Abstract
Background
We aimed to evaluate the incidence, predictive factors, and impact of acute kidney injury (AKI) after thoracic endovascular aortic repair (TEVAR).
Methods
A total of 53 patients who underwent 57 TEVAR operations between 2008 and 2015 were reviewed for the incidence of AKI as defined by the RIFLE (risk, injury, failure, loss, and end-stage kidney disease risk) consensus criteria. The estimated glomerular filtration rate was determined in the perioperative period. Comorbidities and postoperative outcomes were retrospectively reviewed.
Results
Underlying aortic pathologies included 21 degenerative aortic aneurysms, 20 blunt traumatic aortic injuries, six type B aortic dissections, five type B intramural hematomas, three endoleaks and two miscellaneous diseases. The mean age of the patients was 61.2±17.5 years (range, 15 to 85 years). AKI was identified in 13 (22.8%) of 57 patients. There was an association of preoperative stroke and postoperative paraparesis and paraplegia with AKI. The average intensive care unit (ICU) stay in patients with AKI was significantly longer than in patients without AKI (5.3 vs. 12.7 days, p=0.017). The 30-day mortality rate in patients with AKI was significantly higher than patients without AKI (23.1% vs. 4.5%, p=0.038); however, AKI did not impact long-term survival.
Conclusion
Preoperative stroke and postoperative paraparesis and paraplegia were identified as predictors for AKI. Patients with AKI experienced longer average ICU stays and greater 30-day mortality than those without AKI. Perioperative identification of high-risk patients, as well as nephroprotective strategies to reduce the incidence of AKI, should be considered as important aspects of a successful TEVAR procedure.
Keywords: Thoracic endovascular aortic repair, Acute kidney injury, RIFLE
INTRODUCTION
Thoracic endovascular aortic repair (TEVAR) has emerged as an alternative to conventional open surgery for treatment of various thoracic aortic diseases such as degenerative aortic aneurysm, type B aortic dissection, and type B intramural hematoma. The suggested advantages of TEVAR include shorter operative time, reduced duration of general anesthesia, shorter hospital stay, less blood loss, and avoidance of cardiopulmonary bypass, aortic cross clamping, invasive thoracotomy, thoracoabdominal incision, and hypothermic arrest [1–3]. The incidence of acute kidney injury (AKI) after TEVAR has been reported to be 1% to 34% [4]. This relatively common complication of TEVAR is associated with prolonged hospital stays and increased risk of mortality [5–7]. The goal of this clinical research was to investigate the risk factors and impact of AKI after TEVAR.
METHODS
1) Patient population and procedural details
Between April 2008 and February 2015, 53 patients underwent 57 TEVAR operations at our institution. Two of these 53 patients suffered from post-TEVAR endoleak and two other patients were operated on for progressive new-onset penetrating aortic ulceration. The 57 TEVAR cases consisted of 21 aortic aneurysms, 20 traumatic aortic injuries, six type B aortic dissections, five Type B intramural hematomas, three endoleaks and two miscellaneous diseases (Behcet’s aortitis and anastomotic pseudoaneurysm); these details and the location of the TEVAR are described in Tables 1 and 2.
Table 1.
Underlying pathology in patients undergoing thoracic endovascular aortic repair (N=57)
| Aortic pathology | Value |
|---|---|
| Aortic aneurysm | 21 (36.8) |
| Traumatic aortic injury | 20 (35.1) |
| Type B aortic dissection | 6 (10.5) |
| Intramural hematoma | 5 (8.8) |
| Endoleak | 3 (5.3) |
| Miscellaneous | 2 (3.5) |
Values are presented as number (%).
Table 2.
Aortic aneurysm classification according to location (N=21)
| Aneurysm location | Value |
|---|---|
| Aortic arch | 8 (38.1) |
| Proximal descending aorta | 2 (9.5) |
| Descending thoracic aorta | 11 (52.4) |
| Thoracoabdominal aorta | 0 |
Values are presented as number (%).
To reduce postoperative AKI, patients were given 60 mL/hr hydration fluid from the day before surgery, and 600 mg N-acetylcysteine was intravenously injected 12 hours before and just prior to surgery. Iodixanol (Visipaque; GE Healthcare, Milwaukee, WI, USA), an iso-osmolar-contrast medium with reduced nephrotoxicity, was injected during angiogram by contrast media injector (Mark V Provis; Medrad Inc., Indianola, PA, USA).
All patients underwent TEVAR operations using a portable C-arm fluoroscopic device (OEC 9900 Elite, GE Healthcare) under general anesthesia. To reduce postoperative paraparesis or paraplegia, lumbar cerebrospinal fluid (CSF) drainage was performed in all except three patients: in one case, the patient’s bleeding tendency was very high and the other two had cervical and lumbar spine fractures. During the operation, CSF pressure was sustained at 12 cm H2O and CSF drainage was maintained postoperatively for three days. The femoral artery was cannulated with a percutaneous sheath through a small longitudinal incision. A sheath was introduced percutaneously on the contralateral side. To accurately identify the site of deployment, a pigtail catheter was introduced into a percutaneous sheath and a soft guidewire was introduced into the cannulated sheath. Using a Bern catheter, the soft guide wire was then exchanged for a stiff one; subsequently, an endovascular stent graft (S & G Biotech, Seoul, Korea) was introduced to the target lesion. To prevent the stent graft from being pushed back, systolic blood pressure (BP) was maintained below 100 mmHg during the deployment. The proximal landing zone was secured at least 2 cm proximal to the site of the intimal tear. After stent graft deployment, an angiogram was performed to assess the outcome and mean BP was maintained above 90 mmHg to ensure cerebrospinal perfusion. After the procedure, patients were transferred to the intensive care unit (ICU).
2) Definitions
The baseline estimated glomerular filtration rate (eGFR) was calculated by the Cockcroft-Gault equation (eGFR= sex×[140-age]/serum creatinine×weight/72, where male sex=1 and female=0.85). Patients with AKI were defined by a 25% decrease in eGFR or a ≥1.5-fold increase in serum creatinine, compared with baseline, up to 48 hours following the procedure (risk, injury, failure, loss, and end-stage kidney disease [RIFLE] classification) (Table 3), or the need for continuous renal replacement therapy during hospitalization. Chronic renal failure was defined as a baseline eGFR ≤60 mL/min/1.73 m2. Symptoms were classified as acute phase if they occurred within the 14 days prior to TEVAR and as chronic phase if TEVAR was conducted 14 days after symptoms first occurred. A complicated dissection was defined as a case in which the patient had continuous or repeated back pain despite maximal antihypertensive therapy, uncontrolled hypertension, malperfusion syndromes, or (imminent) rupture [8].
Table 3.
RIFLEa) criteria for the classification of acute kidney injury
| Class | GFR criteria |
|---|---|
| Risk | Plasma creatinine increase 1.5×from baseline or GFR decline >25% |
| Injury | Plasma creatinine increase 2×from baseline or GFR decline >50% |
| Failure | Plasma creatinine increase 3×from baseline or GFR decline >75% or acute plasma creatinine >4 mg/dL |
| Loss | Persistent acute renal failure=complete loss of kidney function requiring dialysis for >4 weeks but >3 months |
| End-stage | End-stage kidney disease requiring dialysis for >3 months |
GFR, glomerular filtration rate.
Risk, injury, failure, loss, and end-stage kidney disease risk.
3) Statistical analysis
IBM SPSS Statistics software ver. 21.0 (IBM Co., Armonk, NY, USA) was used for statistical analysis of the data. Categorical variables were analyzed using a chi-square test and continuous variables were analyzed using a two-sample t-test. Variables with a p-value of less than 0.1 in univariate analysis were included in a multivariate analysis. Survival analysis was performed using the Kaplan-Meier log-rank method.
RESULTS
1) Patient characteristics
The mean age of the patients was 61.2±17.5 years (range, 15 to 85 years) and 46 of them were male. The mean pre-operative eGFR was 79.4±32.1 mL/min/1.73 m2. The underlying disease(s) and baseline characteristics of patients undergoing TEVAR are shown in Table 4. Common morbidities were hypertension (68.4%), current smoking (42.1%), hyperlipidemia (19.3%), diabetes (17.5%), previous stroke (14.0%), and coronary artery disease (8.8%).
Table 4.
Demographics and comorbidities
| Variable | Non-AKI (n=44) | AKI (n=13) | Total (n=57) | p-value |
|---|---|---|---|---|
| Age (yr) | 60.3±17.7 | 64.4±17.5 | 0.469 | |
| Sex (female) | 7 (15.9) | 4 (30.8) | 11 (19.3) | 0.233 |
| HTN | 29 (65.9) | 10 (76.9) | 39 (68.4) | 0.453 |
| HTN grade 1 (SBP 140–159 mmHg) | 19 (43.2) | 4 (30.8) | 23 (40.4) | 0.423 |
| HTN grade 2 (SBP 160–179 mmHg) | 3 (6.8) | 2 (15.4) | 5 (8.8) | 0.337 |
| HTN grade 3 (SBP >180 mmHg) | 1 (3.4) | 0 | 1 (1.8) | 0.583 |
| Diabetes mellitus | 8 (18.2) | 2 (15.4) | 10 (17.5) | 0.816 |
| Hyperlipidemia | 9 (20.5) | 2 (15.4) | 11 (19.4) | 0.684 |
| Current smoker | 19 (43.2) | 5 (38.5) | 24 (42.1) | 0.762 |
| Coronary artery disease | 4 (9.1) | 1 (7.7) | 5 (8.8) | 0.876 |
| Previous percutaneous coronary intervention | 3 (6.8) | 1 (7.7) | 4 (7.0) | 0.914 |
| Previous myocardial infarction | 0 | 1 (7.7) | 1 (1.8) | 0.063 |
| Previous history of heart operation | 6 (13.6) | 0 | 6 (10.5) | 0.159 |
| Previous stroke | 4 (9.1) | 4 (30.8) | 8 (14.0) | 0.048 |
| Chronic obstructive pulmonary disease | 6 (13.6) | 0 | 6 (10.5) | 0.159 |
| Preoperative chronic kidney disease (eGFR≤60) | 11 (25.0) | 4 (30.8) | 15 (26.3) | 0.746 |
| Preoperative eGFR ≥90 | 19 (43.1) | 4 (30.8) | 23 (40.4) | 0.423 |
| Preoperative eGFR 60–89 | 15 (34.1) | 5 (38.5) | 20 (35.1) | 0.772 |
| Preoperative eGFR 30–59 | 9 (20.5) | 2 (15.4) | 11 (19.3) | 0.684 |
| Preoperative eGFR ≤29 | 1 (2.3) | 2 (15.4) | 3 (5.3) | 0.063 |
| Acute dissection | 23 (52.3) | 4 (30.8) | 27 (47.4) | 0.172 |
| Complicated dissection | 11 (25.0) | 4 (30.8) | 15 (26.3) | 0.678 |
| Acute phase | 27 (61.4) | 10 (76.9) | 37 (64.9) | 0.302 |
| Chronic phase | 17 (38.6) | 3 (23.1) | 20 (35.1) | 0.302 |
Values are presented as mean±standard deviation or number (%).
AKI, acute kidney injury; HTN, hypertension; SBP, systolic blood pressure; eGFR, estimated glomerular filtration rate.
Postoperative paraparesis and paraplegia developed in five patients (8.8%; three developed paraparesis and two paraplegia). Among these patients, two (40%) were in the non-AKI group. Four of them recovered completely but one patient with paraplegia only recovered partially, with paraparesis remaining.
2) Acute kidney injury predictors and incidence
AKI was identified in 13 of 57 cases (overall incidence, 22.8%; risk, 11; injury, 2; failure/loss/end-stage, 0), with 4 patients (7%) requiring temporary renal replacement therapy. Univariate analysis identified previous stroke (p=0.048) and postoperative paraparesis and paraplegia (p=0.038) as predictors of postoperative AKI. Hypertension grade, preoperative chronic kidney disease (CKD), complicated dissection, and acute and chronic phase were not significantly different between AKI and non-AKI groups (Table 4).
Surprisingly, no significant difference in the volume of contrast medium used was observed between the two groups (Table 5). Multivariate analysis did not identify any statistically significant risk factors for the development of AKI.
Table 5.
Intraoperative and postoperative risk factors
| Variable | Non-AKI (n=44) | AKI (n=13) | All (n=57) | p-value |
|---|---|---|---|---|
| Postoperative stroke | 0 | 1 (7.7) | 1 (1.8) | 0.063 |
| Malperfusion complication | 2 (4.5) | 0 | 2 (3.5) | 0.434 |
| Postoperative paraparesis and paraplegia | 2 (4.5) | 3 (23.1) | 5 (8.8) | 0.038 |
| Multi-organ failure | 2 (4.5) | 2 (15.4) | 4 (7.0) | 0.148 |
| Blood transfusion | 13 (29.5) | 4 (30.8) | 17 (29.8) | 0.932 |
| Stent length (mm) | 141±57 | 160±88 | - | 0.346 |
| Contrast medium volume (mL) | 168±120 | 145±85 | - | 0.508 |
| Contrast medium volume per body mass index | 7.36±5.14 | 6.65±4.11 | - | 0.654 |
Values are presented as number (%) or mean±standard deviation.
AKI, acute kidney injury.
3) Impact of postoperative acute kidney injury
The average ICU stay in patients without AKI was 5.3 days, which was significantly lower than the average of 12.7 days for those with AKI (p=0.017) (Table 6). The average hospital stay in patients without AKI was 22 days, compared with 45.8 days in those with AKI.
Table 6.
Impact of postoperative AKI
| Variable | Non-AKI (n=44) | AKI (n=13) | All (total=57) | p-value |
|---|---|---|---|---|
| Intensive care unit stay (day) | 5.3±7.9 | 12.7±20.5 | 7.0±12.2 | 0.017 |
| Hospital stay (day) | 22.0±23.1 | 45.8±93.6 | 27.4±48.9 | 0.607 |
| 30-Day mortality | 2 (4.5) | 3 (23.1) | 5 (8.8) | 0.038 |
Values are presented as mean±standard deviation or number (%).
AKI, acute kidney injury.
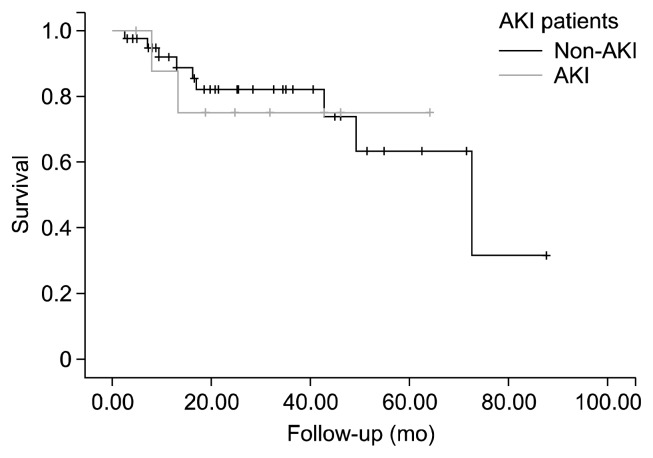
The overall 30-day mortality was 8.8% (5 of 57 cases). The 30-day mortality occurred in two of the 44 cases without AKI (4.5%) and three of the 13 cases with AKI (23.1%); this difference was statistically significant (p=0.038) (Table 6). However, Kaplan-Meier analysis showed survival of 91.9%, 82.0%, and 63.3% at 1, 3, and 5 years, respectively, for patients with AKI and 87.5%, 75.0%, and 75.0% at 1, 3, and 5 years for patients without AKI; these data were not significantly different between the two groups (log-rank=0.922) (Fig. 1).
Fig. 1.
The impact of postoperative AKI on late mortality. AKI, acute kidney injury.
DISCUSSION
Thoracic endovascular aortic repair has emerged as a less invasive alternative to open aortic surgery for the treatment of thoracic aortic disease [3,4,9–11]. The occurrence of AKI after TEVAR markedly increases hospital stay and is independently associated with the risk of mortality [4]. However, data on the risk factors and impact of AKI after TEVAR have not yet been published by The Korean Society for Thoracic and Cardiovascular Surgery.
We used the RIFLE classification, a sensitive index of renal function used to predict long-term survival that was proposed by the Acute Dialysis Quality Initiative [5,12] that is the preferred diagnostic tool for monitoring the progression and the severity of AKI after cardiothoracic surgery [13–16].
In previous studies, the incidence of AKI varies from 14% to 30.8%. In our study, AKI incidence was 22.8% and the perioperative risk factors for AKI after TEVAR were preoperative stroke and postoperative paraparesis and paraplegia.
AKI developed in half of the patients with preoperative stroke. The pathophysiology of AKI in these patients with preoperative stroke is unclear; however, stroke and renal dysfunction share vascular risk factors such as hypertension, diabetes, dyslipidemia, aging, and obesity [17,18]. Moreover, the kidney and brain are vulnerable to arteriosclerotic injury with similar microvascular functional and anatomical aspects [17,19].
In our study, three of five patients with postoperative paraparesis and paraplegia developed AKI. Postoperative paraparesis or paraplegia after TEVAR is a serious complication caused by spinal cord ischemia. The rate of paraplegia ranges from 0% to 13.3% among reported case series [20,21]. Probable mechanisms of ischemic damage to the spinal cord include perioperative hypotension and embolization during device introduction and deployment, and long stent graft coverage blocking critical extrinsic vertebral, intercostal, and lumbar supply to the anterior spinal artery [22]. The reasons for the frequent occurrence of AKI in patients with postoperative paraplegia are unclear; however, insufficient renal blood flow is a likely element of the pathophysiology of AKI.
Significant risk factors for AKI after TEVAR reported in other studies include preoperative CKD, thoracoabdominal extent, postoperative transfusion [12], intraoperative hypotension, stroke, sepsis, lengthy procedures and number of stents [1], acute dissection, complicated dissection, and malperfusion complications.
It is unsurprising that preoperative CKD is a significant risk factor in previous studies; however, in our study, preoperative eGFR ≤29 was not associated with significant risk for AKI. This may be because interventions to prevent AKI such as minimizing contrast use, stopping nephrotoxic agents, hydration, and permissive hypertension were more common in patients with low eGFR.
Postoperative transfusion has been previously associated with AKI. During transfusion of red blood cells, cellular and molecular components of allogeneic blood induce and intensify inflammatory responses, including the release of pro-inflammatory cytokines, which may cause kidney damage [12,23].
Contrast-induced nephropathy is a well-known iatrogenic cause of renal dysfunction. Contrast media induces vasoconstriction by inhibiting nitric oxide-mediated vasodilation and sustains vasoconstriction by altering intracellular calcium and adenosine concentrations in kidney smooth muscle cells [24]. In our study, we used the minimum amount of contrast medium possible, and our results indicate that the volume of contrast medium used was not a significant risk factor for AKI.
Limitations of this study include its retrospective design and the relatively small number of patients. Our results are in concordance with those of other studies, and further research will investigate more extensively the impact of factors such as preoperative eGFR of ≤29, previous MI, and postoperative stroke, which were not statistically significantly associated with risk for AKI in the present study.
In conclusion, preoperative stroke and postoperative paraparesis and paraplegia are significant risk factors for AKI after TEVAR. Optimizing perioperative blood pressure, blood glucose, and blood lipid levels, avoiding hypotension to maximize organ perfusion, and careful manipulation of endovascular devices to reduce embolization of atherosclerotic debris to various organs including the kidney represent important protective strategies to reduce the incidence of AKI. Because AKI increases the duration of hospital stays as well as morbidity and mortality, an awareness of the various risk factors for AKI and aggressive efforts to minimize the incidence, severity, and duration of AKI in patients with these risk factors represent the cornerstone of successful TEVAR intervention.
ACKNOWLEDGMENTS
This study was supported by a Grant of the Samsung Vein Clinic Network (Daejeon, Anyang, Cheongju, Cheonan; Fund No. KTCS04-040).
Footnotes
CONFLICT OF INTEREST
No potential conflict of interest relevant to this article was reported.
REFERENCES
- 1.Pisimisis GT, Khoynezhad A, Bashir K, Kruse MJ, Donayre CE, White RA. Incidence and risk factors of renal dysfunction after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. 2010;140(6 Suppl):S161–7. doi: 10.1016/j.jtcvs.2010.10.014. [DOI] [PubMed] [Google Scholar]
- 2.Song TK, Donayre CE, Walot I, et al. Endograft exclusion of acute and chronic descending thoracic aortic dissections. J Vasc Surg. 2006;43:247–58. doi: 10.1016/j.jvs.2005.10.065. [DOI] [PubMed] [Google Scholar]
- 3.Greenberg RK, O’Neill S, Walker E, et al. Endovascular repair of thoracic aortic lesions with the Zenith TX1 and TX2 thoracic grafts: intermediate-term results. J Vasc Surg. 2005;41:589–96. doi: 10.1016/j.jvs.2005.01.043. [DOI] [PubMed] [Google Scholar]
- 4.Drews JD, Patel HJ, Williams DM, Dasika NL, Deeb GM. The impact of acute renal failure on early and late outcomes after thoracic aortic endovascular repair. Ann Thorac Surg. 2014;97:2027–33. doi: 10.1016/j.athoracsur.2014.02.045. [DOI] [PubMed] [Google Scholar]
- 5.Zhu JC, Chen SL, Jin GZ, et al. Acute renal injury after thoracic endovascular aortic repair of Stanford type B aortic dissection: incidence, risk factors, and prognosis. J Formos Med Assoc. 2014;113:612–9. doi: 10.1016/j.jfma.2014.01.017. [DOI] [PubMed] [Google Scholar]
- 6.Martin MC, Giles KA, Pomposelli FB, Hamdan AD, Wyers MC, Schermerhorn ML. National outcomes after open repair of abdominal aortic aneurysms with visceral or renal bypass. Ann Vasc Surg. 2010;24:106–12. doi: 10.1016/j.avsg.2009.09.008. [DOI] [PubMed] [Google Scholar]
- 7.Ellenberger C, Schweizer A, Diaper J, et al. Incidence, risk factors and prognosis of changes in serum creatinine early after aortic abdominal surgery. Intensive Care Med. 2006;32:1808–16. doi: 10.1007/s00134-006-0308-1. [DOI] [PubMed] [Google Scholar]
- 8.Khoynezhad A, Donayre CE, Smith J, Kopchok GE, Walot I, White RA. Risk factors for early and late mortality after thoracic endovascular aortic repair. J Thorac Cardiovasc Surg. 2008;135:1103–9. doi: 10.1016/j.jtcvs.2008.02.001. [DOI] [PubMed] [Google Scholar]
- 9.Bavaria JE, Appoo JJ, Makaroun MS, et al. Endovascular stent grafting versus open surgical repair of descending thoracic aortic aneurysms in low-risk patients: a multicenter comparative trial. J Thorac Cardiovasc Surg. 2007;133:369–77. doi: 10.1016/j.jtcvs.2006.07.040. [DOI] [PubMed] [Google Scholar]
- 10.Patel HJ, Williams DM, Upchurch GR, Jr, et al. Long-term results from a 12-year experience with endovascular therapy for thoracic aortic disease. Ann Thorac Surg. 2006;82:2147–53. doi: 10.1016/j.athoracsur.2006.06.046. [DOI] [PubMed] [Google Scholar]
- 11.Patel HJ, Sood V, Williams DM, Dasika NL, Diener AC, Deeb GM. Late outcomes with repair of penetrating thoracic aortic ulcers: the merits of an endovascular approach. Ann Thorac Surg. 2012;94:516–22. doi: 10.1016/j.athoracsur.2012.03.074. [DOI] [PubMed] [Google Scholar]
- 12.Barbash IM, Ben-Dor I, Dvir D, et al. Incidence and predictors of acute kidney injury after transcatheter aortic valve replacement. Am Heart J. 2012;163:1031–6. doi: 10.1016/j.ahj.2012.01.009. [DOI] [PubMed] [Google Scholar]
- 13.Piffaretti G, Mariscalco G, Bonardelli S, et al. Predictors and outcomes of acute kidney injury after thoracic aortic endograft repair. J Vasc Surg. 2012;56:1527–34. doi: 10.1016/j.jvs.2012.05.106. [DOI] [PubMed] [Google Scholar]
- 14.Bellomo R, Kellum JA, Ronco C. Defining and classifying acute renal failure: from advocacy to consensus and validation of the RIFLE criteria. Intensive Care Med. 2007;33:409–13. doi: 10.1007/s00134-006-0478-x. [DOI] [PubMed] [Google Scholar]
- 15.Levey AS, Stevens LA, Schmid CH, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–12. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mariscalco G, Lorusso R, Dominici C, Renzulli A, Sala A. Acute kidney injury: a relevant complication after cardiac surgery. Ann Thorac Surg. 2011;92:1539–47. doi: 10.1016/j.athoracsur.2011.04.123. [DOI] [PubMed] [Google Scholar]
- 17.Ma S, Zhao H, Ji X, Luo Y. Peripheral to central: organ interactions in stroke pathophysiology. Exp Neurol. 2015;272:41–9. doi: 10.1016/j.expneurol.2015.05.014. [DOI] [PubMed] [Google Scholar]
- 18.Ninomiya T. Risk of stroke in kidney disease. Contrib Nephrol. 2013;179:58–66. doi: 10.1159/000346724. [DOI] [PubMed] [Google Scholar]
- 19.Mogi M, Horiuchi M. Clinical Interaction between Brain and Kidney in Small Vessel Disease. Cardiol Res Pract. 2011;2011:306189. doi: 10.4061/2011/306189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bobadilla JL, Wynn M, Tefera G, Acher CW. Low incidence of paraplegia after thoracic endovascular aneurysm repair with proactive spinal cord protective protocols. J Vasc Surg. 2013;57:1537–42. doi: 10.1016/j.jvs.2012.12.032. [DOI] [PubMed] [Google Scholar]
- 21.Rizvi AZ, Sullivan TM. Incidence, prevention, and management in spinal cord protection during TEVAR. J Vasc Surg. 2010;52(4 Suppl):86S–90S. doi: 10.1016/j.jvs.2010.06.148. [DOI] [PubMed] [Google Scholar]
- 22.Drinkwater SL, Goebells A, Haydar A, et al. The incidence of spinal cord ischaemia following thoracic and thoracoabdominal aortic endovascular intervention. Eur J Vasc Endovasc Surg. 2010;40:729–35. doi: 10.1016/j.ejvs.2010.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Nuis RJ, Rodes-Cabau J, Sinning JM, et al. Blood transfusion and the risk of acute kidney injury after transcatheter aortic valve implantation. Circ Cardiovasc Interv. 2012;5:680–8. doi: 10.1161/CIRCINTERVENTIONS.112.971291. [DOI] [PubMed] [Google Scholar]
- 24.Azzalini L, Spagnoli V, Ly HQ. Contrast-induced nephropathy: from pathophysiology to preventive strategies. Can J Cardiol. 2015 May 23; doi: 10.1016/j.cjca.2015.05.013. [Epub]. http://dx.doi.org/10.1016/j.cjca.2015.05.013. [DOI] [PubMed]