Abstract
Background
Clinical trials have been criticized on various counts. Any attempt to improve how trials are conducted or reported requires—amongst other things—an understanding of the number, the nature and the location of those that sponsor them or collaborate on them. Here we sought to identify the nature and location of each sponsor/collaborator.
Methods and Findings
We examined the 'sponsor/collaborator' field for the 69,160 drug trials that were registered with ClinicalTrials.gov over a 9-year period (2005–2014). Of the 12,823 unique sponsors, 56% had sponsored only one and 27% had sponsored 2–5 trials each. Just 18% were involved with six or more trials each, and we have (arbitrarily) labeled these organizations as 'more experienced' in sponsoring/collaborating on trials. These 18% (2,266 sponsors/collaborators) were analyzed further: (a) 951 were corporate organizations and (b) 1,145 were non-corporates (including 31 individuals) with (c) 170 unclassified. Further, we identified the location of each organization in (a) and (b).
Conclusions
Clinical trials are an important part of a nation's research endeavors, and ultimately contribute to the health of its people. Thus, understanding the clinical trial landscape—including the number and nature of sponsors, and how active they are—is important for every country. We believe that policy makers in particular should be interested in this study to understand the current situation, and to use the numbers as a baseline for the evolving landscape, to assess the impact of their strategies in future.
Introduction
Clinical trials have been criticized on various counts. They may fail to follow the guidelines of the International Committee of Medical Journal Editors (ICMJE) [1,2]. Trials are often unable to recruit the planned number of participants per site, leading to an over-dependence on a few sites [3,4]. Even so the trials may have too few participants to yield useful information [5]. Further, it has been estimated that in a large percentage of cases it is the wrong test dose that leads to a failed trial [6]. Often, those conducting a trial have insufficient input from patient organizations [7], may not keep patients informed of the results [8] and may not take enough steps to enroll minorities [9]. Additionally, they may be unaware that subjects are not taking the test drug according to the protocol, or may be enrolled in multiple trials at the same time [10]. Trials may take too long for the participants to benefit [11]. Early results from trials may be leaked [12]. In terms of reporting the results, there may be a publication bias that exaggerates the benefits of a drug candidate or minimizes its risks [13, 14], peer-review of randomized controlled trials may not be up to the mark [15] and journals may not meet the required standards while reporting the results of trials [16].
Such criticism has often been directed at trials conducted in traditional locations such as the United States (US) or Western Europe. However trials are increasingly located in non-traditional nations [17, 18]. Problems reported from some of these locations include the lack of a control group, of informed consent and of a system to report adverse events [19], over- or under-monitoring of a trial [18], reduced safety requirements by the Food and Drug Administration of the US (USFDA) for trials conducted abroad [20], the inexperience of local investigators [21] and insufficient local leadership in conducting trials [22], leading to doubts about their quality [21].
Any attempt to improve how trials are conducted or reported requires—amongst other things—an understanding of the number, the nature and the location of those that sponsor them or collaborate on them. In this study we sought to understand this landscape.
Methods
ClinicalTrials.gov (accessed at http://clinicaltrials.gov and hereafter referred to as CT.gov) is the largest registry of clinical trials [23]. In the information downloaded from CT.gov, we were interested in the 'sponsor/collaborator' field. If a record had multiple names in this field, we treated them at par. For convenience we referred to each organization as a sponsor since there was no way to more precisely identify an organization's role(s).
We accessed CT.gov on 4 November 2015 and did an Advanced Search, with the following filters: (i) 'Study type': Interventional studies; (ii) 'Phase': 0–4; and (iii) 'Record first received': 1/1/2005 to 01/31/2014. This yielded 85,528 records that we downloaded with the options '22 available fields' and as 'Tab-separated values'. From these, we selected the drug-related records, that is those that had as 'intervention' either 'drug' or 'biological'. These came to 69,160 records. This file was processed to yield (a) the list of 107,911 sponsors, including redundancies and (b) the list of 12,823 unique sponsors and their frequencies (S1 Table). After identifying this very large number of sponsors/collaborators, we focused on those that had been involved in six or more trials. We categorized each of such organizations as 'corporate' or 'non-corporate'. We also identified the location of each (except the 31 individuals amongst the non-corporates). In some cases we could not conclusively identify the nature of the organization or its location and these were excluded from further analysis. S1 Text provides further details of the methodology.
Results and Discussion
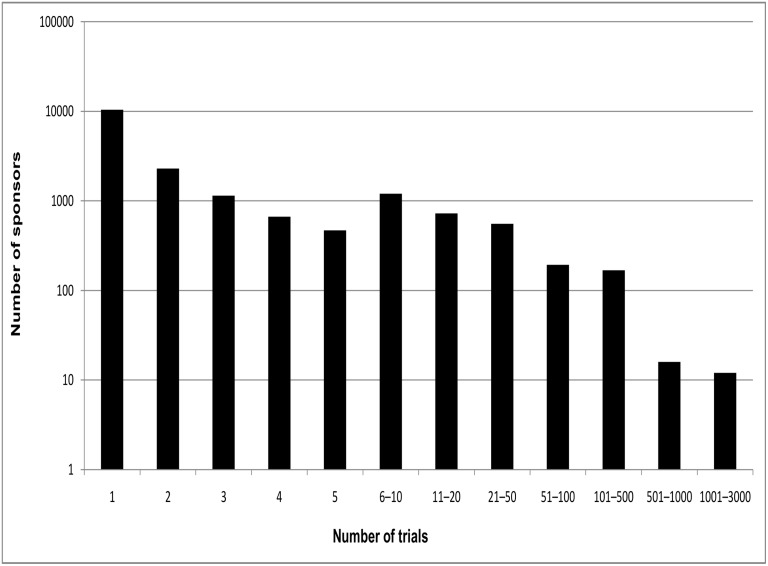
Fig 1 provides a summary of the frequency of occurrence of the sponsors. Of the 12,823 unique sponsors, 7,119 (56%) were involved in a single trial each. A further 3,438 (27%) were associated with 2–5 trials. This left just 2,266 (18%) sponsors that were involved in six or more trials. For convenience we refer to the 2,266 organizations as those with 'more experience', and the rest as those with 'limited experience' as sponsors. Many studies were co-sponsored by multiple organizations, and a significant percentage must have involved a partnership between sponsors with more experience and those with limited experience.
Fig 1. The distribution of sponsors over different numbers of trials.
The sponsor with the largest number of trials (the National Cancer Institute, with 3,498) is not shown.
10,557 organizations sponsored 1–5 trials each, totaling 17,081 trials. We did not analyze this group any further. We sought to classify each of the remaining 2,266 (listed in S2 Table and sorted in S3 Table) as corporate or non-corporate, with the latter including non-profits, government organizations, individuals or 'others' (with the criteria for classification provided in S2 Text). Trials were sponsored by 951 corporates (45,532 occurrences overall) and 1,145 non-corporates (41,812). For 170 entities (3,486) the nature of the organization or its location could not be conclusively determined and they were labeled 'unclassified'. In all, the 2,266 organizations occurred 90,830 times which is 84% of the 107,911 incidences of sponsorship. Thus, the fraction of sponsors with more experience was low (18%), but the fraction of trials in which these experienced sponsors were involved was high (84%).
Corporates occurred 45,532 times and non-corporates 41,812 times, which were, 50% and 46% respectively of the 90,830 total number of occurrences. Since there have been disagreements over whether the private or public sector brings drugs to market [24], these results would be useful to any attempt to quantify the relative contributions of the two sectors. However it is true that without knowing precisely what each organization did, it is impossible to quantify the costs that the two sets of organizations incurred, for example. Separately, a non-profit may have close ties with industry, and in fact may be the non-profit wing of a company. We have not documented the extent of such relationships in this paper.
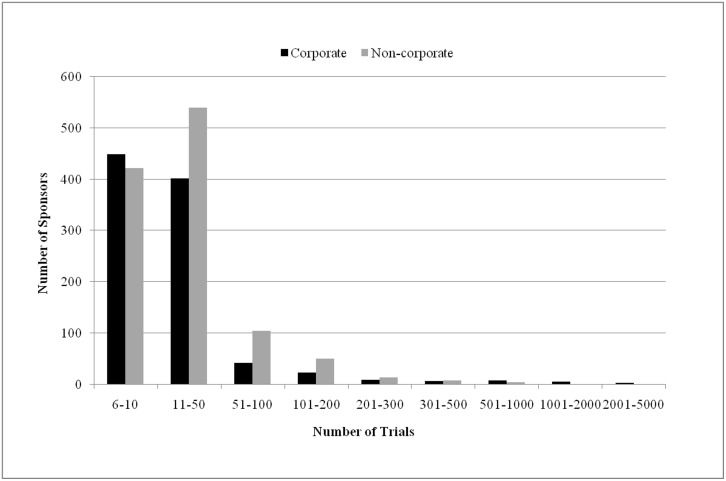
We continued our analysis of the organizations that had sponsored six or more trials each and looked at how many trials were sponsored by the 951 corporates and 1,145 non-corporates (Fig 2). 90% of corporate-sponsored and 84% of non-corporate sponsored studies were by organizations involved in no more than 50 trials each. Only 57 corporates and 78 non-corporates sponsored more than 100 trials each. Further, just a few sponsored more than 1,000 each. The nine corporates were Hoffmann-La Roche (1,058), Eli Lilly and Company (1,269), Novartis Pharmaceuticals (1,439), AstraZeneca (1,619), Sanofi (1,622), Merck Sharp & Dohme Corp. (1,811), Pfizer (2,313), Novartis (2,487) and GlaxoSmithKline (2,930). The non-corporate that did so was the National Cancer Institute (3,498). Thus, the vast majority of sponsors—corporate or non-corporate—were involved in relatively few trials.
Fig 2. The numbers of corporates and non-corporates that sponsored a given number of trials.
Finally, we identified the countries in which the corporate and non-corporate sponsors were based (methodology in S2 Text). In all, the corporates were based in 36 countries and the non-corporates in 63. We list here the top 12 locations for each category (Table 1, with the complete lists in S4 Table). Amongst the corporates, the US was the base for 56% of the sponsors. Also, it was home to an order of magnitude more organizations than the second ranked country, Germany (6%). South Korea, Japan and China are the only Asian countries on this list, with the rest being developed, Western countries. These 12 countries were home to 87% of the corporate sponsors. Among the non-corporates, the US was the base for 33% of the organizations and hosted almost five fold more sponsors than the second-placed country, China (7%). China and Brazil are the two developing countries that made it to this list. The top 12 locations hosted 74% of the sponsors. The dominance of the US in both lists parallels a report that it dominates the 'trials' locations' table, with almost 9-fold more sites than the second-ranked Germany [25]. However countries that have not traditionally been involved in clinical trials made their presence felt.
Table 1. The 12 countries that were home to the most sponsors in the corporate and non-corporate categories.
| No. | Corporates | Non-corporates | ||||||
|---|---|---|---|---|---|---|---|---|
| Country | Number of sponsors | Percentage | Cumulative percentage | Country | Number of sponsors | Percentage | Cumulative percentage | |
| 1 | US | 529 | 55.6 | 55.6 | US | 380 | 33.2 | 33.2 |
| 2 | Germany | 53 | 5.6 | 61.2 | China | 79 | 6.9 | 40.1 |
| 3 | South Korea | 34 | 3.6 | 64.8 | UK | 59 | 5.2 | 45.2 |
| 4 | Canada | 31 | 3.3 | 68 | Canada | 57 | 5.0 | 50.2 |
| 5 | Japan | 31 | 3.3 | 71.3 | France | 57 | 5.0 | 55.2 |
| 6 | UK | 31 | 3.3 | 74.6 | Germany | 51 | 4.5 | 59.7 |
| 7 | France | 29 | 3.0 | 77.6 | Italy | 47 | 4.1 | 63.8 |
| 8 | Switzerland | 24 | 2.5 | 80.1 | Spain | 35 | 3.1 | 66.8 |
| 9 | China | 20 | 2.1 | 82.2 | Denmark | 25 | 2.2 | 69.0 |
| 10 | Australia | 14 | 1.5 | 83.7 | Brazil | 20 | 1.7 | 70.7 |
| 11 | Israel | 14 | 1.5 | 85.2 | Belgium | 19 | 1.7 | 72.4 |
| 12 | Ireland | 13 | 1.4 | 86.5 | Netherlands | 19 | 1.7 | 74.1 |
| Total | 823 | 87 | Total | 848 | 74 | |||
| Overall total | 951 | Overall total | 1145 | |||||
Conclusion
Mainly, this paper provides two categories of information. First, it provides the list of all organizations that were sponsors/collaborators (sponsors) of drug-related interventional trials registered with CT.gov over a recent 9-year period, and the frequency of occurrence of each. Second, it provides the global rankings of countries that hosted (a) the corporate and (b) the non-corporate sponsors that occurred six or more times. We believe that policy makers in particular should be interested in this study to understand the current situation, and to use the numbers as a baseline for the evolving landscape, to assess the impact of their strategies in future.
Supporting Information
The 12,823 unique sponsors and the number of trials sponsored by each.
(XLS)
The 2,266 organizations that sponsored six or more trials and the number of trials sponsored by each.
(XLS)
The 2,266 organizations sorted into (a) corporates (951 entities), (b) non-corporates (1,145) and (c) unclassified entities (170).
(XLS)
For the (a) corporates and (b) non-corporates, the number of sponsors based in a particular country is listed. The percentage hosted by a given country and the cumulative percentage is also provided for each category.
(XLS)
(A) A note on the records downloaded, (B) Determining the number of sponsors, (C) Classifying each organization, and (D) Determining each organization's location.
(DOC)
Acknowledgments
We are deeply grateful to the many individuals around the world who responded to our request for information on the nature of their organization or other organizations in their country. We also thank B. Mehrotra for assistance, H. Subramanya, V. Niranjan and T. S. Sridhar for discussion, and U. S. Bhalla for comment.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by internal funding of the Institute of Bioinformatics and Applied Biotechnology, and in part by a grant from the Department of Biotechnology, Government of India (BTPR12422/MED/31/287/2014, valid November 2014 to 2017). URL: http://www.dbtindia.nic.in/. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Schulman KA, Seils DM, Timbie JW, Sugarman J, Dame LA, Weinfurt KP et al. A national survey of provisions in clinical-trial agreements between medical schools and industry sponsors N Engl J Med 2002; 347: 1335–1341. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. What constitutes full access to data in industry-funded trials? Lancet 2011; 378: 1976. [DOI] [PubMed] [Google Scholar]
- 3.Dal-Re´ R, Moher D, Gluud C, Treweek S, Demotes-Mainard J, Carne´ X. Disclosure of investigators’ recruitment performance in multicenter clinical trials: A further step for research transparency. Plos Med 2011; 8(12): e1001149 10.1371/journal.pmed.1001149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hersher R. Clinicians call for greater transparency in trial recruitment. Nat Med 2012; 18: 186. [DOI] [PubMed] [Google Scholar]
- 5.Couzin-Frankel J. Clinical trials get practical. Science 2015; 348: 382 10.1126/science.348.6233.382 [DOI] [PubMed] [Google Scholar]
- 6.Dolgin E. Adaptive methods help drug sponsors find best treatment dose. Nat Med 2014; 20: 321 10.1038/nm0414-321 [DOI] [PubMed] [Google Scholar]
- 7.Chakradhar S. Training on trials: Patients taught the language of drug development. Nat Med 2015; 21: 209–210. 10.1038/nm0315-209 [DOI] [PubMed] [Google Scholar]
- 8.Chakradhar S. Many returns: Call-ins and breakfasts hand back results to study volunteers. Nat Med 2015; 21: 304–306. 10.1038/nm0415-304 [DOI] [PubMed] [Google Scholar]
- 9.Schork NJ. Time for one-person trials. Nature 2015; 520: 609–610. 10.1038/520609a [DOI] [PubMed] [Google Scholar]
- 10.Servick K. ‘Nonadherence’: A bitter pill for drug trials. Science 2014; 346: 288–289. 10.1126/science.346.6207.288 [DOI] [PubMed] [Google Scholar]
- 11.Servick K.‘Right to Try’ laws bypass FDA for last-ditch treatments. Science 2014; 344: 1329 10.1126/science.344.6190.1329 [DOI] [PubMed] [Google Scholar]
- 12.Bloudoff-Indelicato M. Threat of interim data leaks prompts call for international rules. Nat Med 2015; 21: 200 10.1038/nm0315-200 [DOI] [PubMed] [Google Scholar]
- 13.Doshi P, Dickersin K, Healy D, Vedula SS, Jefferson T. Restoring invisible and abandoned trials: a call for people to publish the findings. BMJ 2013; 346: f2865 10.1136/bmj.f2865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller FG, Brody H. Professional integrity in industry-sponsored clinical trials. Acad Med 2005; 80: 899–904. [DOI] [PubMed] [Google Scholar]
- 15.Patel J. Why training and specialization is needed for peer review: a case study of peer review for randomized controlled trials. BMC Med 2014; 12: 128–134. 10.1186/s12916-014-0128-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Goldacre B. How to get all trials reported: Audit, better data, and individual accountability. Plos Med 2015; 12(4): e1001821 10.1371/journal.pmed.1001821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Tanne J H. US new drug applications increasingly rely on trials conducted abroad. BMJ. 2010. July 6;341:c3616 10.1136/bmj.c3616 [DOI] [PubMed] [Google Scholar]
- 18.Lang T, Siribaddana S. Clinical trials have gone global: Is this a good thing? Plos Med 2012; 9: e1001228 10.1371/journal.pmed.1001228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bagla P. Indian Parliament comes down hard on cervical cancer trial. Science 2013. Available: http://news.sciencemag.org/asiapacific/2013/09/indian-parliament-comes-down-hard-cervical-cancer-trial. Accessed 25 November 2015. [Google Scholar]
- 20.Shah S. Globalization of clinical research by the pharmaceutical industry. Int J Health Serv 2003; 33: 29–36. [DOI] [PubMed] [Google Scholar]
- 21.Glickman SW, McHutchison JG, Peterson ED, Cairns CB, Harrington RA, Califf RM et al. Ethical and scientific implications of the globalization of clinical research. N Engl J Med 2009; 360: 816–823. 10.1056/NEJMsb0803929 [DOI] [PubMed] [Google Scholar]
- 22.Hoekman J, Frenken K, de Zeeuw D, Heerspink HL. The geographical distribution of leadership in globalized clinical trials. Plos One 2012; 7: e45984 10.1371/journal.pone.0045984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Liu G, Chen G, Sinoway LI, Berg A. Assessing the impact of the NIH CTSA program on institutionally sponsored clinical trials. Clin Transl Sci 2013; 6: 196–200. 10.1111/cts.12029 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kessel M. Restoring the pharmaceutical industry’s reputation Nat Biotech 2014; 32: 983–990. [DOI] [PubMed] [Google Scholar]
- 25.Thiers FA, Sinskey AJ, Berndt ER. Trends in the globalization of clinical trials. Nat Rev Drug Discov 2008; 7: 13–14. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The 12,823 unique sponsors and the number of trials sponsored by each.
(XLS)
The 2,266 organizations that sponsored six or more trials and the number of trials sponsored by each.
(XLS)
The 2,266 organizations sorted into (a) corporates (951 entities), (b) non-corporates (1,145) and (c) unclassified entities (170).
(XLS)
For the (a) corporates and (b) non-corporates, the number of sponsors based in a particular country is listed. The percentage hosted by a given country and the cumulative percentage is also provided for each category.
(XLS)
(A) A note on the records downloaded, (B) Determining the number of sponsors, (C) Classifying each organization, and (D) Determining each organization's location.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.