Abstract
Exposure to low or moderate doses of lipopolysaccharides (LPS) renders the host tolerance to a subsequent lethal dose of LPS, which is termed as endotoxin tolerance. It is characterized as the decrease in production of pro-inflammatory cytokines and the increase in production of anti-inflammatory mediators in response to a second LPS challenge. The alteration of cytokine profile protects LPS-primed hosts against a normally lethal dose of subsequent LPS challenge. Nevertheless, whether other environmental factors also trigger endotoxin tolerance remains unclear. Both epidemiologic and experimental studies have provided a link between particulate matter and human health. Here, we speculated on the effect of fine particles priming on endotoxin tolerance in a mouse model.
INTRODUCTION
The inhalation of toxic environmental particles is a worldwide public health problem; both epidemiologic and experimental studies have provided compelling evidence supporting the association between particulate matter (PM) and human health, including mortality and hospital admissions [1], cardiovascular diseases [2,3], type 2 diabetes [4,5], asthma and chronic obstructive pulmonary disease [6,7], and non-alcoholic fatty liver disease [8]. Inflammatory response has been implicated as the key mechanism of PM-mediated healthy problems. Current evidence suggests that inhaled particles trigger innate immune signals in the lung through interacting with toll-like receptors (TLRs), releasing cytokines into circulation and causing systemic inflammatory response [9]; and that direct penetration of leachable components such as reactive oxygen species and stable organic compounds into circulation also contributes to systemic inflammatory response [10].
Particle pollution is a mixture of microscopic solids and liquids droplets suspended in air; it consists of a number of components, including acids, organic chemicals, metals, soils or dust particles, and allergens. According to its aerodynamic diameter, PM is classified into coarse (10 to 2.5 μm; PM10), fine (<2.5 μm; PM2.5), and ultrafine (<0.1 μm; PM0.1) particles. The size of particles is directly linked to their potential for causing health effects. It is believed that fine particles pose the greatest health problems, because they can get and deposit deep into the lung, and may even penetrate into the bloodstream. PM composition and size together influence its adverse effects on public health [11,12].
Endotoxin, also known as lipopolysaccharides (LPS), is a structural component of the gram-negative outer membrane. Leukocytes recognize LPS via TLR4 in the presence of myeloid differentiation factor (MD) 2, triggering a powerful immune reaction [13]. This inflammatory response is tightly regulated and can show different forms, depending on the dose. Exposure to low or moderate doses of LPS renders the host tolerance to a subsequent lethal dose of LPS, which is termed as endotoxin tolerance. It is characterized as the decrease in production of pro-inflammatory cytokines such as TNFα, IL-6 and IL-1β, and the increase in production of anti-inflammatory mediators such as IL-10 in response to a second LPS challenge [14,15]. The alteration of cytokine profile protects LPS-primed hosts against a normally lethal dose of subsequent LPS challenge. Nevertheless, whether other environmental factors also trigger endotoxin tolerance remains unclear. Here, we speculated on the effect of PM2.5 priming on endotoxin tolerance in a mouse model.
METHODS
Animal Care
C57BL/6 mice (6-8 weeks old) were obtained from Jackson Laboratories (Bar Harbor, ME). Animals were maintained at 21°C and exposed to a 12-h light, 12-h dark cycle with free access to water and food. The protocols and the use of animals were approved by and in accordance with the Ohio State University Animal Care and Use Committee.
Intranasal Exposure to PM 2.5
Mice were exposed to PM2.5 by intranasal instillation, which is an effective and noninvasive technique in toxicity studies [16,17]. This instillation technique consists in deliver drop-wise the particle suspension or the vehicle to the nares using a micropipette, while the mouse is in a supine position. Animals were lightly anesthetized with 2% isoflurane and intranasally instilled with 20 μl of free-particle saline or PM2.5 (0.5 μg/μl) saline, for three times per week for eight weeks.
Survival Study
Endotoxic shock was induced by peritoneal injection of LPS (20 μg/g; Escherichia coli serotype 055.B5; Sigma-Aldrich) and mice (n = 10) were monitored up to 84 hours. Survival curves were compared using Kaplan–Meyer log-rank test. All tests were conducted at the two-sided 5% significant level.
RESULTS
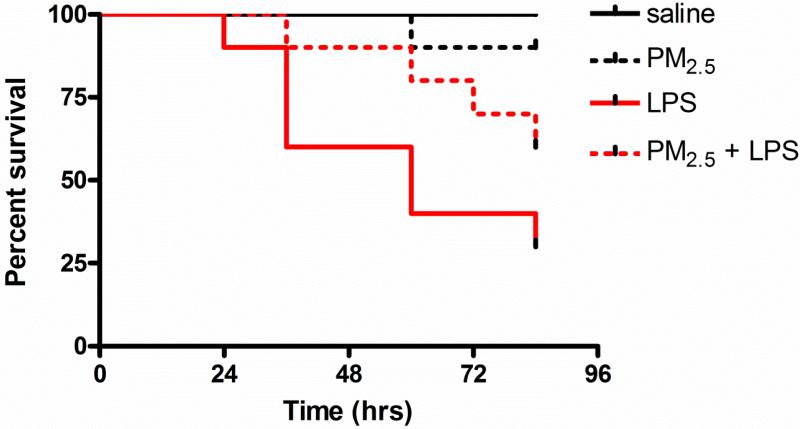
All mice treated with saline without LPS injection survived; one mouse exposed to PM2.5 without LPS injection died (p > 0.05 vs. saline). LPS injection induced a significant decrease in survival rate (p < 0.01 vs. saline); pre-exposure to PM2.5 induced tolerance to death from a subsequent lethal LPS dose, however, these two survival curves were not significantly different (p > 0.05 vs. LPS) (Fig. 1).
Fig.1. PM2.5 priming attenuates LPS-induced mortality in wild-type mice.
LPS: lipopolysaccharides
HYPOTHESIS AND EVALUATION
Our preliminary data showed an evident trend of survival curves between PM2.5- exposure and PM2.5 priming plus LPS treatment, suggesting that PM2.5 priming may cause endotoxin tolerance in mice. To verify this hypothesis, a study with larger sample size is needed. Sample sizes are determined using a two-sided, 0.05-significance level log rank test with 80% statistical power and equal allocation. Based on our preliminary study, 60% of mice survived after 84 hours in PM2.5-exposure group, while only 30% of mice survived in PM2.5 priming plus LPS treatment group. Assuming a constant hazard model and using a log rank test, we would need 40 mice in each group to detect the difference in survival curve. The sample size calculation is conducted using SAS proc power (SAS 9.2, SAS Institute Inc. Cary, NC).
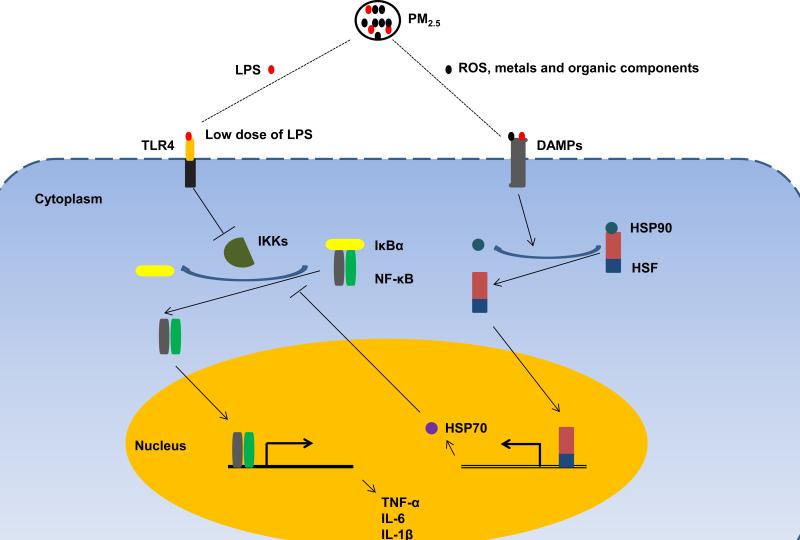
How may PM2.5 priming lead to endotoxin tolerance in mice? Based on our knowledge of this compound, we speculate that at several mechanisms PM2.5 may actively regulate endotoxin tolerance (Fig. 2). On the one hand, PM2.5 is a mixture of various chemical and biological constituents, including a low dose of LPS [18]. In our experiment, pre-exposure to PM2.5 may prime animals with lose dose of LPS, preventing death from a subsequent lethal dose. On the other hand, PM2.5 may induce endotoxin tolerance through regulation of heat-shock response. It has been shown that PM2.5 exposure significantly increases the expression of heat-shock protein (HSP) 70 [19,20]. HSP70, a prominent chaperone protein, functions individually or as part of larger heterocomplexes to maintain protein homeostasis in response to various stress stimuli [21]. In addition, HSP70 exhibits anti-inflammatory effect through interaction with NF-κB complex [22-24].
Fig.2. Proposed mechanisms of PM2.5-mediated endotoxin tolerance.
PM2.5 consists of a number of components, including acids, organic chemicals, metals, ROS and low dose of LPS. On the one hand, pre-exposure to PM2.5 may prime animals with low dose of LPS and contribute to endotoxin tolerance via inhibition of IκBα degradation. On the other, LPS and other components such as ROS, metals and organic chemicals may activate heat-shock response via binding to DAMPs, upregulating the expression of HSP70. It has been shown that HSP70 interrupts NF-κB signaling and inhibits pro-inflammatory cytokine release by stabilizing the complex between NF-κB and its inhibitor IκBα. PM2.5: fine particles; LPS: lipopolysaccharides; ROS: reactive oxygen species; TLR4: toll-like receptor 4; DAMPs: damage-associated molecular patterns; IKKs: IκB kinases; IκBα: κB inhibitor α; NF-κB: nuclear factor-κB; HSP: heat-shock protein; HSF: heat-shock factor; TNF-α: tumor necrosis factor-α; and IL: interleukin.
Additionally, we want to highlight the statistic equation used in this study that may be a very suitable tool to determine the sample size and evaluate the data fidelity for survival study.
Acknowledgments
This work is supported by National Institutes of Health Grant No. ES018900.
REFERENCES
- 1.Atkinson RW, Kang S, Anderson HR, Mills IC, Walton HA. Epidemiological time series studies of PM2.5 and daily mortality and hospital admissions: a systematic review and meta-analysis. Thorax. 2014;69:660–665. doi: 10.1136/thoraxjnl-2013-204492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brook RD, Rajagopalan S, Pope CA, 3rd, et al. Particulate matter air pollution and cardiovascular disease: An update to the scientific statement from the American Heart Association. Circulation. 2010;121:2331–2378. doi: 10.1161/CIR.0b013e3181dbece1. [DOI] [PubMed] [Google Scholar]
- 3.Pedersen M, Stayner L, Slama R, et al. Ambient air pollution and pregnancy-induced hypertensive disorders: a systematic review and meta-analysis. Hypertension. 2014;64:494–500. doi: 10.1161/HYPERTENSIONAHA.114.03545. [DOI] [PubMed] [Google Scholar]
- 4.Liu C, Fonken LK, Wang A, et al. Central IKKbeta inhibition prevents air pollution mediated peripheral inflammation and exaggeration of type II diabetes. Particle and fibre toxicology. 2014;11:53. doi: 10.1186/s12989-014-0053-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Wang B, Xu D, Jing Z, Liu D, Yan S, Wang Y. Effect of long-term exposure to air pollution on type 2 diabetes mellitus risk: a systemic review and meta-analysis of cohort studies. Eur J Endocrinol. 2014;171:R173–182. doi: 10.1530/EJE-14-0365. [DOI] [PubMed] [Google Scholar]
- 6.Kariisa M, Foraker R, Pennell M, et al. Short- and long-term effects of ambient ozone and fine particulate matter on the respiratory health of chronic obstructive pulmonary disease subjects. Arch Environ Occup Health. 2015;70:56–62. doi: 10.1080/19338244.2014.932753. [DOI] [PubMed] [Google Scholar]
- 7.Montoya-Estrada A, Torres-Ramos YD, Flores-Pliego A, et al. Urban PM2.5 activates GAPDH and induces RBC damage in COPD patients. Front Biosci (Schol Ed) 2013;5:638–649. doi: 10.2741/s396. [DOI] [PubMed] [Google Scholar]
- 8.Tarantino G, Capone D, Finelli C. Exposure to ambient air particulate matter and non-alcoholic fatty liver disease. World J Gastroenterol. 2013;19:3951–3956. doi: 10.3748/wjg.v19.i25.3951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kampfrath T, Maiseyeu A, Ying Z, et al. Chronic fine particulate matter exposure induces systemic vascular dysfunction via NADPH oxidase and TLR4 pathways. Circ Res. 2011;108:716–726. doi: 10.1161/CIRCRESAHA.110.237560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Rajagopalan S, Brook RD. Air pollution and type 2 diabetes: mechanistic insights. Diabetes. 2012;61:3037–3045. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Raaschou-Nielsen O, Andersen ZJ, Beelen R, et al. Air pollution and lung cancer incidence in 17 European cohorts: prospective analyses from the European Study of Cohorts for Air Pollution Effects (ESCAPE). Lancet Oncol. 2013;14:813–822. doi: 10.1016/S1470-2045(13)70279-1. [DOI] [PubMed] [Google Scholar]
- 12.Chung Y, Dominici F, Wang Y, Coull BA, Bell ML. Associations between Long-Term Exposure to Chemical Constituents of Fine Particulate Matter (PM) and Mortality in Medicare Enrollees in the Eastern United States. Environmental Health Perspectives. 2015 doi: 10.1289/ehp.1307549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ren W, Wang Z, Hua F, Zhu L. Plasminogen Activator Inhibitor-1 Regulates LPS-Induced TLR4/MD-2 Pathway Activation and Inflammation in Alveolar Macrophages. Inflammation. 2014 doi: 10.1007/s10753-014-0042-8. [DOI] [PubMed] [Google Scholar]
- 14.Xiong Y, Medvedev AE. Induction of endotoxin tolerance in vivo inhibits activation of IRAK4 and increases negative regulators IRAK-M, SHIP-1, and A20. J Leukoc Biol. 2011;90:1141–1148. doi: 10.1189/jlb.0611273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salkowski CA, Detore G, Franks A, Falk MC, Vogel SN. Pulmonary and hepatic gene expression following cecal ligation and puncture: monophosphoryl lipid A prophylaxis attenuates sepsis-induced cytokine and chemokine expression and neutrophil infiltration. Infect Immun. 1998;66:3569–3578. doi: 10.1128/iai.66.8.3569-3578.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schaffer BE, Park KS, Yiu G, et al. Loss of p130 accelerates tumor development in a mouse model for human small-cell lung carcinoma. Cancer Res. 2010;70:3877–3883. doi: 10.1158/0008-5472.CAN-09-4228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Leong BK, Coombs JK, Sabaitis CP, Rop DA, Aaron CS. Quantitative morphometric analysis of pulmonary deposition of aerosol particles inhaled via intratracheal nebulization, intratracheal instillation or nose-only inhalation in rats. J Appl Toxicol. 1998;18:149–160. doi: 10.1002/(sici)1099-1263(199803/04)18:2<149::aid-jat490>3.0.co;2-l. [DOI] [PubMed] [Google Scholar]
- 18.Yang L, Liu G, Lin Z, et al. Pro-inflammatory response and oxidative stress induced by specific components in ambient particulate matter in human bronchial epithelial cells. Environ Toxicol. 2014 doi: 10.1002/tox.22102. [DOI] [PubMed] [Google Scholar]
- 19.Farina F, Sancini G, Mantecca P, Gallinotti D, Camatini M, Palestini P. The acute toxic effects of particulate matter in mouse lung are related to size and season of collection. Toxicol Lett. 2011;202:209–217. doi: 10.1016/j.toxlet.2011.01.031. [DOI] [PubMed] [Google Scholar]
- 20.Sancini G, Farina F, Battaglia C, et al. Health risk assessment for air pollutants: alterations in lung and cardiac gene expression in mice exposed to Milano winter fine particulate matter (PM2.5). PLoS ONE. 2014;9:e109685. doi: 10.1371/journal.pone.0109685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Neef DW, Jaeger AM, Thiele DJ. Heat shock transcription factor 1 as a therapeutic target in neurodegenerative diseases. Nat Rev Drug Discov. 2011;10:930–944. doi: 10.1038/nrd3453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zheng Z, Kim JY, Ma H, Lee JE, Yenari MA. Anti-inflammatory effects of the 70 kDa heat shock protein in experimental stroke. J Cereb Blood Flow Metab. 2008;28:53–63. doi: 10.1038/sj.jcbfm.9600502. [DOI] [PubMed] [Google Scholar]
- 23.Guzhova IV, Darieva ZA, Melo AR, Margulis BA. Major stress protein Hsp70 interacts with NF-kB regulatory complex in human T-lymphoma cells. Cell Stress Chaperones. 1997;2:132–139. doi: 10.1379/1466-1268(1997)002<0132:msphiw>2.3.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ran R, Lu A, Zhang L, et al. Hsp70 promotes TNF-mediated apoptosis by binding IKK gamma and impairing NF-kappa B survival signaling. Genes & Development. 2004;18:1466–1481. doi: 10.1101/gad.1188204. [DOI] [PMC free article] [PubMed] [Google Scholar]