Abstract
OBJECTIVE
Maternal birth trauma to the pelvic floor muscles (PFMs) is a major risk factor for pelvic floor disorders. Modeling and imaging studies suggest that demands placed on PFMs during childbirth exceed their physiologic limits; however many parous women do not sustain PFM injury. Here we determine whether pregnancy induces adaptations in PFM architecture, the strongest predictor of muscle function, and/or intramuscular extracellular matrix (ECM), responsible for load bearing. To establish if parallel changes occur in muscles outside of the PFM, we also examined a hind limb muscle.
STUDY DESIGN
Coccygeus, iliocaudalis, pubocaudalis, and tibialis anterior of 3-month-old Sprague-Dawley virgin, mid-pregnant, and late-pregnant; 6-month-old virgin; and 4- and 12-week postpartum rats (N = 10/group) were fixed in situ and harvested. Major architectural parameters determining muscle’s excursion and force-generating capacity were quantified, namely, normalized fiber length (Lfn), physiologic cross-sectional area, and sarcomere length. Hydroxyproline content was used as a surrogate for intramuscular ECM quantity. Analyses were performed by 2-way analysis of variance with Tukey post hoc testing at a significance level of .05.
RESULTS
Pregnancy induced a significant increase in Lfn in all PFMs by the end of gestation relative to virgin controls. Fibers were elongated by 37% in coccygeus (P < .0001), and by 21% in iliocaudalis and pubocaudalis (P < .0001). Importantly, no Lfn change was observed in the tibialis anterior. Physiologic cross-sectional area and sarcomere length were not affected by pregnancy. By 12 weeks’ postpartum, Lfn of all PFMs returned to the prepregnancy values. Relative to virgin controls, ECM increased by 140% in coccygeus, 52% in iliocaudalis, and 75% in pubocaudalis in late-pregnant group, but remained unchanged across time in the tibialis anterior. Postpartum, ECM collagen content returned to prepregnancy levels in iliocaudalis and pubocaudalis, but continued to be significantly elevated in coccygeus (P < .0001).
CONCLUSION
This study demonstrates that pregnancy induces unique adaptations in the structure of the PFMs, which adjust their architectural design by adding sarcomeres in series to increase fiber length as well as mounting a substantial synthesis of collagen in intramuscular ECM.
Keywords: adaptation, pelvic muscles, pregnancy, rat
The striated pelvic floor muscles (PFMs), comprised of the coccygeus and the muscles of the levator ani complex, are integral for structural support and proper pelvic floor function. Maternal birth trauma to the PFMs negatively impacts their function, which plays an important role in the pathogenesis of pelvic floor disorders.1–3 Modeling and imaging studies estimate that >300% PFM strain is required for vaginal childbirth. Such strain exceeds the physiologic limit of striated muscles and is presumed to cause radiologically detected abnormalities in the PFMs.4–9 These conclusions are consistent with mechanical studies of limb striated muscles, which demonstrate that muscle injury magnitude is a function of fiber strain.10–12 Given the extent of PFM strain necessary for vaginal delivery, it appears that all women should sustain injury to these muscles during childbirth. However, published data indicate that two thirds of women do not demonstrate evidence of PFM damage after spontaneous vaginal delivery. It is, therefore, possible that protective pregnancy-induced adaptations take place in the PFMs to ameliorate the impact of excessive muscle strain.
Adaptation during pregnancy in adjacent to the PFMs vagina and its supportive structures have been demonstrated in both humans and animals, with substantial biomechanical and biochemical changes occurring in preparation for delivery.13–17 To assess changes in the PFMs in pregnancy, clinical studies utilize an imaging parameter referred to as the levator hiatus.18,19 However, dimensions of levator hiatus provide very limited information relevant to PFM structure and function and do not correlate with strength or endurance of PFM contraction.20 Thus, our limited understanding of the impact of pregnancy and delivery on the structure and function of PFMs precludes identification of targets for preventive strategies.
Skeletal muscle architecture refers to the macroscopic arrangement of fibers within a muscle and provides the ability to predict skeletal muscle active force generation.21 Architectural design is dynamic—muscle’s plasticity enables adjustments to be made in response to chronic alterations in use. Skeletal muscle fibers are embedded in a connective tissue network of their extracellular matrix (ECM), which primarily consists of collagen. Intramuscular ECM determines muscle passive mechanical properties and ability to sustain load.22,23 Elucidating the impact of pregnancy on the PFMs architecture and intramuscular ECM will significantly advance our understanding of the mechanics of injury of these muscles due to parturition.
Architectural studies require isolation of the entire muscle and are not feasible in women, requiring the use of animal models. In rat, the organization of pelvic skeletal muscles, coccygeus, iliocaudalis, and pubocaudalis, are anatomically similar to human PFMs.24,25 Furthermore, we recently reported that with respect to major architectural parameters, rat PFM design is similar to human.26 Rat has also been shown to be a reliable model for the study of pregnancy-related changes in cervix, vagina, and vaginal supportive structures.14,27,28 In this study we quantified the structural adaptations that take place in the PFMs throughout pregnancy and determined the extent of recovery after vaginal delivery. Second, to determine whether these changes are specific to the PFMs or occur throughout the body, we examined a hind limb muscle, the tibialis anterior. We hypothesized that, if systemic hormonal effects cause the adaptation, pregnancy-induced alterations would take place in both the PFMs and the tibialis anterior. On the other hand, if local mechanical loading causes the adaptations, PFMs would adjust their architecture, while the tibialis anterior would remain unchanged.
Materials and Methods
The University of California Institutional Animal Care Committee approved all procedures performed. Ten mid-pregnant (10–12 days) and 10 late-pregnant (19–21 days) 3-month-old Sprague-Dawley rats (Rattus norvegicus) were used to characterize changes in PFM architectural endpoints and collagen content of the intramuscular ECM during pregnancy. PFM architecture of 3-month-old Sprague-Dawley female virgin rats, which served as a control group, was previously reported.26 Collagen content of the PFMs of virgin controls was determined using previously preserved tissue. Ten intermediate (4 weeks) and 10 late (12 weeks) postpartum animals were studied to determine whether PFMs returned to prepregnancy state. Previous studies in limb and shoulder muscles demonstrated that, as rats age and increase in size, their muscle fibers also elongate.29 Because 12-week postpartum rats were 6 months old at the time of sacrifice, PFM architecture was determined in 6-month-old virgin rats, which served as a control group for the late-postpartum animals. Virgin and postpartum animals were in similar parts of the menstrual cycle (estrus and metestrus) as determined by vaginal smears. Architectural parameters and collagen content of the tibialis anterior were compared among the same groups. Pregnancy- and delivery-induced changes were defined as the departure from the native PFMs in virgin controls.
Muscle architecture
After animal sacrifice, muscles were fixed in situ (ie, attached to the skeleton) in 10% buffered formaldehyde for 3–5 days after which each muscle was harvested, blotted dry, and weighed. Individual pelvic muscles were identified by tracking each muscle from its origin to insertion, weighed and divided into 3 regions, as previously described.26 Tibialis anterior muscles were identified, harvested, and processed in parallel. The following architectural parameters were quantified: fiber length (Lf), a predictor of contractile velocity and muscle excursion; physiologic cross-sectional area (PCSA), proportional to the maximum force a muscle can generate isometrically; and sarcomere length (Ls), the “molecular machine” that determines a muscle’s active force (ie, the force a muscle produces when stimulated). We measured Lf from fiber bundles dissected from each region of the muscle using electronic digital calipers to the nearest 0.01 mm. Muscle fibers were separated under a dissecting microscope using a ×6 objective and an overall magnification of ×60 (Leica MZ16, Meyer Instruments Inc, Houston, TX) and mounted on a slide for Ls determination by laser diffraction.30 The fixation process shortens Ls only by ~10% of its in vivo length, thus Ls provides information regarding muscle’s in vivo Ls. Ls was also used to calculate the sarcomere number (Sn) in series within fibers to normalize Lf (Lfn) and account for potential differences in length among specimens at the time of fixation. We achieved Lfn by first calculating serial Sn using the following equation: Sn = Lf/Ls. Values for Lfn were then derived from the following equation: Lfn = Sn × Lso, where Lso represents species-specific optimal Ls and is 2.4 μm in rat.31 PCSA was calculated according to the following equation: PCSA = (mass * Lfn)/ρ,32 where ρ = 1.056 g/cm3 in a fixed muscle.33
Intramuscular ECM
Hydroxyproline content was used to determine intramuscular collagen content using a modification of a previously validated protocol.34 Briefly, small tissue samples were randomly taken from 3 regions of each muscle and hydrolyzed in 6 N hydrochloric acid at 110°C for 24 hours. Samples were then pipetted with standards into 96-well plates and incubated with a chloramine T solution, followed by the addition of a p-dime-thylaminobenzaldehyde solution. Hydroxyproline concentration was determined by spectrophotometry at 550 nm, normalized to the wet weight of the tissue sample, and converted to collagen using the constant of 7.46 that defines the number of hydroxyproline residues per collagen molecule. Normalization to wet weight is the preferred method when tissue samples are small because lyophilization further decreases sample mass and increases data variability.
Statistical analyses
Comparison among muscles and conditions was performed by 2-way analysis of variance followed by multiple comparisons with Tukey range test, as appropriate. Comparison of rat body weight between groups was performed by 1-way analysis of variance followed by multiple comparisons with Tukey range test, as appropriate. Significance level (α) was set to 5%. To obtain 80% power (1-β) to detect a 15% difference the sample size of 10/group was calculated utilizing the equation: n= (coefficient of variation)2/(ln [1-δ])2 using the experimental variability from our previous data26 with variables n = sample size; δ = difference desired (15%).35,36 All data were screened for normality and skewness to satisfy the assumptions of the parametric tests used. Results are presented as mean ± SEM, except where noted. All statistical analyses were performed using software (GraphPad Prism, version 6.00; GraphPad Software, San Diego, CA).
Results
Weight varied among the groups (Table 1), with the late-pregnant animals being the heaviest, as expected (P <.0001 relative to 3-month-old virgin rats). Due to the progressive increase in weight of rats with age, weight of postpartum rats was significantly greater than 3-month-old virgin group (P < .0001). Weight of late-postpartum rats did not differ from age-matched 6-month-old virgin animals (P >.1).
TABLE 1.
Comparison of body weight measured as mean – standard error of the mean 3- and 6-month old V, MP, LP, 4 and 12 wks’ PP groups
| Group | Weight, g | Comparison groups | P valuea |
|---|---|---|---|
| V 3-mo old | 201.0 ± 3.7 | V vs MP | .006 |
| MP | 221.5 ± 4.7 | V vs LP | .0001 |
| LP | 274.7 ± 4.8 | V vs 4 wks PP | .0001 |
| PP 4 wks | 236.3 ± 2.5 | V vs 12 wks PP | .0001 |
| PP 12 wks | 254.9 ± 3.2 | V vs V 6 mo | .0001 |
| V 6-mo old | 268.9 ± 4.5 | V 6 mo vs 12 wks PP | .16 |
LP, late-pregnant; MP, mid-pregnant; PP, postpartum; V, virgin.
P values derived from one-way analysis of variance, followed by Tukey’s pairwise comparisons with significance level set to 5%.
Alperin. Pregnancy adaptations in rat pelvic muscles. Am J Obstet Gynecol 2015.
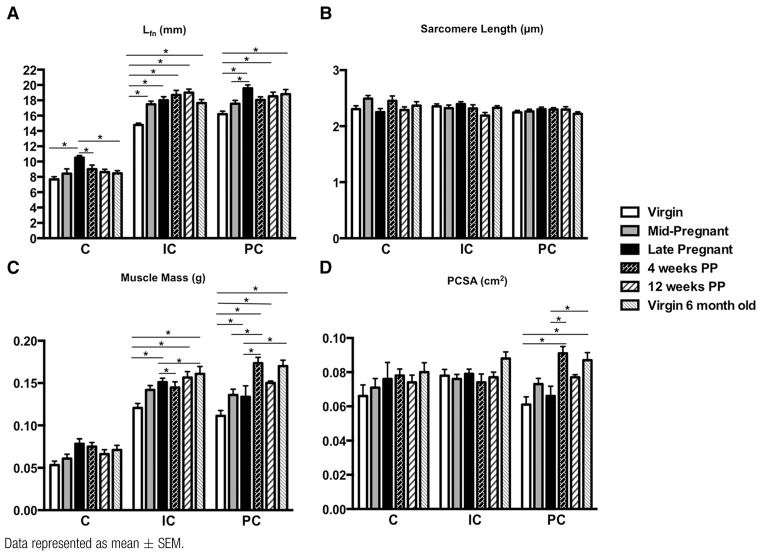
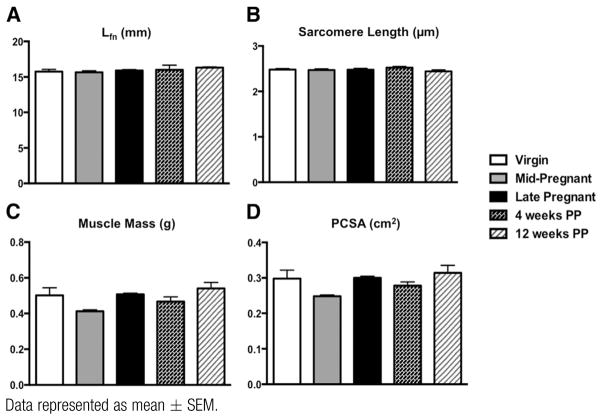
The following major architectural parameters were quantified: Lfn, a predictor of contractile velocity and muscle excursion; PCSA, proportional to the maximum force a muscle can generate; and Ls that determines relative force of active muscle contraction. The most important finding of our study was that pregnancy induces Lfn (serial Sn) change in all 3 PFMs. By mid pregnancy, Lfn was significantly greater only in iliocaudalis, with 18% increase from 14.8 ± 0.24 mm in virgin controls to 17.5 ± 0.41 mm in the mid-pregnant group (P < .0005). This was followed by an additional increase of 3% from mid to late pregnancy (Figure 1, A). Coccygeus and pubocaudalis, on the other hand, demonstrated a smaller change in Lfn by mid pregnancy, with 9.9% increase in coccygeus relative to virgin controls (P >.5) and 8.6% increase in pubocaudalis relative to virgin controls (P>.1). However, fibers of both of these muscles were significantly longer by the end of gestational period relative to virgin controls, with a 37% total increase in coccygeus (P < .0001) and a 21% increase in pubocaudalis (P < .0001) (Figure 1, A). Importantly, PFMs only elongated their fibers by adding sarcomeres in series, as there were no differences in the length of the sarcomeres between the groups (Figure 1, B). Despite the largest increase in Lfn in coccygeus, this muscle returned to its prepregnancy state by 4 weeks’ postpartum (7.7 ± 0.34 mm in virgins vs 9.0 ± 0.53 mm at 4 weeks’ postpartum, P > .2). Instead, fibers of iliocaudalis (14.8 ± 0.24 mm in virgins vs 18.7 ± 0.61 mm at 4 weeks’ postpartum, P < .0001) and pubocaudalis (16.2 ± 0.38 mm in virgins vs 18.1 ± 0.39 mm at 4 weeks’ postpartum, P <.05) remained significantly elongated at 4 weeks after delivery (Figure 1, A). Twelve weeks after delivery, iliocaudalis and pubocaudalis Lfn did not differ between postpartum and age-matched virgin animals (iliocaudalis: 17.6 ± 0.46 mm in 6-month-old virgins vs 19.0 ± 0.45 mm at 12 weeks’ postpartum, P >.5; pubocaudalis 18.8 ± 0.60 mm in 6-month-old virgins vs 18.53 ± 0.53 mm at 12 weeks’ postpartum, P > .5) (Figure 1, A). Notably, the significant Lfn increase observed in all 3 PFMs in late pregnancy was not present in the tibialis anterior, suggesting a local effect. Specifically, tibialis anterior Lfn was 15.7±0.32 mm in virgins vs 15.9 ± 0.13 mm in late-pregnant rats (P >.9) (Figure 2, A).
FIGURE 1.
Comparison of architectural parameters of C, IC, and PC among virgin, pregnant, and PP groups
Data represented as mean ± SEM.
C, coccygeus; IC, iliocaudalis; Lfn, fiber length normalized to optimal sarcomere length; PC, pubocaudalis; PCSA, physiologic cross-sectional area; PP, postpartum.
*Significantly different P values derived from 2-way analysis of variance, followed by Tukey pairwise comparisons with significance level set to 5%.
Alperin. Pregnancy adaptations in rat pelvic muscles. Am J Obstet Gynecol 2015.
FIGURE 2.
Comparison of architectural parameters of tibialis anterior among virgin, pregnant, and PP groups
Data represented as mean ± SEM.
Lfn, fiber length normalized to optimal sarcomere length; PCSA, physiologic cross-sectional area; PP, postpartum.
Alperin. Pregnancy adaptations in rat pelvic muscles. Am J Obstet Gynecol 2015.
Mass of coccygeus did not change due to pregnancy or delivery (Figure 1, C). Mass of iliocaudalis, on the other hand, significantly increased from 0.12±0.005 g in virgin animals to 0.15 ± 0.005 g by late pregnancy (P <.02), with no difference postpartum (0.16 ± 0.007 g at 12 weeks’ postpartum vs 0.16±0.009 g in 6-month-old virgin rats, P >.5) (Figure 1, C). The increase in pubocaudalis mass by late pregnancy was not significant: 0.11 ± 0.006 g in virgins vs 0.13 ± 0.013 g in late pregnant rats, P > .1. However, a substantial increase in mass was observed at 4 weeks’ postpartum (0.17 ± 0.007 g, P < .0001). Mass of pubocaudalis did not differ from virgin values by late-postpartum period (0.15 ± 0.002 g at 12 weeks’ postpartum vs 0.17 ± 0.007 g in 6-month-old virgin rats, P > .1) (Figure 1, C). There were no changes in PCSA during pregnancy or 12 weeks after delivery in any of the PFMs (Figure 1, D). The tibialis anterior did not differ among groups with respect to Ls, muscle mass, or PCSA (Figure 2, B to D).
There were no differences in intramuscular ECM collagen content between individual PFMs in virgin controls; however all 3 PFMs contained significantly less collagen relative to the tibialis anterior: coccygeus vs tibialis anterior P <.001; iliocaudalis vs tibialis anterior P < .005; pubocaudalis vs tibialis anterior P < .005 (Figure 3). Similar to the differential response of the muscle contractile component in pelvic and non-pelvic muscles, pregnancy impact on collagen of the intramuscular ECM also varied. Total collagen content increased drastically in all 3 PFMs in pregnant animals relative to virgin controls, while remaining unchanged in the tibialis anterior (Table 2). Because collagen content in the tibialis anterior was unchanged by the end of gestational period and at 12 weeks’ postpartum, it was not assessed in mid-pregnant and 4 weeks’ postpartum groups. By mid pregnancy, ECM collagen content was substantially increased only in coccygeus, rising from prepregnancy level of 3.1 ± 0.47 μg/mg to 5.9 ± 0.66 μg/mg; P =.0005. By the end of the gestational period, the amount of ECM collagen more than doubled in coccygeus, reaching 7.5 ± 0.65 μg/mg. Compared to virgin controls, intramuscular collagen content increased by 52% in iliocaudalis in late-pregnant group (from 2.9 ± 0.21 μg/mg to 4.3 ± 0.12 μg/mg; P = .04) and by 75% in pubocaudalis (from 3.5 ± 0.52 μg/mg to 6.1 ± 0.62 μg/mg; P =.001). The dramatic increase in ECM collagen observed in pregnancy was no longer present by 4 weeks’ postpartum in iliocaudalis and pubocaudalis (Table 2). In the postpartum period, ECM collagen content of coccygeus decreased from its peak value in late pregnancy, but continued to be significantly elevated relative to virgin controls, with 6.7 ± 0.68 μg/mg at 4 weeks and 6.37 ± 0.55 μg/mg at 12 weeks’ postpartum (P <.0001).
FIGURE 3.
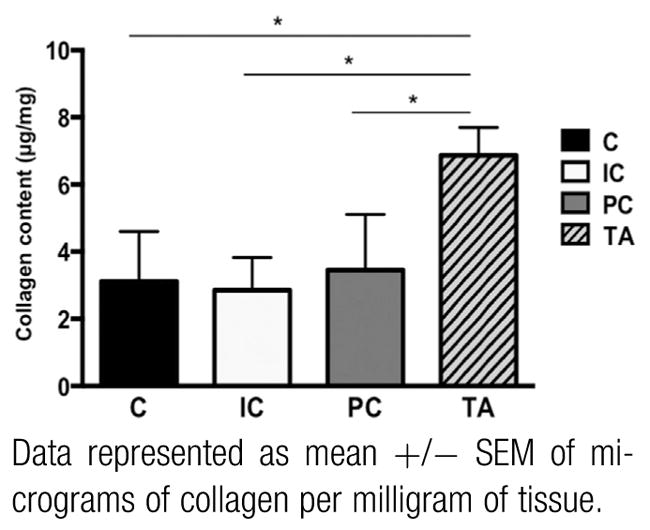
Intramuscular collagen content of C, IC, PC, and TA in virgin controls
Data represented as mean +/− SEM of micrograms of collagen per milligram of tissue.
C, coccygeus; IC, iliocaudalis; PC, pubocaudalis; TA, tibialis anterior.
*Significantly different P values derived from repeated measures analysis of variance, followed by Tukey pairwise comparisons with significance level set to 5%.
Alperin. Pregnancy adaptations in rat pelvic muscles. Am J Obstet Gynecol 2015.
TABLE 2.
Comparison of intramuscular ECM collagen content between virgin, MP, LP, 4 wks PP and 12 wks PP groups
| Group | Coccygeus | Iliocaudalis | Pubocaudalis | Tibialis anterior |
|---|---|---|---|---|
| Virgin | 3.11 ± 0.47 | 2.87 ± 0.27 | 3.46 ± 0.52 | 6.87 ± 0.37 |
| MP | 5.87 ± 0.66 | 3.18 ± 0.11 | 4.90 ± 0.53 | — |
| LP | 7.45 ± 0.65 | 4.34 ± 0.12 | 6.05 ± 0.62 | 7.02 ± 0.54 |
| 4 wks PP | 6.67 ± 0.68 | 2.90 ± 0.22 | 4.14 ± 0.32 | — |
| 12 wks PP | 6.37 ± 0.55 | 2.86 ± 0.34 | 4.96 ± 0.32 | 5.33 ± 0.41 |
| Virgin vs MP, P valuea | .0005 | > .9999 | .2667 | — |
| Virgin vs LP, P valuea | < .0001 | .0198 | .0014 | .9729 |
| MP vs LP, P valuea | .1704 | .0846 | .5891 | — |
| LP vs 4 wks PP, P valuea | .9367 | .0246 | .0449 | — |
| LP vs 12 wks PP, P valuea | .6706 | .0196 | .6559 | .0510 |
| Virgin vs 4 wks PP, P valuea | < .0001 | > .9999 | .9724 | — |
| Virgin vs 12 wks PP, P valuea | < .0001 | > .9999 | .2219 | .0736 |
Data presented as mean +/− SEM of micrograms of collagen per milligram of tissue.
ECM, extracellular matrix; LP, late-pregnant; MP, mid-pregnant; PP, postpartum.
P values derived from two-way analysis of variance, followed by Tukey’s pairwise comparisons with significance level set to 5%.
Alperin. Pregnancy adaptations in rat pelvic muscles. Am J Obstet Gynecol 2015.
Comment
This study demonstrates that pregnancy induces unique adaptations in the intrinsic structure of the striated PFMs. Our results show that, under functional conditions of pregnancy, PFMs adjust their architectural design—specifically, increasing their Lf by adding sarcomeres in series. The largest change in Lf occurred in coccygeus, as this muscle has the shortest fibers when compared to other PFMs. The above adaptation suggests that the muscles would have increased excursion, and may allow muscles to maintain maximum force production despite the increase in mechanical strain observed during parturition. Definitive answers to this question await measurement of Ls during parturition since current models do not have the resolution to address this issue. Muscle injury is primarily caused by excessive sarcomere strain,11,37 which is likely to occur during parturition. Increased Lf is protective against such damage since large mechanical deformations are distributed across a greater Sn.
In contrast to other tissues (vagina, cervix, nipples), collagen content of the intramuscular ECM increased in pregnancy in the PFMs. This expansion accompanies the increase in Lf and presumably also occurs in response to a greater mechanical load exerted on the PFMs in pregnancy. Increased ECM collagen content serves to stabilize the elongated fibers and has a potential to shield muscle fibers from excessive mechanical strain during parturition by providing a parallel elastic element that limits fiber strain. The largest increase in collagen content occurred in coccygeus, corresponding to the biggest Lf increase in this muscle. The accumulation of collagen in the intramuscular ECM persisted in this muscle postpartum, likely due to the tremendous rise during pregnancy. While increased collagen content in the intramuscular ECM is potentially a favorable adaptation during pregnancy and at the time of vaginal delivery, persistent increase in collagen in the late-postpartum period is pathological as it indicates the presence of fibrosis. This can have a detrimental impact on muscle function, as long-term increased stiffness of fibrotic muscles leads to muscle atrophy, reduced force production, decreased excursion, and compromised passive mechanical properties.38–40 In future studies, we will examine the impact of postpartum fibrosis on muscle’s passive mechanical properties.
As an initial step into probing potential mechanisms that govern pregnancy-induced adaptations in the PFMs, we evaluated the tibialis anterior muscle throughout pregnancy. The changes observed in the PFMs were not present in this ankle dorsiflexor. The results suggest that pregnancy-induced adaptations in the pelvic muscles are caused by the local increase in mechanical loading and are not due to the diffuse effects of altered systemic hormonal milieu in pregnancy. It is, however, possible that systemic mediators differentially impact pelvic vs nonpelvic striated muscles.
We are aware of the limitations inherent to the use of a quadrupedal animal model, which also differs from human with regard to the size of the fetus relative to the size of maternal pelvis. However, the rodent model is valuable for establishing the precise structural adaptations that occur in the PFMs throughout pregnancy. This is an important first step toward future work aimed at examining the extent to which such adaptations protect the PFMs from injury during parturition. It is plausible that, to accommodate the delivery of encephalized human fetus while diminishing detrimental effects of excessive muscle strain, the extent of Lf increase in the PFMs in women is even greater than that observed in the rat model. We also appreciate that our specimens were fixed by formaldehyde and, therefore, the spatial and topographical appearance of the PFMs may not correspond to its appearance in vivo due to the loss of muscle tone. Fortunately, these differences in the topology do not appear to affect major determinants of muscle architecture.41
The current study represents a significant shift in the way that PFMs are examined and have substantial implications for future research. Establishing changes that take place in the structure of the PFMs in pregnancy is a prerequisite for moving toward the identification of mechanisms that govern them and the development of strategies to maximize maternal protective adaptations. Determining the impact of pregnancy and vaginal delivery on the PFMs architecture also adds to the understanding of the mechanics of injury of these muscles due to parturition. Finally, our findings can be applied to the computational models of the impact of vaginal deliveries on human PFMs, which are currently limited in that they do not account for pregnancy-induced changes in the architecture and the intramuscular extracellular matrix in these muscles. We suggest that these models will require significant modification to include fiber structural adaptation to represent realistic simulations.
With 3 million vaginal deliveries in the United States annually, it is of utmost importance to gain insights into potentially modifiable protective adaptations that could help prevent injuries of the PFMs during childbirth reducing the associated pathological sequelae.
Acknowledgments
Funding for this research was provided by National Institutes of Health grant numbers K12 HD001259 and R24 HD050837.
Footnotes
The authors report no conflict of interest.
References
- 1.DeLancey JOL, Morgan DM, Fenner DE, et al. Comparison of levator ani muscle defects and function in women with and without pelvic organ prolapse. Obstet Gynecol. 2007;109:295–302. doi: 10.1097/01.AOG.0000250901.57095.ba. [DOI] [PubMed] [Google Scholar]
- 2.Memon HU, Handa VL. Vaginal childbirth and pelvic floor disorders. Womens Health (Lond Engl) 2013;9:265–77. doi: 10.2217/whe.13.17. quiz 276–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kearney R, Fitzpatrick M, Brennan S, et al. Levator ani injury in primiparous women with forceps delivery for fetal distress, forceps for second stage arrest, and spontaneous delivery. Int J Gynaecol Obstet. 2010;111:19–22. doi: 10.1016/j.ijgo.2010.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lien KC, Mooney B, DeLancey JO, Ashton-Miller JA. Levator ani muscle stretch induced by simulated vaginal birth. Obstet Gynecol. 2004;103:31–40. doi: 10.1097/01.AOG.0000109207.22354.65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hoyte L, Damaser MS, Warfield SK, et al. Quantity and distribution of levator ani stretch during simulated vaginal childbirth. Am J Obstet Gynecol. 2008;199:198, e1–5. doi: 10.1016/j.ajog.2008.04.027. [DOI] [PubMed] [Google Scholar]
- 6.Krofta L, Otcenasek M, Kasikova E, Feyereisl J. Pubococcygeus-puborectalis trauma after forceps delivery: evaluation of the levator ani muscle with 3D/4D ultrasound. Int Urogynecol J Pelvic Floor Dysfunct. 2009;20:1175–81. doi: 10.1007/s00192-009-0837-6. [DOI] [PubMed] [Google Scholar]
- 7.Shek KL, Dietz HP. Intrapartum risk factors for levator trauma. BJOG. 2010;117:1485–92. doi: 10.1111/j.1471-0528.2010.02704.x. [DOI] [PubMed] [Google Scholar]
- 8.Dietz HP, Lanzarone V. Levator trauma after vaginal delivery. Obstet Gynecol. 2005;106:707–12. doi: 10.1097/01.AOG.0000178779.62181.01. [DOI] [PubMed] [Google Scholar]
- 9.Cassado Garriga J, Pessarrodona Isern A, Espuna Pons M, Duran Retamal M, Felgueroso Fabregas A, Rodriguez-Carballeira M. Tridimensional sonographic anatomical changes on pelvic floor muscle according to the type of delivery. Int Urogynecol J. 2011;22:1011–8. doi: 10.1007/s00192-011-1413-4. [DOI] [PubMed] [Google Scholar]
- 10.Faulkner JA, Brooks SV, Opiteck JA. Injury to skeletal muscle fibers during contractions: conditions of occurrence and prevention. Phys Ther. 1993;73:911–21. doi: 10.1093/ptj/73.12.911. [DOI] [PubMed] [Google Scholar]
- 11.Warren GL, Hayes DA, Lowe DA, Armstrong RB. Mechanical factors in the initiation of eccentric contraction-induced injury in rat soleus muscle. J Physiol. 1993;464:457–75. doi: 10.1113/jphysiol.1993.sp019645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lieber RL, Friden J. Mechanisms of muscle injury gleaned from animal models. Am J Phys Med Rehabil. 2002;81(Suppl):S70–9. doi: 10.1097/00002060-200211001-00008. [DOI] [PubMed] [Google Scholar]
- 13.Daucher JA, Clark KA, Stolz DB, Meyn LA, Moalli PA. Adaptations of the rat vagina in pregnancy to accommodate delivery. Obstet Gynecol. 2007;109:128–35. doi: 10.1097/01.AOG.0000246798.78839.62. [DOI] [PubMed] [Google Scholar]
- 14.Alperin M, Feola A, Duerr R, Moalli P, Abramowitch S. Pregnancy- and delivery-induced biomechanical changes in rat vagina persist postpartum. Int Urogynecol J. 2010;21:1169–74. doi: 10.1007/s00192-010-1149-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Oliphant SS, Nygaard IE, Zong W, Canavan TP, Moalli PA. Maternal adaptations in preparation for parturition predict uncomplicated spontaneous delivery outcome. Am J Obstet Gynecol. 2014;211:630, e1–7. doi: 10.1016/j.ajog.2014.06.021. [DOI] [PubMed] [Google Scholar]
- 16.O’Boyle AL, O’Boyle JD, Calhoun B, Davis GD. Pelvic organ support in pregnancy and postpartum. Int Urogynecol J Pelvic Floor Dysfunct. 2005;16:69–72. doi: 10.1007/s00192-004-1210-4. [DOI] [PubMed] [Google Scholar]
- 17.Sze EH, Sherard GB, III, Dolezal JM. Pregnancy, labor, delivery, and pelvic organ prolapse. Obstet Gynecol. 2002;100:981–6. doi: 10.1016/s0029-7844(02)02246-9. [DOI] [PubMed] [Google Scholar]
- 18.Shek KL, Kruger J, Dietz HP. The effect of pregnancy on hiatal dimensions and urethral mobility: an observational study. Int Urogynecol J. 2012;23:1561–7. doi: 10.1007/s00192-012-1795-y. [DOI] [PubMed] [Google Scholar]
- 19.Siafarikas F, Staer-Jensen J, Hilde G, Bo K, Ellstrom Engh M. Levator hiatus dimensions in late pregnancy and the process of labor: a 3-and 4-dimensional transperineal ultrasound study. Am J Obstet Gynecol. 2014;210:484, e1–7. doi: 10.1016/j.ajog.2014.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Bo K, Hilde G, Tennfjord MK, Staer-Jensen J, Siafarikas F, Engh ME. Pelvic floor muscle variables and levator hiatus dimensions: a 3/4D transperineal ultrasound cross-sectional study on 300 nulliparous pregnant women. Int Urogynecol J. 2014;25:1357–61. doi: 10.1007/s00192-014-2408-8. [DOI] [PubMed] [Google Scholar]
- 21.Lieber RL, Friden J. Functional and clinical significance of skeletal muscle architecture. Muscle Nerve. 2000;23:1647–66. doi: 10.1002/1097-4598(200011)23:11<1647::aid-mus1>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 22.Gillies AR, Lieber RL. Structure and function of the skeletal muscle extracellular matrix. Muscle Nerve. 2011;44:318–31. doi: 10.1002/mus.22094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Purslow PP. Muscle fascia and force transmission. J Bodyw Mov Ther. 2010;14:411–7. doi: 10.1016/j.jbmt.2010.01.005. [DOI] [PubMed] [Google Scholar]
- 24.Lara-Garcia M, Alvarado M, Cuevas E, et al. The effects of castration and hormone replacement on the cross-sectional area of pubococcygeus muscle fibers in the female rat. Anat Rec (Hoboken) 2011;294:1242–8. doi: 10.1002/ar.21414. [DOI] [PubMed] [Google Scholar]
- 25.Bremer RE, Barber MD, Coates KW, Dolber PC, Thor KB. Innervation of the levator ani and coccygeus muscles of the female rat. Anat Rec A Discov Mol Cell Evol Biol. 2003;275:1031–41. doi: 10.1002/ar.a.10116. [DOI] [PubMed] [Google Scholar]
- 26.Alperin M, Tuttle LJ, Conner BR, et al. Comparison of pelvic muscle architecture between humans and commonly used laboratory species. Int Urogynecol J. 2014;25:1507–15. doi: 10.1007/s00192-014-2423-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kokenyesi R, Woessner JF., Jr Effects of hormonal perturbations on the small dermatan sulfate proteoglycan and mechanical properties of the uterine cervix of late pregnant rats. Connect Tissue Res. 1991;26:199–205. doi: 10.3109/03008209109152438. [DOI] [PubMed] [Google Scholar]
- 28.Lowder JL, Debes KM, Moon DK, Howden N, Abramowitch SD, Moalli PA. Biomechanical adaptations of the rat vagina and supportive tissues in pregnancy to accommodate delivery. Obstet Gynecol. 2007;109:136–43. doi: 10.1097/01.AOG.0000250472.96672.6c. [DOI] [PubMed] [Google Scholar]
- 29.Swan MA, Sato E, Galatz LM, Thomopoulos S, Ward SR. The effect of age on rat rotator cuff muscle architecture. J Shoulder Elbow Surg. 2014;23:1786–91. doi: 10.1016/j.jse.2014.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lieber RL, Yeh Y, Baskin RJ. Sarcomere length determination using laser diffraction: effect of beam and fiber diameter. Biophys J. 1984;45:1007–16. doi: 10.1016/S0006-3495(84)84246-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Burkholder TJ, Lieber RL. Sarcomere length operating range of vertebrate muscles during movement. J Exp Biol. 2001;204:1529–36. doi: 10.1242/jeb.204.9.1529. [DOI] [PubMed] [Google Scholar]
- 32.Powell PL, Roy RR, Kanim P, Bello MA, Edgerton VR. Predictability of skeletal muscle tension from architectural determinations in guinea pig hindlimbs. J Appl Physiol Respir Environ Exerc Physiol. 1984;57:1715–21. doi: 10.1152/jappl.1984.57.6.1715. [DOI] [PubMed] [Google Scholar]
- 33.Ward SR, Lieber RL. Density and hydration of fresh and fixed human skeletal muscle. J Biomech. 2005;38:2317–20. doi: 10.1016/j.jbiomech.2004.10.001. [DOI] [PubMed] [Google Scholar]
- 34.Edwards CA. Modified assay for determination of hydroxyproline in a tissue hydrolyzate. Clin Chim Acta. 1980;104:161–7. doi: 10.1016/0009-8981(80)90192-8. [DOI] [PubMed] [Google Scholar]
- 35.Lieber RL. Statistical significance and statistical power in hypothesis testing. J Orthop Res. 1990;8:304–9. doi: 10.1002/jor.1100080221. [DOI] [PubMed] [Google Scholar]
- 36.Tuttle LJ, Ward SR, Lieber RL. Sample size considerations in human muscle architecture studies. Muscle Nerve. 2012;45:742–5. doi: 10.1002/mus.23283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lieber RL, Friden J. Muscle damage is not a function of muscle force but active muscle strain. J Appl Physiol. 1985;1993:520–6. doi: 10.1152/jappl.1993.74.2.520. [DOI] [PubMed] [Google Scholar]
- 38.Lieber RL, Ward SR. Cellular mechanisms of tissue fibrosis, 4: structural and functional consequences of skeletal muscle fibrosis. Am J Physiol Cell Physiol. 2013;305:C241–52. doi: 10.1152/ajpcell.00173.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lieber RL, Runesson E, Einarsson F, Friden J. Inferior mechanical properties of spastic muscle bundles due to hypertrophic but compromised extracellular matrix material. Muscle Nerve. 2003;28:464–71. doi: 10.1002/mus.10446. [DOI] [PubMed] [Google Scholar]
- 40.Järvinen TAH, Józsa L, Kannus P, Järvinen TLN, Järvinen M. Organization and distribution of intramuscular connective tissue in normal and immobilized skeletal muscles. J Muscle Res Cell Motil. 2002;23:245–54. doi: 10.1023/a:1020904518336. [DOI] [PubMed] [Google Scholar]
- 41.Janda S, van der Helm FC, de Blok SB. Measuring morphological parameters of the pelvic floor for finite element modeling purposes. J Biomech. 2003;36:749–57. doi: 10.1016/s0021-9290(03)00008-3. [DOI] [PubMed] [Google Scholar]