Abstract
Background
The mechanisms leading to delayed rupture, distal emboli and intraparenchymal hemorrhage in relation to pipeline embolization device (PED) placement remain debatable and poorly understood. The aim of this study was to identify clinical and procedural predictors of these perioperative complications.
Methods
We conducted a retrospective review of consecutive patients who underwent PED placement. We utilized a non-commercial platelet aggregation method measuring adenosine diphosphate (ADP)% inhibition for evaluation of clopidogrel response. To our knowledge, this is the first study to test ADP in neurovascular procedures. Multivariable regression analysis was used to identify the strongest predictor of three separate outcomes: (1) thrombotic complications, (2) hemorrhagic complications, and (3) aneurysm mass effect exacerbation
Results
Permanent complication-related morbidity and mortality at 3 months was 6% (3/48). No specific predictors of hemorrhagic complications were identified. In the univariate analysis, the strongest predictors of thrombotic complications were: ADP % inhibition <49 (p = 0.01), aneurysm size (p = 0.04) and fluoroscopy time (p = 0.002). In the final multivariate analysis, among all baseline variables, fluoroscopy time exceeding 52 min was the only factor associated with thrombotic complications (p = 0.007). Aneurysm size ≥18 mm was the single predictor of mass effect exacerbation (p = 0.039).
Conclusions
Procedural complexity, reflected by fluoroscopy time, is the strongest predictor of thrombotic complications in this study. ADP% inhibition is a reliable method of testing clopidogrel response in neurovascular procedures and values of <50% may predict thrombotic complications. Interval mass effect exacerbation after PED placement may be anticipated in large aneurysms exceeding 18 mm.
Keywords: Aneurysm, flow diversion, pipeline, embolization, complications, intracranial hemorrhage, mass effect
Background and rationale
The pipeline embolization device (PED) recently gained worldwide acceptance based on several initial long-term observational studies, demonstrating a successful permanent intracranial aneurysm occlusion rate with acceptable morbidity and mortality.1,2 However, despite the promising initial results, there have been multiple case reports and studies describing immediate or delayed complications, including ischemic strokes, intraparenchymal hematomas and interval growth and aneurysm rupture following PED procedures.3-5 Various factors have been implied; however, the currently available data is limited and the precise pathophysiologic explanation for these complications remain unclear.
The aim of the present study is to further elucidate the mechanisms of PED-associated complications, based on individual clinical, anatomic, physiologic, and procedural characteristics in a cohort of consecutive patients treated with PED.
Methods
We conducted a retrospective analysis of consecutive patients who underwent PED procedures in our institution. The patients were included in the study if at least one PED was delivered successfully and only if pre-procedural adenosine diphosphate (ADP) values were available. Given these strict inclusion criteria, total of 45 patients who underwent 48 procedures were included in this study. The analyzed clinical and procedural factors included: age, history of hypertension, history of smoking, aneurysm size, aneurysm mass effect, ADP% inhibition, alternative antiplatelet regimen, fluoroscopy time, adjunctive coiling, post-PED angioplasty and aneurysm angiographic outcome. Aneurysm size was defined by 3D angiography. All patients received double antiplatelet therapy with aspirin 325 mg daily and clopidogrel 75 mg daily for at least three consecutive days prior to the day of the planned procedure. Thrombotic complications were defined as any intra-procedural events requiring escalation of care (such as intra-arterial infusion of antithrombotic medications), or any ischemic strokes causing clinical symptoms. Hemorrhagic complications were defined as any systemic hemorrhages requiring escalation of care (such as blood transfusions or prolonged ICU stay), and any SICH (intracranial hemorrhage (ICH) associated with clinical symptoms). Mass effect exacerbation was defined as any clinical symptoms attributable to a new or pre-existing aneurysm mass effect on adjacent cranial nerves or brain tissue. Patients were followed up closely within the first 30 days after the procedure and subsequently every 6–12 months via direct encounter, or via communication with the referring physician.
PED placement procedure
All procedures were performed under general anesthesia. Pre-intervention diagnostic angiogram was performed in standard bi-plane and 3D rotational projections (Phillips). Additional 3D angiographic reconstruction was used for procedural planning, including measurement of the parent vessel and the target aneurysm, as well as virtual device placement. 6–8 FR shuttle sheath, Navien intermediate catheter, and Marksman microcatheter was the most commonly used tiraxial system. Adjunctive coiling was performed via a jailed microcatheter in selected patients. Post-deployment angioplasty was performed when inadequate wall apposition was observed. All patients received intravenous heparin during the procedure.
Platelet aggregation assessment
Unlike the previously published studies,6,7 we utilized light transmission aggregometry as a non-commercial platelet aggregation testing, measuring clopidogrel-induced ADP% inhibition in comparison with healthy subjects in our hospital laboratory. This method is considered the “gold standard” in vitro testing of platelet aggregation.8 To our knowledge, ADP inhibition has not been studied in PED or any other intracranial procedures as a method for clopidogrel aggregation. This is the first article in the literature to investigate its significance in neuroendovascular procedures. The pre-procedural values of % inhibition were determined by comparison with a matched healthy subject naïve to antiplatelet medications, using the following formula: % inhibition = (control minus patient) divided by control × 100. Based on the widely variable values for ADP% inhibition, patients were divided into three groups in the present study: (1) poor responders: <50%, (2) satisfactory responders: ≥ 50% ≤ 75%, and (3) over responders: ≥ 75%. Some patients in the poor responders group were switched to an alternative antiplatelet regimen based on individual clinicians’ preference (either double clopidogrel dosage or a different ADP inhibitor, such as Ticagrelor or Prasugrel). These patients taking alternative antiplatelet therapy were categorized in a separate group.
This study was approved by the institutional review board of the local institution and was conducted in compliance with the Health Information Portability and Accountability Act.
Statistical analysis
Univariate comparisons between categorical variables were made using Fisher’s exact test and the chi-square test, and between continuous variables using the Mann–Whitney U test. All variables from the univariate analysis with p ≤ 0.2 were selected for inclusion in the step-wise logistic regression model. The logistic regression equation was used to determine specific cut off values for continuous variables (ADP, fluoroscopy time, and aneurysm size) included in the model
Results
A total of 45 patients who underwent 48 consecutive procedures met the study criteria. Clinical and procedural details, outcomes, and complications are summarized in Table 1. Long-term clinical data was available in 96% (46/48), and long-term angiographic data was available in 94% (45/48) of all performed procedures (one patient expired and two patients were lost to follow-up after the initial 30 days visit). The average clinical follow-up duration was 1142 days, or 3 years and 1 month. The overall morbidity and mortality in our cohort was 6.3% (3/48).
Table 1.
Summary of clinical and procedural details, outcomes and complications.
| Clinical and procedural details | |
|---|---|
| Age (median) | 58 |
| Female | 76% (35/46) |
| Aneurysm size (median) | 14.5 (2–38) mm |
| Post-PED placement angioplasty | 44% (21/48) |
| Adjunctive coiling | 27% (13/48) |
| Number of PED/patient (median) | 1.3 (1–5) |
| Fluoroscopy time (median) | 39 (15–314) min |
| Outcomes | |
| Occlusion rate, 3–6 months | 78% (35/45) |
| Mass effect exacerbation | 30% (5/15) |
| Symptomatic improvement of mass effect on cranial nerves | 46% (7/15) |
| Complications | |
| Delayed rupture (SAH) | 2% (1/48) |
| Intraparenchymal hematoma | 4% (2/48) |
| Symptomatic intracranial hemorrhage | 6% (3/48) |
| Thrombotic complications | 12.5% (6/48) |
| Hemorrhagic complications | 17% (8/48) |
| Symptomatic ischemic stroke | 6% (3/48) |
| Any stroke affecting mRS at 90 days | 6% (3/48) |
Hemorrhagic complications
Hemorrhagic complications, including intracranial and systemic hemorrhage requiring escalation of care, occurred in 15% (7/48) of all procedures, including 2/48 (4%) patients with symptomatic intraparenchymal hematomas (IPH). Both IPH were located in a remote vascular territory in reference to the treated aneurysm, one of which was fatal. Symptomatic ICH occurred in 3/48 procedures (6%) (Table 1). Given the small number of ICH, and higher number of hemorrhagic complications (including systemic hemorrhages requiring escalation of care), we did two separate analyses: SICH alone, and hemorrhagic complications (presumably induced by dual antiplatelet therapy (DAPT)). No predictors of either hemorrhage type were identified.
Thrombotic complications
Thrombotic complications occurred at a rate of 13% (6/48), of which only 3/48 (6%) were symptomatic. In the initial univariate analysis, the strongest predictors of thrombotic complications included: (1) ADP% inhibition as a continuous variable with a cutoff value of 49%, (2) poor clopidogrel response pre-defined as < 50% ADP inhibition, (3) aneurysm size, and (4) fluoroscopy time. In the final multivariate analysis, among all baseline clinical and procedural variables, fluoroscopy time exceeding 52 min was the only factor associated with thrombotic complications (Table 2).
Table 2.
Thrombotic complications analysis.
| Thrombotic complications: univariate analysis | ||
|---|---|---|
| Factors | p-value | |
| AGE | 0.07 | |
| HTN | 0.67 | |
| Smoking | 0.62 | |
| Aneurysm size | 0.04 | |
| Mass effect | 0.09 | |
| ADP% inhibition (<49) | 0.01 | |
| ADP ≥75% | 0.08 | |
| ADP 50–75% | 0.55 | |
| ADP <50% | 0.05 | |
| Double plavix or alternative agent | 0.16 | |
| Coils | 0.13 | |
| Fluoroscopy time | 0.002 | |
| Angioplasty | 0.16 | |
| Thrombotic complications: Logistic regression | ||
| Fluoroscopy time | OR 1.061 95% CI (1.009–1.116) | 0.007 |
Mass effect evolution
We found that 15/46 (33%) of patients had mass effect prior to PED placement. Of the five patients who had new or worsened mass effect, four experienced complete symptomatic resolution, while one had a delayed aneurysm rupture requiring vessel sacrifice (Figure 1). Some 7/15 (46%) patients with pre-existing mass effect eventually experienced symptomatic improvement. Among all baseline clinical and procedural factors, the strongest predictor of new or worsened mass effect was aneurysm size ≥ 18 mm (p = 0.039; OR 1.121; 95% CI 1.006–1.250) (Table 3).
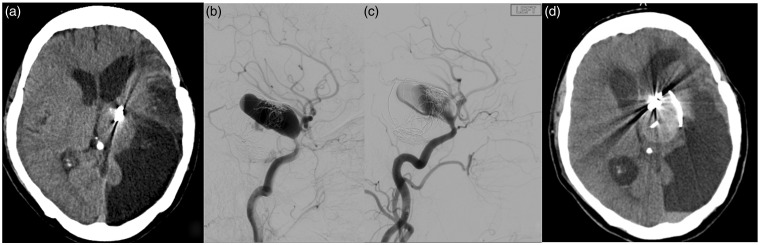
Figure 1.
Mass effect exacerbation and delayed rupture.
Middle-aged patient with previously ruptured and partially coiled giant Left P-comm aneurysm. The prior hospitalization was complicated by symptomatic vasospasm resulting in ischemic stroke. The patient was lost to follow-up for many years and eventually presented to our clinic with worsened R hemiparesis and partial 3rd cranial nerve palsy due to mass effect on the midbrain (a) from the recanalized giant aneurysm ((b) Pre-PED angiogram). PED embolization resulted in significant intra-aneurysmal contrast stagnation ((c) Post-PED angiogram). However, the patient presented to ED 5 months later with worse R hemiparesis and obtundation due to interval aneurysm growth and rupture (d).
Table 3.
Mass effect exacerbation analysis.
| Mass effect exacerbation: univariate analysis | ||
|---|---|---|
| Factors | p-value | |
| AGE | 0.2 | |
| HTN | 0.68 | |
| Smoking | 0.68 | |
| Aneurysm size | 0.022 | |
| Mass effect | 0.14 | |
| ADP | 0.39 | |
| ADP >75 | 0.76 | |
| ADP 50–75 | 0.14 | |
| ADP <50 | 0.74 | |
| Double plavix or alternative agent | 0.29 | |
| Coils | 0.175 | |
| Fluoroscopy time | 0.97 | |
| Angioplasty | 0.08 | |
| Mass effect exacerbation: Logistic regression | ||
| Aneurysm size | OR 1.121 95% CI (1.006–1.250) | p = 0.039 |
Discussion
Thrombotic complications
The most frequently detected complication type in our study was thrombotic, and was observed either during the procedure (device thrombosis requiring additional intra-arterial antithrombotic infusion), or within the immediate postoperative period (symptomatic ischemic strokes). Given the unique construct of this flow-diverting device, these finding are not unexpected. The PED is composed of bimetallic cobalt chromium and platinum tungsten with approximately 30% porosity, resulting in much higher metal-to-wall coverage and increased thrombogenicity as compared with all other intracranial stents. As such, thromboembolic events were anticipated and we took the extra precaution of measuring the degree of platelet aggregation in each individual patient prior to the planned procedure. We pre-defined poor clopidogrel response as ADP < 50%. The accuracy of this value for predicting thrombotic complication was confirmed in our univariate analysis: we identified ADP cutoff value of 49%, which nearly matched our pre-specified definition of poor clopidogrel response as ADP < 50%. We also found strong correlation with ADP% as a continuous variable.
Among all baseline characteristics included in the final multivariate analysis, procedural duration (reflected by fluoroscopy time) had the strongest impact on thrombotic complications in our cohort. Similar findings have been demonstrated by Tan et al.9 This phenomenon can be explained by prolonged deployment time in a setting of challenging anatomy, associated with prolonged exposure of partially or improperly unsheathed device. Achieving optimal flow diversion is heavily dependent on adequate delivery and deployment with optimal wall apposition. Given the braided nature of the device, inadequate deployment may lead to great variability in the arterial wall coverage, particularly in high degree of curved arterial segments, complex fusiform and large dysplastic saccular aneurysms, and locations associated with significant changes in the parent vessel diameter.10 Cases with such challenging anatomic features may require longer deployment time for achieving optimal PED placement. In addition, similarly to all other endovascular procedures, obtaining proper access is key for procedural success. Difficult aortic arch and proximal CCA/ICA tortuosity can be independent factors associated with higher risk of thromboembolic complications, as evidenced by prior experience with carotid stenting.11 As such, the results of our study confirm that thrombotic complications in relation to PED are directly influenced by the procedural complexity, and less strongly associated with poor clopidogrel response, defined as ADP < 49%.
Hemorrhagic complications
Despite the relatively high occurrence of hemorrhagic complications, no specific clinical or procedural predictors were identified. We also did not find correlation between alternative antiplatelet regimen and hemorrhagic complication as reported by Akbari et al.12 Although our data does not demonstrate direct correlation between the clopidogrel-induced platelet inhibition measured by ADP, the systemic coagulopathic effect of the DAPT is likely dependent on individual physiologic characteristics and varies among different patients. The increased hemorrhagic risk of DAPT with aspirin plus clopidogrel has been well established in large-scale randomized trials.13 However, the reported rate of moderate to severe bleeding in these trials does not exceed 5%, which is significantly lower than the 15% rate observed in our cohort. These findings warrant further investigation regarding a possible procedure-related coagulopathic effect and associated increased hemorrhagic risk.
Intraparenchymal hematoma (IPH) occurred in 2/48 (4%) procedures in our cohort, one of which was fatal (Figure 2). Similar to other studies, this complication type was the most serious and unfortunately unpredictable.4,14 Both IPH in our cohort were delayed (3 days and 3 weeks after the PED procedure, respectively), and occurred in the contralateral hemisphere in reference to the treated aneurysms. There were no specific technical difficulties during the procedure and the precise mechanism leading to this serious and sometimes fatal complication remains unclear. Several plausible mechanisms for delayed IPH after PED have been proposed, including thromboemboli causing ischemic stroke and subsequent hemorrhagic transformation, foreign body embolization, hemodynamic alteration with loss of compliance within the parent vasculature, and hemorrhagic tendency due to DAPT.4,14,15 Although most reported IPH occur within the ipsilateral hemisphere in reference to the treated aneurysm, contralateral hematomas have also been described.16 Both patients with IPH in our cohort had prominent communicating arteries and, despite the remote contralateral location, these mechanisms are potentially applicable to our patients. It is also important to emphasize that both intraparenchymal hematomas observed in this cohort occurred in patients with other systemic hemorrhages, indicating causal association with the DAPT-induced systemic coagulopathy. Potential correlation can be made with delayed fatal intracranial hemorrhage (ICH) occurrence after carotid artery stenting (CAS), which is also associated with distal embolization, DAPT-induced coagulopathy, and hemodynamic changes within the parent vasculature.17 However, the rate of symptomatic ICH in CAS is only 0.3–0.5%.18 This major discrepancy of ICH occurrence between CAS and PED can be potentially attributed to higher incidence of thrombotic complications with PED and subsequent hemorrhagic conversion. Although the pathophysiologic mechanism of intracranial hematoma with the PED procedure is likely multifactorial, distal thromboemboli may play pivotal role in its occurrence (Figure 3).
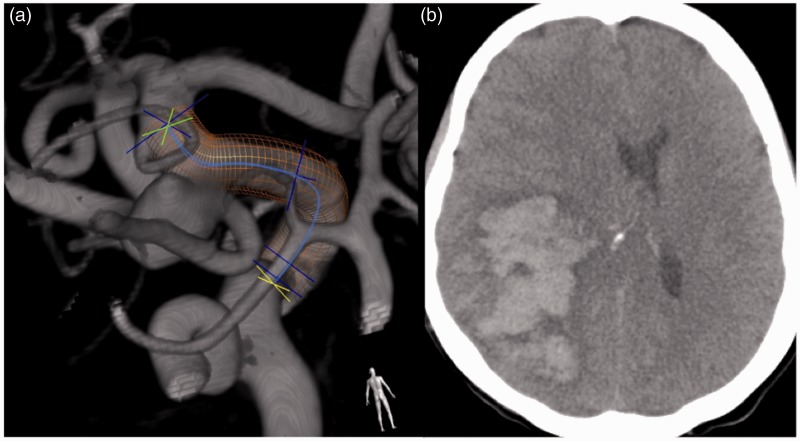
Figure 2.
Contralateral symptomatic ICH
Middle-aged patient with incidentally discovered 9 mm LEFT P-comm aneurysm (a). The patient had a large groin hematoma requiring transfusion and close ICU monitoring after the procedure. On POD #3 the patient had an acute L hemiparesis and became obtunded. Computed tomography demonstrated large RIGHT hemispheric ICH, resulting in fatal outcome (b).
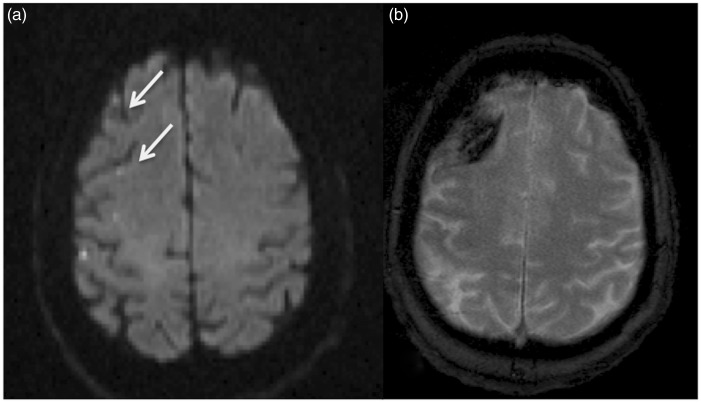
Figure 3.
Hemorrhage at site of the initial ischemic infarct.
Elderly patient with R carotid ophthalmic aneurysm. The patient had transient L arm drift on POD # 1. MRI demonstrated several foci of restricted diffusion in the R frontal lobe (a). The patient was readmitted 6 months later with seizures and repeat MRI demonstrated hemorrhage within the initially noted ischemic infarcts (b).
Transient mass effect exacerbation
Mass effect exacerbation has not been studied systematically in any endovascular embolization procedures for intracranial aneurysms. This phenomenon has been described only in isolated case reports with flow diversion and coil embolization.19-23 To our knowledge, this is the first article to investigate this phenomenon in multivariate regression model.
In parallel with the previously described aneurysmal wall changes after endosaccular embolization, a plausible pathophysiologic explanation of such occurrence is hemodynamically induced intra- and perianeurysmal inflammatory changes within an already partially thrombosed large and giant aneurysmal sac, leading to adventitial neovascularization, mural weakening and ultimately delayed rupture.24 One of the mechanisms by which flow diversion may lead to increased mural inflammatory response is through wall shear disturbance, which renders the vascular endothelium susceptible to accelerated inflammation by altered expression of pro-inflammatory and oxidant/anti-oxidant pathways, as well as low expression of major protective factors.25 Despite the traditional focus on vascular lumen, there is substantial evidence that the pathology underlying the formation, growth and rupture of an aneurysm likely resides within the vessel wall.26 The presence of partial thrombosis within the aneurysm sac, typically seen in large and giant aneurysms, has been independently associated with risk of rupture due to enhanced inflammation, as evidenced by distinct imaging findings of wall enhancement and perifocal edema.27 Some authors correlated such pathological factors leading to rupture of partially thrombosed intracranial aneurysm with Abdominal Aortic Aneurysm (AAA), harboring intraluminal thrombus (ILT).24,28 The process leading to deposition and progression of ILT in AAA has been associated with low oscillatory shear stress.29 In intracranial aneurysms, low shear stress and decreased flow conditions are inversely proportional to the aneurysm size.30 Our data confirms that the aneurysm size remains the key factor in predicting perifocal inflammatory response and potential wall fragility after flow diversion, manifested clinically by new or exacerbated mass effect.
Limitations
One of the aims of this study was to investigate the ADP values as the only available non-commercial method of platelet aggregation. Although the total number of PED patients treated by the group is approaching 100, we did not include many patients from our satellite hospital, where the platelet aggregation was studied via the usual commercial methods. Given the strict inclusion criteria, the main limitation of our study is the relatively small number of analyzed procedures (N = 48) and the retrospective review of the prospectively collected data. Another limitation is the possibility of erroneous significance of the results due to the multiple analyses in this relatively small cohort of patients.
Conclusions
The overall rates of symptomatic post-procedural complications and permanent complication-related morbidity of 6.3% in our cohort is similar to the reported data in recent meta-analysis of endovascular treatment of intracranial aneurysms with flow diverters.31 Complete thrombosis at 6 months after PED was 78%, which is similar to other previously reported data.1,2,32,33 Our study confirms that PED embolization remains the treatment of choice for challenging intracranial aneurysm not amenable to alternative endovascular or surgical treatment, with acceptable success and complications rate. In addition, despite the transient exacerbation and/or de novo mass effect occurrence in large and giant intracranial aneurysms, PED ultimately led to complete resolution of symptomatic mass effect with 46% success rate in our cohort.
Our study provides important insights into the potential mechanisms leading to periprocedural complications associated with intracranial flow diversion. Moreover, our findings can be utilized in clinical practice for prediction and avoidance of such complications, pre-procedural patient counseling and ultimately improvement of clinical outcome:
Procedural complexity, reflected by the prolonged duration, in combination with suboptimal platelet aggregation can lead to increased risk of thrombotic complication. Extra precautions, such as proximal embolic protection and alternative antiplatelet regimen may be appropriate for such patients with challenging anatomy and poor platelet aggregation.
ADP% inhibition is a reliable method of testing clopidogrel response in neurovascular procedures and values of <50% may predict thrombotic complications.
New or worsened mass effect after PED placement may be expected in patients with intracranial aneurysm exceeding 18 mm in size. Although this phenomenon is uncommon and transient in most cases, physicians should be aware of it as perioperative administration of steroids and close follow-up may be warranted.
Acknowledgments
This study was approved by the institutional review board of the local institution and was conducted in compliance with the Health Information Portability and Accountability Act. The research materials related to this paper are stored in password-protected files and are available upon request.
Funding
The authors received no financial support for the research, authorship, and/or publication of this article.
Declaration of conflicting interests
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Lylyk P, Miranda C, Ceratto R, et al. Curative endovascular reconstruction of cerebral aneurysms with the pipeline embolization device: The Buenos Aires experience. Neurosurgery 2009; 64: 632–642. discussion 42–3; quiz N6. [DOI] [PubMed] [Google Scholar]
- 2.Nelson PK, Lylyk P, Szikora I, et al. The pipeline embolization device for the intracranial treatment of aneurysms trial. AJNR Am J Neuroradiol 2011; 32: 34–40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lubicz B, Collignon L, Raphaeli G, et al. Pipeline flow-diverter stent for endovascular treatment of intracranial aneurysms: Preliminary experience in 20 patients with 27 aneurysms. World Neurosurg 2011; 76: 114–119. [DOI] [PubMed] [Google Scholar]
- 4.Velat GJ, Fargen KM, Lawson MF, et al. Delayed intraparenchymal hemorrhage following pipeline embolization device treatment for a giant recanalized ophthalmic aneurysm. J Neurointerv Surg 2012; 4: e24. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui AH, Kan P, Abla AA, et al. Complications after treatment with pipeline embolization for giant distal intracranial aneurysms with or without coil embolization. Neurosurgery 2012; 71: E509–E513; discussion E13. [DOI] [PubMed] [Google Scholar]
- 6.Delgado Almandoz JE, Crandall BM, Scholz JM, et al. Pre-procedure P2Y12 reaction units value predicts perioperative thromboembolic and hemorrhagic complications in patients with cerebral aneurysms treated with the Pipeline Embolization Device. J Neurointerv Surg 2013; 5(Suppl 3): iii3–iii10. [DOI] [PubMed] [Google Scholar]
- 7.McTaggart RA, Choudhri OA, Marcellus ML, et al. Use of thromboelastography to tailor dual-antiplatelet therapy in patients undergoing treatment of intracranial aneurysms with the Pipeline embolization device. J Neurointerv Surg 2015; 7: 425–430. [DOI] [PubMed] [Google Scholar]
- 8.Frontroth JP. Light transmission aggregometry. Methods Mol Biol 2013; 992: 227–240. [DOI] [PubMed] [Google Scholar]
- 9.Tan LA, Keigher KM, Munich SA, et al. Thromboembolic complications with Pipeline Embolization Device placement: Impact of procedure time, number of stents and pre-procedure P2Y12 reaction unit (PRU) value. J Neurointerv Surg 2015; 7: 217–221. [DOI] [PubMed] [Google Scholar]
- 10.Shapiro M, Raz E, Becske T, et al. Variable porosity of the pipeline embolization device in straight and curved vessels: A guide for optimal deployment strategy. AJNR Am J Neuroradiol 2014; 35: 727–733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Werner M, Bausback Y, Braunlich S, et al. Anatomic variables contributing to a higher periprocedural incidence of stroke and TIA in carotid artery stenting: Single center experience of 833 consecutive cases. Catheter Cardiovasc Interv 2012; 80: 321–328. [DOI] [PubMed] [Google Scholar]
- 12.Akbari SH, Reynolds MR, Kadkhodayan Y, et al. Hemorrhagic complications after prasugrel (Effient) therapy for vascular neurointerventional procedures. J Neurointerv Surg 2013; 5: 337–343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Berger PB, Bhatt DL, Fuster V, et al. Bleeding complications with dual antiplatelet therapy among patients with stable vascular disease or risk factors for vascular disease: Results from the Clopidogrel for High Atherothrombotic Risk and Ischemic Stabilization, Management, and Avoidance (CHARISMA) trial. Circulation 2010; 121: 2575–2583. [DOI] [PubMed] [Google Scholar]
- 14.Cruz JP, O'Kelly C, Kelly M, et al. Pipeline embolization device in aneurysmal subarachnoid hemorrhage. AJNR Am J Neuroradiol 2013; 34: 271–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hu YC, Deshmukh VR, Albuquerque FC, et al. Histopathological assessment of fatal ipsilateral intraparenchymal hemorrhages after the treatment of supraclinoid aneurysms with the Pipeline Embolization Device. J Neurosurg 2014; 120: 365–374. [DOI] [PubMed] [Google Scholar]
- 16.Chitale R, Gonzalez LF, Randazzo C, et al. Single center experience with pipeline stent: Feasibility, technique, and complications. Neurosurgery 2012; 71: 679–691. discussion 91. [DOI] [PubMed] [Google Scholar]
- 17.Day JS, Adams HP Jr. Delayed catastrophic intracerebral hemorrhage preceded by progressive recovery after carotid stenting for acute ischemic stroke. J Stroke Cerebrovasc Dis 2012; 21: 151–154. [DOI] [PubMed] [Google Scholar]
- 18.Clark WM, Brott TG. Intracranial hemorrhage complicating carotid artery stenting and carotid endarterectomy. Stroke 2011; 42: 2720–2721. [DOI] [PubMed] [Google Scholar]
- 19.Hirasawa T, Tsubokawa T, Katayama Y, et al. Growth of a giant aneurysm following complete thrombosis by detachable balloon occlusion. Surg Neurol 1992; 38: 283–286. [DOI] [PubMed] [Google Scholar]
- 20.Iihara K, Murao K, Sakai N, et al. Continued growth of and increased symptoms from a thrombosed giant aneurysm of the vertebral artery after complete endovascular occlusion and trapping: The role of vasa vasorum. Case report. J Neurosurg 2003; 98: 407–413. [DOI] [PubMed] [Google Scholar]
- 21.Dehdashti AR, Thines L, Willinsky RA, et al. Symptomatic enlargement of an occluded giant carotido-ophthalmic aneurysm after endovascular treatment: The vasa vasorum theory. Acta Neurochir (Wien) 2009; 151: 1153–1158. [DOI] [PubMed] [Google Scholar]
- 22.Meckel S, McAuliffe W, Fiorella D, et al. Endovascular treatment of complex aneurysms at the vertebrobasilar junction with flow-diverting stents: Initial experience. Neurosurgery 2013; 73: 386–394. [DOI] [PubMed] [Google Scholar]
- 23.Hammoud D, Gailloud P, Olivi A, et al. Acute vasogenic edema induced by thrombosis of a giant intracranial aneurysm: A cause of pseudostroke after therapeutic occlusion of the parent vessel. AJNR Am J Neuroradiol 2003; 24: 1237–1239. [PMC free article] [PubMed] [Google Scholar]
- 24.Kulcsar Z, Houdart E, Bonafe A, et al. Intra-aneurysmal thrombosis as a possible cause of delayed aneurysm rupture after flow-diversion treatment. AJNR Am J Neuroradiol 2011; 32: 20–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Davies PF, Civelek M, Fang Y, et al. The atherosusceptible endothelium: Endothelial phenotypes in complex haemodynamic shear stress regions in vivo. Cardiovasc Res 2013; 99: 315–327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Krings T, Mandell DM, Kiehl TR, et al. Intracranial aneurysms: From vessel wall pathology to therapeutic approach. Nat Rev Neurol 2011; 7: 547–559. [DOI] [PubMed] [Google Scholar]
- 27.Krings T, Alvarez H, Reinacher P, et al. Growth and rupture mechanism of partially thrombosed aneurysms. Intervent Neuroradiol 2007; 13: 117–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chow M, McDougall C, O'Kelly C, et al. Delayed spontaneous rupture of a posterior inferior cerebellar artery aneurysm following treatment with flow diversion: A clinicopathologic study. AJNR Am J Neuroradiol 2012; 33: E46–E51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Arzani A, Suh GY, Dalman RL, et al. A longitudinal comparison of hemodynamics and intraluminal thrombus deposition in abdominal aortic aneurysms. Am J Physiol Heart Circ Physiol 2014; 307: H1786–H1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tateshima S, Chien A, Sayre J, et al. The effect of aneurysm geometry on the intra-aneurysmal flow condition. Neuroradiology 2010; 52: 1135–1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Brinjikji W, Murad MH, Lanzino G, et al. Endovascular treatment of intracranial aneurysms with flow diverters: A meta-analysis. Stroke 2013; 44: 442–447. [DOI] [PubMed] [Google Scholar]
- 32.Kallmes DF, Hanel R, Lopes D, et al. International retrospective study of the pipeline embolization device: A multicenter aneurysm treatment study. AJNR Am J Neuroradiol 2015; 36: 108–115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Becske T, Kallmes DF, Saatci I, et al. Pipeline for uncoilable or failed aneurysms: Results from a multicenter clinical trial. Radiology 2013; 267: 858–868. [DOI] [PubMed] [Google Scholar]