriovenous shunts were related to metameric lesions.9 However, our patient did not exhibit a metameric lesion associated with complex AVFs, although there was a contralateral typical dAVF. Nevertheless, multiple shunts in the same myelomere could favor the possibility of metameric lesions.4 Another explanation for the complex AVF is that it evolves from a dAVF into a more complex form with intradural shunts.4 The development of dural AVFs might be associated with venous hypertension. Lawton et al. reported that venous hypertension could induce angiogenesis that results in dAVFs in experimental models.10 Sato et al. postulated that venous hypoxia secondary to venous hypertension could induce additional pial shunts in patients with dAVFs at the craniocervical junction.3 A sump effect by the dAVFs could also evolve into complex AVFs. Hassler et al. suggested that venous drainage close to the nerve root sleeve could increase the sump effect.11 In such circumstances, blood supply originated from the radicular or radiculomedullary arteries may occur near the origin of the venous drainage.4,12 The absence of the C1 dorsal root may explain the complex angiographic architecture. Onda et al. reported two cases involving high cervical complex dural AVFs without the C1 dorsal root.4 In such cases, the anterior radicular branch of the VA could anastomose with the ASA and PSA4. For our patient, the C1 dorsal root was not observed during the operation. Accordingly, we think a serial connection of the radicular branch arteries with the right-sided PSA and left-sided ASA could attribute to the complex AVFs.
Kim et al. reported a similar complex AVF which presented with subarachnoid hemorrhage.13 The superselective angiographies of the C2 dural feeder from the right VA and ASA from the left VA revealed dural and pial shunts of the AVF with same venous drainage. However, AVF in our report showed dominant pial feeders from the ASA and PSA, and additional supply recruited from dural branches, combined with a coexistent dAVF on the opposite side. Considering that the main feeders to the shunt were from the pial branches of the ASA and PSA, we cannot rule out the possibility that one or a part of recruited arteries was a pial branch.
Small, tortuous feeding arteries and arteries originating from the VA at an acute angle can be technically challenging for endovascular treatment in patients with complex AVFs.3,14 Surgical disconnection and coagulation of the fistula could be a more appropriate treatment option for cases with a small shunt fistula, small tortuous feeding arteries, and a dominant pial AV shunt. Therefore, meticulous angiographic evaluation is necessary for accurate diagnosis and proper treatment for patients suspected of having an AVF at craniocervical junction.
Acknowledgments
The authors thank Dr. Roh-Eul Yoo and Dr. Sung-Eun Kim for their help with English editing and image preparation.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
References
- 1.Krings T, Geibprasert S. Spinal dural arteriovenous fistulas. Am J Neuroradiol 2009; 30: 639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Oshita J, Yamaguchi S, Ohba S, et al. Mirror-image spinal dural arteriovenous fistulas at the craniocervical junction: case report and review of the literature. Neurosurgery 2011; 69: E1166–E1171. [DOI] [PubMed] [Google Scholar]
- 3.Sato K, Endo T, Niizuma K, et al. Concurrent dural and perimedullary arteriovenous fistulas at the craniocervical junction: case series with special reference to angioarchitecture. J Neurosurg 2013; 118: 451–459. [DOI] [PubMed] [Google Scholar]
- 4.Onda K, Yoshida Y, Watanabe K, et al. High cervical arteriovenous fistulas fed by dural and spinal arteries and draining into a single medullary vein: report of 3 cases. J Neurosurg Spine 2014; 20: 256–264. [DOI] [PubMed] [Google Scholar]
- 5.Ohba S, Onozuka S, Horiguchi T, et al. Perimedullary arteriovenous fistula at the craniocervical junction – case report. Neurol Med Chir 2011; 51: 299–301. [DOI] [PubMed] [Google Scholar]
- 6.Aviv RI, Shad A, Tomlinson G, et al. Cervical dural arteriovenous fistulae manifesting as subarachnoid hemorrhage: report of two cases and literature review. Am J Neuroradiol 2004; 25: 854–858. [PMC free article] [PubMed] [Google Scholar]
- 7.Kinouchi H, Mizoi K, Takahashi A, et al. Dural arteriovenous shunts at the craniocervical junction. J Neurosurg 1998; 89: 755–761. [DOI] [PubMed] [Google Scholar]
- 8.Rodesch G, Hurth M, Alvarez H, et al. Classification of spinal cord arteriovenous shunts: proposal for a reappraisal–the Bicetre experience with 155 consecutive patients treated between 1981 and 1999. Neurosurgery 2002; 51: 374–379. [PubMed] [Google Scholar]
- 9.Matsumaru Y, Pongpech S, Laothamas J, et al. Multifocal and metameric spinal cord arteriovenous malformations. Review of 19 cases. Interv Neuroradiol 1999; 5: 27–34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lawton MT, Jacobowitz R, Spetzler RF. Redefined role of angiogenesis in the pathogenesis of dural arteriovenous malformations. J Neurosurg 1997; 87: 267–274. [DOI] [PubMed] [Google Scholar]
- 11.Hassler W, Thron A, Grote EH. Hemodynamics of spinal dural arteriovenous fistulas. An intraoperative study. J Neurosurg 1989; 70: 360–370. [DOI] [PubMed] [Google Scholar]
- 12.Garcia-Monaco R, Rodesch G, Terbrugge K, et al. Multifocal dural arteriovenous shunts in children. Child's Nerv Syst 1991; 7: 425–431. [DOI] [PubMed] [Google Scholar]
- 13.Kim DJ, Willinsky R, Geibprasert S, et al. Angiographic characteristics and treatment of cervical spinal dural arteriovenous shunts. Am J Neuroradiol 2010; 31: 1512–1515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mitsuhashi Y, Aurboonyawat T, Pereira VM, et al. Dural arteriovenous fistulas draining into the petrosal vein or bridging vein of the medulla: possible homologs of spinal dural arteriovenous fistulas. J Neurosurg 2009; 111: 889–899. [DOI] [PubMed] [Google Scholar]