Abstract
Posttraumatic stress disorder (PTSD) is a psychiatric illness whose prevalence in women is more than twice the rate as men. Despite a burgeoning literature characterizing sex differences in PTSD incidence and its disproportionate burden on society, there is a dearth of literature describing biological mechanisms underlying these disparities. However, the recent identification of biomarkers of PTSD by translational neuroscientists offers a promising opportunity to explore sex interactions in PTSD phenotypes. A notable observation is that individuals with PTSD show deficits in their ability to inhibit conditioned fear responding after extinction training. Given that extinction procedures, via exposure-based cognitive behavioral therapy, make up one of the predominant modes of treatment in PTSD, there is a critical need for more research on sex interactions in this form of fear regulation. An emerging hypothesis is that fluctuating gonadal hormones, especially estrogen, in the menstrual cycle may play a critical role in fear extinction and, hence, PTSD vulnerability and symptom severity in women. The current review discusses how the study of putative activational effects of estrogen on fear extinction may be harnessed to advance the search for better treatments for PTSD in women. We conclude that estrogen treatment may be a putative pharmacological adjunct in extinction based therapies, and should be tracked in the menstrual cycle during the course of PTSD treatment.
Keywords: Extinction, Fear conditioning, Estrogen, PTSD, Anxiety, Sex differences
Introduction
It is widely understood that women have a significantly higher occurrence of anxiety disorders than men (1, 2). Posttraumatic stress disorder (PTSD), a severe and debilitating anxiety disorder that may develop following a traumatic experience, is one of the most sex-polarized psychiatric illnesses, with women having more than twice the prevalence than men (3-6). Women are also more likely to experience chronic PTSD that persists for more than a year (4, 5, 7) and carry a greater burden of this illness on the healthcare infrastructure (1). While there is much debate about what factors contribute to these disparities –from sociocultural and environmental influences (8) to sexual dimorphisms in physiology – there is, surprisingly, very little known about the biological mechanisms that underlie sex differences in PTSD. The overreliance of male subjects in preclinical animal models and in clinical research has, thus far, limited our progress in understanding the nature of psychiatric illnesses that disproportionately affect women. Recently, however, a few studies have linked the cyclical release of ovarian hormones to PTSD vulnerability in women (9-11). These findings are particularly important given that anxiety disorders tend to emerge around puberty, when sex organs begin to release hormones that have activational effects on brain activity. The current review discusses how this recent work – along with an accumulating line of evidence suggesting activational effects of gonadal steroid hormones on female emotional regulation – may be harnessed to advance the search for better treatments for PTSD in women.
The role of fluctuating estrogen on anxiety-related behaviors
Sex differences in rates of anxiety disorders typically emerge after puberty, when circulating ovarian hormones increase (12, 13), implicating activational sex steroids (i.e., estrogens, progestogens, and androgens) in the expression of psychopathology (14). Among the sex steroids, estrogen has come forth as a leading candidate for the study of gonadal hormone influence on female brain and behavior. At sexual maturity, females experience cyclic fluctuations in estrogen secretions throughout their reproductive cycle, ending in rapid decline during menopause. Interestingly, women are more likely to report symptoms of depression and anxiety during pre-menstrual, post-partum and peri- and post-menopausal periods, when estrogen levels are relatively low (14-20).
Neuroimaging studies show greater activation of neural networks involved in fear excitation when women are scanned during the early follicular phase of their menstrual cycle (marked by low estrogen levels) relative to those scanned mid-cycle (high estrogen levels) (21, 22). Moreover, studies using rodent models of anxiety have shown that females in the pro-estrus phase of their cycle (marked by high estrogen levels) show less fear- and anxiety-related behaviors relative to females in the metestrus or diestrus phases (lower estrogen levels; (23-26). Together, these findings suggest that cyclical secretions of estrogen across the reproductive cycle may play a role in women's increased vulnerability to, and the severity of, PTSD symptoms after psychological trauma. However, very few studies have taken into account the estrous cycle, or studied phasic estrogen levels in laboratory-based models that explicitly probe for PTSD phenotypes. In fact, many studies intentionally exclude female subjects to eliminate this source of variability. However, a more inclusive research approach is critical for understanding sex interactions in PTSD vulnerability. In the following sections, we discuss an effective approach for identifying biomarkers for PTSD, and describe research to date that have used this approach to examine an activational role of fluctuating estrogen on PTSD risk in women.
Use of fear conditioning models to study biomarkers for PTSD
Most individuals who experience trauma do not develop PTSD in its aftermath. Therefore, a major goal in psychiatric research is to identify what factors confer resilience or vulnerability to traumatic life events. The three primary symptom clusters that characterize PTSD, although differentially characterized by the Diagnostic and Statistical Manual for Mental Disorders (DSM) versions IV and 5, include: (1) persistent and intrusive recollections of the traumatic event, (2) hyperactivity and increased arousal, usually in response to trauma reminders, and (3) avoidance of sensory cues associated with the traumatic event (27); dominant features of the disorder that reflect memory-related mechanisms. Hence, many translational researchers have focused on the neural bases of fear-motivated learning and memory to better understand the biological underpinnings of this disorder. It is generally believed that traumatic memories form when individuals contemporaneously experience neutral stimuli with highly aversive stimuli in their environment. Many behavioral scientists attribute this to associative learning, a phenomenon that has been extensively modeled in animals including rodents and non-human primates (28-30).
In Pavlovian fear conditioning, a subject is exposed to a previously neutral conditioned stimulus (CS), which is often presented in the form of a discrete cue, such as an odor, light, or tone (cued fear conditioning), or a distinctive environment (context fear conditioning), that overlaps in time with an aversive unconditioned stimulus (US), such as a footshock or an aversive airblast. Consequently, subjects show a species-specific response, such as increased startle or freezing behavior, in the presence of the reinforced CS due to its prior association with the US. The CS may also elicit autonomic (e.g., changes in heart rate or blood pressure) and endocrine (e.g., hormone release) responses. Thus, memory is inferred from a quantifiable behavioral or physiological change observed in the presence versus the absence of the CS.
In the clinical presentation of PTSD, fear responses closely resemble those observed in fear conditioning models (i.e., increased arousal and hyper-reactivity in the presence of trauma-related cues). A major challenge in PTSD research has been to understand the neural mechanisms involved in the reduction of these conditioned fear responses, and finding tools in which one can inhibit or eliminate maladaptive behaviors associated with unwanted fear memories.
Fear extinction is one of the leading experimental models for measuring the ability to suppress a conditioned fear response. In this model, the amplitude and frequency of conditioned fear responses may be gradually reduced by repeated presentations of the previously CS in the absence of the aversive US. Our laboratory has consistently found that people with PTSD show deficits in fear inhibition (31, 32) and extinction (33), suggesting that impaired inhibition of conditioned fear may be a biomarker of PTSD (34).
Although fear extinction models have been critical in shaping our understanding of the neurobiological basis of fear regulation, there are very few studies that examined sex differences or observed the female behavioral response using this paradigm. Given that extinction procedures comprise some of the evidence-based modes of treatment of PTSD and other trauma-, stressor-, and anxiety-related disorders (i.e., exposure-based cognitive behavioral therapy or CBT), there is a critical need for more research on sex interactions in fear conditioning and extinction processes.
Sex interactions in fear conditioning
In general, male rats outperform females in tests of conditioned fear acquisition. In contextual fear conditioning, male rats have shown enhanced acquisition (35, 36), which has been associated with increased long-term potentiation (LTP) in the hippocampus compared to females (35). This effect was not altered by castration of the adult male rat (37). However, ovariectomized (OVX) female rats showed enhanced fear expression, similar to males; an effect that normalized back to control levels with estrogen replacement (38). A similar pattern of results was found in tone-induced cued fear conditioning (35, 36, 39, 40). These findings suggest a role of estrogen in reducing the over-expression of fear following acquisition in females via synaptic plasticity, but indicate no such function of testosterone in males.
Conflicting findings have been reported when conditioned fear retention was measured and when the estrous cycle of females was taken into account. That is, female rats that were conditioned and tested during proestrus (when estrogen levels are at their peak) showed less contextual fear retention compared to both males and estrus females (when estrogen levels are low; (41). However, there was no sex difference in cued fear retention (41). Together, these findings implicate an important role of estrogen in hippocampal-dependent fear learning and memory.
Role of sex hormones in conditioned fear extinction
Fear extinction is believed to be a form of hippocampal-dependent learning in concert with brain areas including prefrontal cortical regions (42). Given previous evidence of estrogen involvement in other hippocampal-dependent fear conditioning tasks (35-37, 41), it is reasonable to hypothesize a role for estrogen in this model. Nevertheless, few studies have used fear extinction paradigms to examine sex and gonadal hormone interactions. In one of the first studies to implicate sex steroids in fear extinction processes, Rivas-Arancibia and Vazquez-Pereyra (43) found that both testosterone and estradiol treatment facilitated extinction of a single-trial passive avoidance task in male rats. Since steroid hormone treatments were given prior to avoidance training and then again before each of 10 weekly extinction trials, it is difficult to disentangle the mechanism of action of steroid hormones on fear acquisition versus extinction acquisition and/or retention (43). Also, the role of sex differences in extinction was not addressed as only male rats were examined.
Sex differences in fear extinction have since been reported in rats, whereby naturally cycling female rats exhibited impairments in extinction recall compared to males (39, 44). However, female estrous cycle phase was not an experimental variable in those studies. A formative study by Chang and colleagues (45) found significant sex and estrous phase differences in contextual fear extinction in rats. Specifically, they replicated previous findings that male rats exhibited enhanced context fear acquisition relative to naturally cycling females. However, when fear extinction rate was determined in relation to estrous stage, females in proestrus and estrus extinguished more rapidly than males and females in diestrus (45). In further support for a role of estrogen in extinction, they found that OVX females treated with estradiol extinguished more rapidly than both vehicle-treated and progesterone-treated OVX females. In addition, this facilitating effect of estradiol on extinction was replicated when OVX females were treated with a selective estrogen receptor (ER) β agonist, but not with a selective ERα agonist (45). These findings strongly implicate estrogen in fear extinction modulation due to ERβ activation.
In perhaps the most comprehensive set of studies examining sex and estrous cycle influences on fear extinction, Milad and colleagues have established an important role of estrogen in extinction mechanisms in female rats and women (see (46) for review). Importantly, they demonstrated that when cycle phase was not factored in their analyses, no differences in fear extinction were found between males and females. However, sex differences were evident when females were divided into low and high estradiol groups based on their phase in estrous or menstrual cycle (47-50). Specifically, females in the high estradiol group expressed extinction similar to males, and both groups expressed greater extinction retention than females in the low estradiol group (48, 49). A recent study that examined fear conditioning and extinction while collecting saliva hormonal levels also found that low estradiol, but not progesterone, levels were associated with higher fear responses during extinction (51). Moreover, this study found that women who had low levels of estrogen during the observation of violent film clips had more intrusive thoughts about these scenes in the following days; this finding is of high relevance to the re-experiencing symptoms of PTSD (51). It may appear contradictory that females, who should be protected by higher estrogen levels relative to men, show higher vulnerability to PTSD. However, taking into account the cyclic nature of estrogen in females and the more constant endogenous estrogen levels in males (albeit on average lower than those in females) these data suggest a phasic vulnerability in women. These findings underscore the importance of taking cycle phase into consideration when examining sex interactions in fear learning and memory. Given the additional observation that administration of selective ERβ but not ERα agonists to female rats in the metestrus phase (low estrogen) improved extinction recall (50) and that blockade of ERβ and ERα in proestrus females impaired extinction recall (48), there is convincing evidence to conclude that activational estrogen exposure is critical for fear extinction in females, and ERβ is a critical site of activation. These observations, tying fear extinction to estrogen activity at ERβ, hold clinical promise for the use of estrogen as a pharmacological adjunct in PTSD treatment. More clinical research is clearly needed to characterize estrogen function in women who are at risk for or have developed PTSD.
Estrogen and PTSD
It has been established that PTSD is a disorder of the fear memory circuitry and that individuals with PTSD show deficits in extinction. Neurobiological models of PTSD hold that the maladaptive fear responses observed in PTSD are due to heightened activity in the amygdala (52) in concert with impaired inhibitory control of the amygdala from prefrontal cortical regions (53). This fear regulatory network has been well-characterized as playing a critical role in extinction learning and memory (54, 55). Estrogen receptors, both ERα and ERβ, are densely expressed in the amygdala, hypothalamus, and hippocampal cortical regions (56-59), and express sexually dimorphic intracellular processing (60, 61), suggesting sex-specific estrogen modulation of emotion and cognition. However, the functional consequences of estrogen-induced plastic changes within the fear extinction network are not fully understood. Estrogen may influence extinction via genomic mechanisms by binding to classical nuclear receptors and initiating long-lasting transcription-related events. However, recent advances suggest that estrogen may also act at membrane receptors (which are similar or identical to classical receptors) to initiate rapid effects via signal transduction pathways (62, 63). The fact that acute estrogen administration induces rapid and reversible changes in synaptic plasticity within the extinction network (64, 65) and in extinction memory (48-50) suggests that non-genomic estrogen activity at membrane receptors may largely mediate these facilitatory effects. A number of candidate cellular and molecular markers have been linked to estrogen activity within the extinction network, such as increased NMDA- (66) and AMPA- (67) receptor activity, and increases in key synaptic proteins such as MAPK-dependent CREB phosphorylation (68, 69) and brain-derived neurotrophic factor (BDNF) expression (63, 70, 71). Nevertheless, a great deal of research is needed to delineate the precise mechanisms underlying estrogen modulation of fear extinction. By establishing a role of estrogen in this neural circuitry and in extinction function, there is a unique opportunity to pharmacologically target this behavioral phenotype in women with PTSD (72).
Functional magnetic resonance imaging studies (fMRI) have found sex differences in fear-associated neural activation patterns (46, 73, 74). Changes in brain activity in areas associated with fear regulation occur across the menstrual cycle in time with fluctuating gonadal hormones (21, 75). Women who are ovulating (high estrogen) show attenuated activity in the amygdala and related subcortical regions and increased activity in those regions during the early follicular phase of their menstrual cycle (low estrogen) (21, 22). Together, these findings establish a dynamic neuromodulatory role of estrogen on female brain function during emotion processing, and provide firm rationale for research examining the role of estrogen in PTSD vulnerability and symptom severity. In addition to the above neural activation, peripheral biomarkers are also influenced by menstrual cycle changes. For example, the acoustic startle reflex, which is mediated by amygdala activity, is enhanced during periods of lower estrogen (76, 77).
Current work from our group and others has employed a translational approach to characterize biomarkers of PTSD in a clinical population using fear potentiation of the acoustic startle response. By measuring conditioned fear in a traumatized sample, we are well suited to examine estrogen influences on PTSD risk in women. We previously found that people with PTSD show impairments in their ability to inhibit conditioned fear responses in the presence of safety cues (78, 79), and also show deficits in fear extinction (79). In addition, women with PTSD exhibit higher expression of conditioned fear compared to men with PTSD (80). In the first study to examine estrogen interactions in these behavioral phenotypes in a clinical sample, we recently identified a potential role for estrogen in PTSD vulnerability and severity in women (9). Specifically, the mechanism underlying this role is still not well understood, but recent evidence suggests an interaction between ovarian hormone levels and genetic risk. For example, a single nucleotide polymorphism rs2267735 in the pituitary adenylate cyclase activating protein (PACAP) receptor gene, ADCYAP1R1, located within a canonical estrogen response element, is associated with increased PTSD symptoms (81), brain activation to fearful stimuli (82), and elevated startle responses (81). In addition, plasma levels of the PACAP peptide were positively associated with increased PTSD symptom expression (81). The PACAP—PAC1R pathway has been linked to numerous fear- and anxiety-related phenotypes with several animal models demonstrating a role of the PACAP pathway in controlling HPA-axis activation during stress responses, activation of adrenal medulla activation in the periphery, and corticosterone regulation ((83).
Notably, the sex differences in ADCYAP1R1 effects on startle responses were not observed in prepubertal children, emphasizing the relevance of activational effects of ovarian hormones (84). The interaction between PACAP receptor genomics and environmental influences ((85) remains under investigation and there have been some recent conflicting reports in the recent literature (86). Nevertheless, this type of gene by environment (G×E) analyses will better inform the tailoring of fear-and anxiety-disorder treatments that better account for genomic, hormonal, and environmental complexity and heterogeneity.
In a separate series of studies, we assessed fear conditioning and extinction in traumatized women with and without PTSD and assayed their serum estradiol levels. We then divided the sample into high versus low levels of estradiol at the time of testing (9). Our findings showed that high levels of estrogen was protective against the extinction deficit observed in women with PTSD and low estrogen, so that they did not differ from trauma controls. Figure 1 summarizes the effects of plasma estrogen levels on fear extinction in traumatized women within our psychophysiological paradigm and others in the literature. The optimal range of estrogen on in vivo fear learning and fear extinction in humans remains to be elucidated. Certainly, there are potentially adverse effects of estrogen levels that are too high that must be explored.
Figure 1.
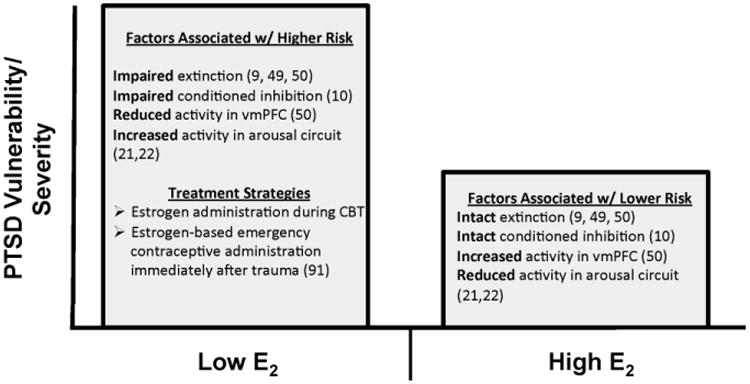
Schematic summarizing risk factors related to estrogen levels in PTSD vulnerability and severity based on preclinical and clinical investigations (9-10; 45-51). Although not depicted in the figure, it is understood that these factors operate within the context of a larger gene by environment perspective and that the beneficial “dose” range of estrogen must be further explored.
Clinical significance
The association between low estrogen and fear extinction has great clinical significance, as they suggest that elevating estrogen levels in some patients may prove beneficial in rescuing extinction deficits observed in PTSD. A recent proof-of-concept study found that administration of synthetic estrogen to normally cycling healthy volunteers increased extinction recall after fear extinction training (87). Due to the fact that clinical exposure therapy recruits neural learning mechanisms that mediate conditioned fear extinction, the adjuvant use of estrogen could facilitate therapeutic effects in fewer sessions. Such effects have been found with other pharmacological agents that enhance learning, such as d-cycloserine (DCS; (88). Although the latter study by Rothbaum and colleagues (2014) did not demonstrate superior effects of DCS, as compared to placebo, in terms of clinical treatment response in general, the Rothbaum group found that DCS augmentation of extinction-based psychotherapy reduced acoustic startle reactivity more than did alprazolam or placebo. Thus, in similar clinical trial paradigms, it remains possible that estrogen could be used specifically in women with low circulating levels and high PTSD symptoms. Similar studies have found that estradiol administration significantly alleviated symptoms of depression in peri-menopausal women (89, 90). A recent naturalistic study compared sexual assault victims who either received or declined estrogen-based emergency contraceptives immediately after the assault and found that those who took estrogen had significantly lower PTSD symptoms 6 months later(91). The studies reviewed here support the use of estrogen as an individualized treatment strategy in women with PTSD.
Limitations/Future Directions
The facilitation of extinction learning through pharmacological and non-pharmacological manipulations has been the focus of translational research for several years. As discussed previously, interest in DCS was renewed within the pharmacological arena given its early clinical success ((92) whereas non-pharmacological efforts were driven by recent work in the area of reconsolidation updating as a means of enhancing fear extinction ((93-95). An important series of next steps will be further investigation of updating fear memories through reconsolidation in humans as well as determining the degree to which estrogen manipulations can enhance extinction learning and prevent the return of fear.
Follow-up studies will also address influences that have not been directly investigated in well-controlled studies. This includes, but is not limited to, a focus on other hormones and hormone-hormone interactions (e.g., testosterone and progesterone), assessment of hormonal metabolites (e.g.,allopregnanolone), and the within-subjects study of fear processing across phases of the menstrual cycle. Sex differences as indexed by estrogen levels can appear contradictory, given that women should, in general, have higher estrogen levels than men, yet are more vulnerable. A study by Milad and others (49) compared women with high and low estrogen to men and found that women with high estrogen had equally successful levels of extinction recall to men; Both groups differed from low estrogen women. It is likely that endogenous estrogen in men, combined with the cyclic nature of estrogen in women, accounts for these apparently counterintuitive findings.
Given that prolonged estrogen treatment has been previously linked to adverse health consequences, such as cancer, stroke, liver disease and lupus (96), its mental health benefits must be carefully balanced against other disease risks. Many factors must be taken into consideration when designing estrogen-based PTSD therapies, including dose and route of administration, as well as timing, and duration of estrogen exposure. In addition, individualized treatment strategies should be designed within the context of preexisting factors such as patients'; PTSD symptom severity, possible family history of disease and the existence of genetic polymorphisms associated with disease risk. Considering the enormous burden of mental health disease on society, there is an urgent need for more clinical research examining the benefit potential of estrogen treatment while identifying those factors that predict health risks (97) keeping in mind the potential benefits of non-drug approaches, such as the systematic monitoring of menstrual cycle phase during therapy.
Conclusions
To summarize, translational studies of stress, trauma, and anxiety have often excluded female subjects due to the degree of variance introduced by fluctuating hormone levels. With a focus on both animal and human studies, this review not only highlights the importance of shifting focus to sex differences in laboratory investigations of psychiatric illness vulnerability and symptom severity, but suggests how such a focus may improve treatment outcome for specific traumatized populations. In this review, we focused on observed effects of estrogen; however, this research could be incorporated into an expanded optimization of treatment that includes assessment of genetic and environmental risk. There is strong evidence that estrogen levels are highly influential during and after the extinction of conditioned fear and, as such, clinical researchers and clinicians should capitalize on this potential avenue of therapeutic intervention.
Acknowledgments
This work was funded in part by the Brain and Behavior Foundation (formerly NARSAD; S.D.N. and T.J.), the Department of Defense (DOD)/Congressionally Directed Medical Research Program (CDMRP, Award # W81XWH-08-2-0170) (PI, S.D.N.), the Emory University Research Committee, a PHS Grant (UL1 RR025008) from the Clinical and Translational Science Award program, National Institutes of Health, National Center for Research Resources (S.D.N.), National Institutes of Mental Health (F32 MH100880, PI, E.M.G., R01 MH100122, PI, T.J.).
Footnotes
Financial Disclosures: Drs. Glover, Norrholm, and Jovanovic report no biomedical financial interests or potential conflicts of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.McLean CP, Asnaani A, Litz BT, Hofmann SG. Gender differences in anxiety disorders: Prevalence, course of illness, comorbidity and burden of illness. Journal of psychiatric research. 2011;45:1027–1035. doi: 10.1016/j.jpsychires.2011.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Solomon MB, Herman JP. Sex differences in psychopathology: Of gonads, adrenals, and mental illness. Physiology & Behavior. 2009;97:250–258. doi: 10.1016/j.physbeh.2009.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Resnick HS, Kilpatrick DG, Dansky BS, Saunders BE, Best CL. Prevalence of civilian trauma and posttraumatic stress disorder in a representative national sample of women. J Consult Clin Psychol. 1993;61:984–991. doi: 10.1037//0022-006x.61.6.984. [DOI] [PubMed] [Google Scholar]
- 4.Kessler RC, Sonnega A, Bromet E, Hughes M, Nelson CB. Posttraumatic stress disorder in the National Comorbidity Survey. Archives of general psychiatry. 1995;52:1048–1060. doi: 10.1001/archpsyc.1995.03950240066012. [DOI] [PubMed] [Google Scholar]
- 5.Breslau N, Kessler RC, Chilcoat HD, Schultz LR, Davis GC, Andreski P. Trauma and posttraumatic stress disorder in the community: the 1996 Detroit Area Survey of Trauma. Archives of general psychiatry. 1998;55:626–632. doi: 10.1001/archpsyc.55.7.626. [DOI] [PubMed] [Google Scholar]
- 6.Kilpatrick DG, Resnick HS, Milanak ME, Miller MW, Keyes KM, Friedman MJ. National Estimates of Exposure to Traumatic Events and PTSD Prevalence Using DSM-IV and DSM-5 Criteria. Journal of traumatic stress. 2013;26:537–547. doi: 10.1002/jts.21848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Breslau N, Davis GC. Posttraumatic stress disorder in an urban population of young adults: risk factors for chronicity. The American journal of psychiatry. 1992;149:671–675. doi: 10.1176/ajp.149.5.671. [DOI] [PubMed] [Google Scholar]
- 8.Laredo SA, Villalon Landeros R, Trainor BC. Rapid effects of estrogens on behavior: environmental modulation and molecular mechanisms. Front Neuroendocrinol. 2014;35:447–458. doi: 10.1016/j.yfrne.2014.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, et al. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological psychiatry. 2012;72:19–24. doi: 10.1016/j.biopsych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Glover EM, Mercer KB, Norrholm SD, Davis M, Duncan E, Bradley B, et al. Inhibition of fear is differentially associated with cycling estrogen levels in women. J Psychiatry Neurosci. 2013;38:120129. doi: 10.1503/jpn.120129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Shvil E, Sullivan GM, Schafer S, Markowitz JC, Campeas M, Wager TD, et al. Sex differences in extinction recall in posttraumatic stress disorder: a pilot fMRI study. Neurobiol Learn Mem. 2014;113:101–108. doi: 10.1016/j.nlm.2014.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Earls F. Sex differences in psychiatric disorders: origins and developmental influences. Psychiatr Dev. 1987;5:1–23. [PubMed] [Google Scholar]
- 13.Paus T, Keshavan M, Giedd JN. Why do many psychiatric disorders emerge during adolescence? Nat Rev Neurosci. 2008;9:947–957. doi: 10.1038/nrn2513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Epperson CN, Kim DR, Bale TL. Estradiol modulation of monoamine metabolism: one possible mechanism underlying sex differences in risk for depression and dementia. JAMA Psychiatry. 2014;71:869–870. doi: 10.1001/jamapsychiatry.2014.729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cloitre M, Yonkers KA, Pearlstein T, Altemus M, Davidson KW, Pigott TA, et al. Women and anxiety disorders: implications for diagnosis and treatment. CNS Spectr. 2004;9:1–16. [PubMed] [Google Scholar]
- 16.Dean C, Kendell RE. The symptomatology of puerperal illnesses. Br J Psychiatry. 1981;139:128–133. doi: 10.1192/bjp.139.2.128. [DOI] [PubMed] [Google Scholar]
- 17.Douma SL, Husband C, O'Donnell ME, Barwin BN, Woodend AK. Estrogen-related mood disorders: reproductive life cycle factors. Advances in Nursing Science. 2005;28:364–375. doi: 10.1097/00012272-200510000-00008. [DOI] [PubMed] [Google Scholar]
- 18.Pigott T. Anxiety disorders in women. The Psychiatric clinics of North America. 2003;26:621–672. doi: 10.1016/s0193-953x(03)00040-6. [DOI] [PubMed] [Google Scholar]
- 19.Rapkin AJ, Mikacich JA, Moatakef-Imani B, Rasgon N. The clinical nature and formal diagnosis of premenstrual, postpartum, and perimenopausal affective disorders. Curr Psychiatry Rep. 2002;4:419–428. doi: 10.1007/s11920-002-0069-7. [DOI] [PubMed] [Google Scholar]
- 20.Yonkers KA. Anxiety symptoms and anxiety disorders: how are they related to premenstrual disorders? The Journal of clinical psychiatry. 1997;58:62–67. [PubMed] [Google Scholar]
- 21.Goldstein JM, Jerram M, Poldrack RA, Ahern T, Kennedy DN, Seidman LJ, et al. Hormonal cycle modulates arousal circuitry in women using functional magnetic resonance imaging. The Journal of Neuroscience. 2005;25:9309–9316. doi: 10.1523/JNEUROSCI.2239-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Goldstein JM, Jerram M, Abbs B, Whitfield-Gabrieli S, Makris N. Sex differences in stress response circuitry activation dependent on female hormonal cycle. J Neurosci. 2010;30:431–438. doi: 10.1523/JNEUROSCI.3021-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Frye CA, Petralia SM, Rhodes ME. Estrous cycle and sex differences in performance on anxiety tasks coincide with increases in hippocampal progesterone and 3α,5α-THP. Pharmacology Biochemistry and Behavior. 2000;67:587–596. doi: 10.1016/s0091-3057(00)00392-0. [DOI] [PubMed] [Google Scholar]
- 24.Walf AA, Paris JJ, Frye CA. Chronic estradiol replacement to aged female rats reduces anxiety-like and depression-like behavior and enhances cognitive performance. Psychoneuroendocrinology. 2009;34:909–916. doi: 10.1016/j.psyneuen.2009.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Marcondes FK, Miguel KJ, Melo LL, Spadari-Bratfisch RC. Estrous cycle influences the response of female rats in the elevated plus-maze test. Physiology and Behavior. 2001;74:435–440. doi: 10.1016/s0031-9384(01)00593-5. [DOI] [PubMed] [Google Scholar]
- 26.Mora S, Dussaubat N, Diaz-Veliz G. Effects of the estrous cycle and ovarian hormones on behavioral indices of anxiety in female rats. Psychoneuroendocrinology. 1996;21:609–620. doi: 10.1016/s0306-4530(96)00015-7. [DOI] [PubMed] [Google Scholar]
- 27.Layton B, Krikorian R. Memory Mechanisms in Posttraumatic Stress Disorder. J Neuropsychiatry Clin Neurosci. 2002;14:254–261. doi: 10.1176/jnp.14.3.254. [DOI] [PubMed] [Google Scholar]
- 28.Davis M. Animal models of anxiety based on classical conditioning: the conditioned emotional response (CER) and fear-potentiated startle effect. Pharmacol Ther. 1990;47:147–165. doi: 10.1016/0163-7258(90)90084-f. [DOI] [PubMed] [Google Scholar]
- 29.Rasmusson AM, Charney DS. Animal models of relevance to PTSD. Annals of the New York Academy of Sciences. 1997;821:332–351. doi: 10.1111/j.1749-6632.1997.tb48290.x. [DOI] [PubMed] [Google Scholar]
- 30.Rescorla RA, Wagner AR. A theory of Pavlovian conditioning: variations in the effectiveness of reinforcement and nonreinforcement. In: Black AH, Prokasy WF, editors. Classical conditioning II: Current theory and research. New York, NY: Appleton-Century-Crofts; 1972. pp. 64–99. [Google Scholar]
- 31.Jovanovic T, Norrholm SD, Fennell JE, Keyes M, Fiallos A, Myers KM, et al. Posttraumatic stress disorder may be associated with impaired fear inhibition: relation to symptom severity. Psychiatry Research. 2009;167:151–160. doi: 10.1016/j.psychres.2007.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Jovanovic T, Ressler KJ. How the neurocircuitry and genetics of fear inhibition may inform our understanding of PTSD. The American journal of psychiatry. 2010;167:648–662. doi: 10.1176/appi.ajp.2009.09071074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Norrholm SD, Jovanovic T, Olin IW, Sands LA, Karapanou I, Bradley B, et al. Fear extinction in traumatized civilians with posttraumatic stress disorder: relation to symptom severity. Biological psychiatry. 2011;69:556–563. doi: 10.1016/j.biopsych.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Briscione MA, Jovanovic T, Norrholm SD. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Frontiers in Psychiatry. 2014 doi: 10.3389/fpsyt.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maren S, DeOca B, Fanselow MS. Sex differences in hippocampal long-term potentiation (LTP) and Pavolvian fear conditioning in rats: positive correlation between LTP and contextual learning. Brain Research. 1994;661:25–34. doi: 10.1016/0006-8993(94)91176-2. [DOI] [PubMed] [Google Scholar]
- 36.Pryce CR, Lehmann J, Feldon J. Effect of sex on fear conditioning is similar for context and discrete CS in Wistar, Lewis and Fischer rat strains. Pharmacology, Biochemistry & Behavior. 1999;64:753–759. doi: 10.1016/s0091-3057(99)00147-1. [DOI] [PubMed] [Google Scholar]
- 37.Anagnostaras SG, Maren S, DeCola JP, Lane NI, Gale GD, Schlinger BA, et al. Testicular hormones do not regulate sexually dimorphic Pavlovian fear conditioning or perforant-path long-term potentiation in adult male rats. Behavioural brain research. 1998;92:1–9. doi: 10.1016/s0166-4328(97)00115-0. [DOI] [PubMed] [Google Scholar]
- 38.Gupta RR, Sen S, Diepenhorst LL, Rudick CN, Maren S. Estrogen modulates sexually dimorphic contextual fear conditioning and hippocampal long-term potentiation (LTP) in rats(1) Brain Res. 2001;888:356–365. doi: 10.1016/s0006-8993(00)03116-4. [DOI] [PubMed] [Google Scholar]
- 39.Baran SE, Armstrong CE, Niren DC, Conrad CD. Prefrontal cortex lesions and sex differences in fear extinction and perseveration. Learn Mem. 2010;17:267–278. doi: 10.1101/lm.1778010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jasnow AM, Schulkin J, Pfaff DW. Estrogen facilitates fear conditioning and increases corticotropin-releasing hormone mRNA expression in the central amygdala in female mice. Hormones and behavior. 2006;49:197–205. doi: 10.1016/j.yhbeh.2005.06.005. [DOI] [PubMed] [Google Scholar]
- 41.Markus EJ, Zecevic M. Sex differences and estrous cycle changes in hippocampus-dependent fear conditioning. Psychobiology. 1997;25:246–252. [Google Scholar]
- 42.Myers KM, Davis M. Behavioral and Neural Analysis of Extinction. Neuron. 2002;36:567–584. doi: 10.1016/s0896-6273(02)01064-4. [DOI] [PubMed] [Google Scholar]
- 43.Rivas-Arancibia S, Vazquez-Pereyra F. Hormonal modulation of extinction responses induced by sexual steroid hormones in rats. Life sciences. 1994;54:PL363–367. doi: 10.1016/0024-3205(94)90036-1. [DOI] [PubMed] [Google Scholar]
- 44.Baran SE, Armstrong CE, Niren DC, Hanna JJ, Conrad CD. Chronic stress and sex differences on the recall of fear conditioning and extinction. Neurobiol Learn Mem. 2009;91:323–332. doi: 10.1016/j.nlm.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Chang YJ, Yang CH, Liang YC, Yeh CM, Huang CC, Hsu KS. Estrogen modulates sexually dimorphic contextual fear extinction in rats through estrogen receptor beta. Hippocampus. 2009;19:1142–1150. doi: 10.1002/hipo.20581. [DOI] [PubMed] [Google Scholar]
- 46.Lebron-Milad K, Milad MR. Sex differences, gonadal hormones and the fear extinction network: implications for anxiety disorders. Biology of mood & anxiety disorders. 2012;2:3. doi: 10.1186/2045-5380-2-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Milad MR, Goldstein JM, Orr SP, Wedig MM, Klibanski A, Pitman RK, et al. Fear conditioning and extinction: influence of sex and menstrual cycle in healthy humans. Behavioral Neuroscience. 2006;120:1196–1203. doi: 10.1037/0735-7044.120.5.1196. [DOI] [PubMed] [Google Scholar]
- 48.Milad MR, Igoe SA, Lebron-Milad K, Novales JE. Estrous cycle phase and gonadal hormones influence conditioned fear extinction. Neuroscience. 2009;164:887–895. doi: 10.1016/j.neuroscience.2009.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Milad MR, Zeidan MA, Contero A, Pitman RK, Klibanski A, Rauch SL, et al. The influence of gonadal hormones on conditioned fear extinction in healthy humans. Neuroscience. 2010;168:652–658. doi: 10.1016/j.neuroscience.2010.04.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zeidan MA, Igoe SA, Linnman C, VItalo A, Levine JB, Klibanski A, et al. Estradiol modulates medial prefrontal cortex and amygdala activity during fear extinction in women and female rats. Biological psychiatry. 2011;70:920–927. doi: 10.1016/j.biopsych.2011.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Wegerer M, Kerschbaum H, Blechert J, Wilhelm FH. Low levels of estradiol are associated with elevated conditioned responding during fear extinction and with intrusive memories in daily life. Neurobiol Learn Mem. 2014;116:145–154. doi: 10.1016/j.nlm.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB, et al. Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biological Psychiatry. 2000;47:769–776. doi: 10.1016/s0006-3223(00)00828-3. [DOI] [PubMed] [Google Scholar]
- 53.Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B, et al. A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry. 2005;62:273–281. doi: 10.1001/archpsyc.62.3.273. [DOI] [PubMed] [Google Scholar]
- 54.Milad MG, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- 55.Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: role of the amygdala and vmPFC. Neuron. 2004;43:897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- 56.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, et al. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 57.Ostlund H, Keller E, Hurd YL. Estrogen receptor gene expression in relation to neuropsychiatric disorders. Annals of the New York Academy of Sciences. 2003;1007:54–63. doi: 10.1196/annals.1286.006. [DOI] [PubMed] [Google Scholar]
- 58.Osterlund MK, Hurd YL. Estrogen receptors in the human forebrain and the relation to neuropsychiatric disorders. Prog Neurobiol. 2001;64:251–267. doi: 10.1016/s0301-0082(00)00059-9. [DOI] [PubMed] [Google Scholar]
- 59.Weiser MJ, Foradori CD, Handa RJ. Estrogen receptor beta in the brain: from form to function. Brain Res Rev. 2008;57:309–320. doi: 10.1016/j.brainresrev.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gillies GE, McArthur S. Estrogen actions in the brain and the basis for differential action in men and women: a case for sex-specific medicines. Pharmacol Rev. 2010;62:155–198. doi: 10.1124/pr.109.002071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Kruijver FP, Balesar R, Espila AM, Unmehopa UA, Swaab DF. Estrogen receptor-alpha distribution in the human hypothalamus in relation to sex and endocrine status. J Comp Neurol. 2002;454:115–139. doi: 10.1002/cne.10416. [DOI] [PubMed] [Google Scholar]
- 62.Inagaki T, Gautreaux C, Luine V. Acute estrogen treatment facilitates recognition memory consolidation and alters monoamine levels in memory-related brain areas. Hormones and behavior. 2010;58:415–426. doi: 10.1016/j.yhbeh.2010.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Luine VN, Frankfurt M. Estrogens facilitate memory processing through membrane mediated mechanisms and alterations in spine density. Front Neuroendocrinol. 2012;33:388–402. doi: 10.1016/j.yfrne.2012.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kramar EA, Babayan AH, Gall CM, Lynch G. Estrogen promotes learning-related plasticity by modifying the synaptic cytoskeleton. Neuroscience. 2013;239:3–16. doi: 10.1016/j.neuroscience.2012.10.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Srivastava DP, Woolfrey KM, Evans PD. Mechanisms underlying the interactions between rapid estrogenic and BDNF control of synaptic connectivity. Neuroscience. 2013;239:17–33. doi: 10.1016/j.neuroscience.2012.12.004. [DOI] [PubMed] [Google Scholar]
- 66.Romeo RD, McCarthy JB, Wang A, Milner TA, McEwen BS. Sex differences in hippocampal estradiol-induced N-methyl-D-aspartic acid binding and ultrastructural localization of estrogen receptor-alpha. Neuroendocrinology. 2005;81:391–399. doi: 10.1159/000089557. [DOI] [PubMed] [Google Scholar]
- 67.Liu F, Day M, Muniz LC, Bitran D, Arias R, Revilla-Sanchez R, et al. Activation of estrogen receptor-beta regulates hippocampal synaptic plasticity and improves memory. Nature neuroscience. 2008;11:334–343. doi: 10.1038/nn2057. [DOI] [PubMed] [Google Scholar]
- 68.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mermelstein PG. Membrane-localised oestrogen receptor alpha and beta influence neuronal activity through activation of metabotropic glutamate receptors. Journal of neuroendocrinology. 2009;21:257–262. doi: 10.1111/j.1365-2826.2009.01838.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Scharfman HE, MacLusky NJ. Estrogen and brain-derived neurotrophic factor (BDNF) in hippocampus: complexity of steroid hormone-growth factor interactions in the adult CNS. Front Neuroendocrinol. 2006;27:415–435. doi: 10.1016/j.yfrne.2006.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Soliman F, Glatt CE, Bath KG, Levita L, Jones RM, Pattwell SS, et al. A genetic variant BDNF polymorphism alters extinction learning in both mouse and human. Science (New York, NY. 2010;327:863–866. doi: 10.1126/science.1181886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bryant RA, Felmingham KL, Silove D, Creamer M, O'Donnell M, McFarlane AC. The association between menstural cycle and traumatic memories. Journal of Affective Disorders. 2011;131:398–401. doi: 10.1016/j.jad.2010.10.049. [DOI] [PubMed] [Google Scholar]
- 73.Cahill L. Sex-related influences on the neurobiology of emotionally influenced memory. Annals of the New York Academy of Sciences. 2003;985:163–173. doi: 10.1111/j.1749-6632.2003.tb07080.x. [DOI] [PubMed] [Google Scholar]
- 74.Felmingham K, Williams LM, Kemp AH, Liddell B, Falconer EM, Peduto A, et al. Neural responses to masked fear faces: sex differences and trauma exposure in posttraumatic stress disorder. J Abnormal Psychol. 2010;119:241–247. doi: 10.1037/a0017551. [DOI] [PubMed] [Google Scholar]
- 75.Butler T, Pan H, Epstein J, Protopopescu X, Tuescher O, Goldstein M, et al. Fear-related activity in subgenual anterior cingulate differs between men and women. Neuro-Report. 2005;16:1233–1236. doi: 10.1097/00001756-200508010-00020. [DOI] [PubMed] [Google Scholar]
- 76.Jovanovic T, Szilagyi S, Chakravorty S, Fiallos AM, Lewison BJ, Parwani A, Schwartz MP, Gonzenbach S, Rotrosen JP, Duncan EJ. Menstrual cycle phase effects on prepulse inhibition of acoustic startle. Psychophysiology. 2004;41:401–406. doi: 10.1111/1469-8986.2004.00166.x. [DOI] [PubMed] [Google Scholar]
- 77.Epperson CN, Pittman B, Czarkowski KA, Stiklus S, Krystal JH, Grillon C. Luteal-phase accentuation of acoustic startle response in women with premenstrual dysphoric disorder. Neuropsychopharmacology. 2007;32:2190–2198. doi: 10.1038/sj.npp.1301351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, et al. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and anxiety. 2011 doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, et al. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and anxiety. 2010;27:244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, et al. Sex differences in fear conditioning in posttraumatic stress disorder. Journal of psychiatric research. 2013;47:64–71. doi: 10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, et al. Post-traumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470:492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Stevens JS, Almli LM, Fani N, Gutman DA, Bradley B, Norrholm SD, et al. PACAP receptor gene polymorphism impacts fear responses in the amygdala and hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 2014;111:3158–3163. doi: 10.1073/pnas.1318954111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Almli LM, Mercer KB, Kerley K, Feng H, Bradley B, Conneely KN, et al. ADCYAP1R1 genotype associates with post-traumatic stress symptoms in highly traumatized African-American females. Am J Med Genet B Neuropsychiatr Genet. 2013;162B:262–272. doi: 10.1002/ajmg.b.32145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, et al. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Mol Psychiatry. 2012 doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Uddin M, Chang SC, Zhang C, Ressler K, Mercer KB, Galea S, et al. Adcyap1r1 genotype, posttraumatic stress disorder, and depression among women exposed to childhood maltreatment. Depression and anxiety. 2013;30:251–258. doi: 10.1002/da.22037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Chang SC, Xie P, Anton RF, De Vivo I, Farrer LA, Kranzler HR, et al. No association between ADCYAP1R1 and post-traumatic stress disorder in two independent samples. Mol Psychiatry. 2012;17:239–241. doi: 10.1038/mp.2011.118. [DOI] [PubMed] [Google Scholar]
- 87.Graham BM, Milad MR. Blockade of estrogen by hormonal contraceptives impairs fear extinction in female rats and women. Biological psychiatry. 2013;73:371–378. doi: 10.1016/j.biopsych.2012.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Rothbaum BO, Price M, Jovanovic T, Norrholm SD, Gerardi M, Dunlop B, et al. A Randomized, Double-Blind Evaluation of d-Cycloserine or Alprazolam Combined With Virtual Reality Exposure Therapy for Posttraumatic Stress Disorder in Iraq and Afghanistan War Veterans. The American journal of psychiatry. 2014 doi: 10.1176/appi.ajp.2014.13121625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Schmidt PJ, Nieman L, Danaceau MA, Tobin MB, Roca CA, Murphy JH, et al. Estrogen replacement in perimenopause-related depression: a preliminary report. American Journal of Obstetrics & Gynecology. 2000;183:414–420. doi: 10.1067/mob.2000.106004. [DOI] [PubMed] [Google Scholar]
- 90.Soares CN, Almeida OP, Joffe H, Cohen LS. Efficacy of estradiol for the treatment of depressive disorders in perimenopausal women: a double-blind, randomized, placebo-controlled trial. Archives of General Psychiatry. 2001;58:529–534. doi: 10.1001/archpsyc.58.6.529. [DOI] [PubMed] [Google Scholar]
- 91.Ferree NK, Wheeler M, Cahill L. The influence of emergency contraception on post traumatic stress symptoms following sexual assault. Journal of forensic nursing. 2012;8:122–130. doi: 10.1111/j.1939-3938.2012.01134.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Ressler KJ, Rothbaum BO, Tannenbaum L, Anderson P, Graap K, Zimand E, et al. Cognitive enhancers as adjuncts to psychotherapy: use of D-cycloserine in phobic individuals to facilitate extinction of fear. Archives of general psychiatry. 2004;61:1136–1144. doi: 10.1001/archpsyc.61.11.1136. [DOI] [PubMed] [Google Scholar]
- 93.Monfils MH, Cowansage KK, Klann E, LeDoux JE. Extinction-Reconsolidation Boundaries: Key to Persistent Attenuation of Fear Memories. Science. 2009;324:951–955. doi: 10.1126/science.1167975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Schiller D, Monfils MH, Raio CM, Johnson DC, LeDoux JE, Phelps EA. Preventing the return of fear in humans using reconsolidation update mechanisms. Nature. 2009 doi: 10.1038/nature08637. advance online publication. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Warren VT, Anderson KM, Kwon C, Bosshardt L, Jovanovic T, Bradley B, et al. Human fear extinction and return of fear using reconsolidation update mechanisms: The contribution of on-line expectancy ratings. Neurobiol Learn Mem. 2014;113:165–173. doi: 10.1016/j.nlm.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Parke A, Abernethy K. Hormone replacement therapy: risks and benefits. Nurse Prescribing. 2008;6:433–439. [Google Scholar]
- 97.Rubinow DR, Girdler SS. Hormones, heart disease, and health: individualized medicine versus throwing the baby out with the bathwater. Depression and anxiety. 2011;28:E1–E15. doi: 10.1002/da.20833. [DOI] [PubMed] [Google Scholar]


