Abstract
The most common primary malignant cardiac tumor is the cardiac sarcoma, which is mesenchymal in origin. This case report describes a patient with hyperacute stroke caused by cardiac sarcoma who underwent rapid recanalization through mechanical aspiration thrombectomy with Penumbra catheter.
Keywords: Cardiac tumor, stroke, thrombectomy
Introduction
Primary cardiac tumors are rare entities with reported incidences of 0.001% to 0.03%.1 The most common primary malignant cardiac tumor is the cardiac sarcoma, which is mesenchymal in origin.2 Signs and symptoms of cardiac sarcoma can include dyspnea on exertion, syncope, fever, weight loss, and severe dizziness in addition to valvular abnormalities, arrhythmias, and embolic phenomena. Cardioembolic stroke has been reported previously in patients with cardiac sarcoma.3–6 This report describes a patient with hyperacute stroke caused by embolized cardiac sarcoma who underwent rapid recanalization through mechanical aspiration thrombectomy (MAT) with Penumbra catheter.
Case report
A 55-year-old man with no history of hypertension, diabetes, atrial fibrillation, or coronary artery disease, was admitted to the hospital because of a cardiac tumor with multiple pulmonary nodules and splenic infarction on chest computed tomography (CT) scan (Figure 1(a)). The patient underwent percutaneous lung biopsy, and the tumor was confirmed as an unclassified sarcoma. He presented left hemiparesis 1 day after lung biopsy. The National Institute of Health Stroke Scale (NIHSS) score was 22. Non-contrast-enhanced brain CT showed no evidence of intracranial hemorrhage. Intravenous recombinant tissue plasminogen activator was administered 50 minutes after symptom onset, but no symptomatic improvement was seen.
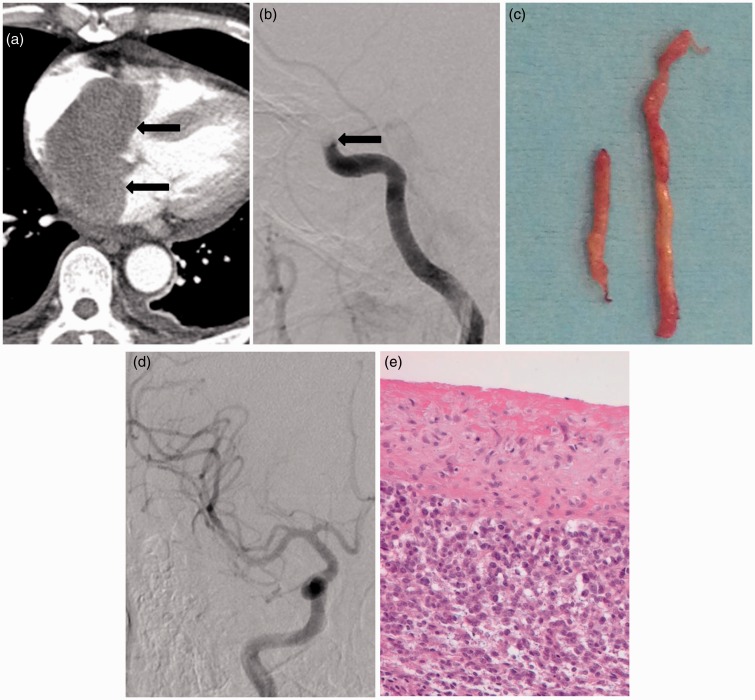
Figure 1.
A 55-year-old man with a cardiac tumor and hyperacute stroke of right middle cerebral artery territory.
(a) Contrast-enhanced chest CT scan shows a well-defined solid tumor in the left atrium (arrows).
(b) Cerebral angiography shows a complete occlusion of the right distal internal carotid artery (arrow).
(c) Gross appearance of emboli shows yellowish, gelatinous, and hard tissue.
(d) Final angiogram after aspiration thrombectomy using Penumbra catheter demonstrating complete recanalization of the right internal carotid artery and middle cerebral artery territories.
(e) Histologic section showing high-grade undifferentiated sarcoma (HE, x 400).
Diffusion-weighted imaging of the brain revealed hyperintense foci in the right basal ganglia, the left periventricular white matter and the left parietal lobe. Diffusion–perfusion mismatch was shown in the right middle cerebral artery (MCA) territory. Cerebral angiography for endovascular recanalization showed complete occlusion of the right distal internal carotid artery (ICA) (Figure 1(b)). Endovascular treatment was initiated 120 minutes after the onset of symptoms. A triple coaxial system assembled by combining an outermost 80 cm-long 8 F sheath (Shuttle-SL; Cook, Bloomington, IN, USA), a middle 100 cm-long 8 F guide catheter (Guider Softip; Stryker, Natick, MA, USA), and an inner 125 cm-long selection catheter (Headhunter; Cook, Bloomington, IN, USA), was placed in the common carotid artery and ICA for aspiration thrombectomy. The guiding catheter was placed in the distal ICA without vessel spasm. A double coaxial system assembled by combining an outer Penumbra catheter and an inner Rebar 18 micro-catheter (Covidien/ev3, Irvine, CA, USA) was advanced to the level of the thrombus using a 0.014 inch micro-guidewire (Synchro; Stryker Neurovascular, Freemont, CA, USA). At the level of the thrombus, the micro-guidewire and micro-catheter were advanced more distally through the thrombus up to distal MCA in order to get sufficient support to track the Penumbra reperfusion catheter. Thereafter, we gently advanced the Penumbra catheter into the thrombus until wedged tightly. Subsequently, the micro-catheter and micro-guidewire were removed and a 20 ml syringe was connected to the proximal hub of the Penumbra catheter (Figure 1(c)). Continuous manual aspiration was performed with maintaining the vacuum state between the tip of Penumbra catheter and the thrombus while gently withdrawing the Penumbra catheter through the guide catheter and resulted in recanalization (Figure 1(d)). Non-contrast CT scans of the brain, obtained immediately after the procedure, showed a small amount of intracerebral hemorrhage in the right basal ganglia. Mental status was improved immediately after the procedure. The thrombus retrieved from the intracranial artery was high-grade undifferentiated sarcoma and confirmed as a tumor thrombus (Figure 1(e)).
Discussion
Recently, several authors reported successful outcomes in patients with hyperacute ischemic stroke from cardiac myxomas which were treated with MAT.7–9 Based on review of current literature, this is the first report of endovascular MAT used for a stroke caused by a biopsy-confirmed cardiac sarcoma and tumor thrombus.
Primary cardiac sarcomas account for 30% of the primary malignant tumors of the heart.2 Cardiac sarcomas have been further classified into angiosarcomas, fibrosarcomas, rhabdomyosarcomas, leiomyosarcomas, liposarcomas, pleomorphic malignant fibrous histiocytomas/pleomorphic undifferentiated sarcomas, and synovial sarcomas based on the classification proposed by WHO in 2004.2 Primary cardiac sarcoma can arise at any age, with a reported mean age of 41 years. There is no specific sex predilection.6 Life expectancy in cardiac sarcoma patients is poor, with a median survival of 24 months after complete resection compared with a median survival of only 10 months in patients with incomplete or no tumor resection. Clinical manifestations occur due to different mechanisms including obstruction of blood flow through the heart, valvular dysfunction, local invasion causing arrhythmia or pericardial effusion with/without tamponade, tumor embolism and systemic involvement.6 Several cases of stroke secondary to embolized cardiac sarcomas have been described previously.3,5,6
More recent clinical trial results have been positive with improved outcomes of endovascular therapy using stent retrievers.10–12 Compared with the recent outcomes of stent-retrievers thrombectomy, the MAT technique we used displayed promising results in recanalization rate, favorable outcome, and safety.4,13–15 Kang et al.4 reported the clinical safety and effectiveness of forced suction thrombectomy using the Penumbra catheter in patients with acute intracranial obstruction. This modified technique resulted in 100% recanalization and a 45.5% favorable functional outcome, which is comparable with or better than the previous standard Penumbra technique. Also, short procedural times and rapid recanalization make the modified Penumbra technique a promising new innovation. In this case, MAT with Penumbra catheter was used and achieved complete recanalization. Emboli caused by a cardiac sarcoma may be composed of fragments of the myxoma itself, a thrombus, or both. The emboli in this case were yellow in color and a pathologic examination demonstrated a cellular and highly vascular undifferentiated spindle cell neoplasm.
Conclusion
In this case, MAT with Penumbra catheter was successfully used for a patient with a hyperacute infarction caused by embolized cardiac sarcoma. For hard emboli, aspiration thrombectomy may be considered a safe approach which allows for rapid recanalization.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Burke A and Virmani R. Tumors of the heart and great vessels. In: Atlas of tumor pathology. 3rd series. Washington (DC): American Registry of Pathology, 1996, pp. 1–98.
- 2.Hoffmerier A, Deiters S, Schmidt C, et al. Radical resection of cardiac sarcoma. Thorac Cardiovasc Surg 2004; 52: 77–81. [DOI] [PubMed] [Google Scholar]
- 3.Jassal DS, Thakrar A, Neilan TG, et al. Cardioembolic stroke in patient with spindle cell sarcoma of the left atrium. J Am Soc Echocardiogr 2007; 20: 438.e1–e4. [DOI] [PubMed] [Google Scholar]
- 4.Kang DH, Hwang YH, Kim YS, et al. Direct thrombus retrieval using the reperfusion catheter of the penumbra system: Forced-suction thrombectomy in acute ischemic stroke. AJNR Am J Neuroradiol 2010; 32: 283–287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pickering L, Cox I, Pandha H. Left atrial sarcoma presenting as cerebral infarction. Lancet Oncol 2001; 2: 705–706. [DOI] [PubMed] [Google Scholar]
- 6.Wu JC, Fishbein MC, Child JS. An unusual cause of stroke from a left atrial mass. J Am Soc Echocardiogr 2007; 20: 537.e1–e2. [DOI] [PubMed] [Google Scholar]
- 7.Baek SH, Park S, Lee NJ, et al. Effective mechanical thrombectomy in a patient with hyperacute stroke associated with cardiac myxoma. J Stroke Cerebrovasc Dis 2014; 23: e417–e4199. [DOI] [PubMed] [Google Scholar]
- 8.Garcia-Ptacek S, Matias-Guiu JA, Valencia-Sánchez C, et al. Mechanical endovascular treatment of acute stroke due to cardiac myxoma. J Neurointerv Surg 2014; 6: e1–e4. [DOI] [PubMed] [Google Scholar]
- 9.van den Wijngaard I, Wermer M, van Walderveen M, et al. Intra-arterial treatment in a child with embolic stroke due to atrial myxoma. Interv Neuroradiol 2104; 20: 345–351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Berkhemer OA, Fransen PSS, Beumer D, et al. a randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015; 372: 11–20. [DOI] [PubMed] [Google Scholar]
- 11.Campbell BCV, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015; 372: 1009–1018. [DOI] [PubMed] [Google Scholar]
- 12.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015; 372: 1019–1030. [DOI] [PubMed] [Google Scholar]
- 13.Jankowitz B, Aghaebrahim A, Zirra A, et al. Manual aspiration thrombectomy: Adjunctive endovascular recanalization technique in acute stroke interventions. Stroke 2012; 43: 1408–1411. [DOI] [PubMed] [Google Scholar]
- 14.Jankowitz B, Grandhi R, Horev A, et al. Primary manual aspiration thrombectomy (MAT) for acute ischemic stroke: Safety, feasibility and outcomes in 112 consecutive patients. J Neurointerv Surg 2015; 7: 27–31. [DOI] [PubMed] [Google Scholar]
- 15.Turk AS, Frei D, Fiorella D, et al. ADAPT FAST study: A direct aspiration first pass technique for acute stroke thrombectomy. J Neurointerv Surg 2014; 6: 260–264. [DOI] [PubMed] [Google Scholar]