Abstract
Purpose
To determine the corneal regenerative capacity of sequentially generated primary, secondary, and tertiary limbal explant outgrowths in a limbal stem cell deficiency (LSCD) surgical model.
Methods
Two-millimeter-long limbal shallow biopsies were surgically excised from the upper quadrant of the right eye of rabbits and set on preserved amniotic membrane for explant culture. After the generation of primary outgrowth, the biopsies were sequentially transferred to new amniotic membrane to generate secondary and then tertiary outgrowths. Eighteen rabbits were subjected to a 360° limbal peritomy extending into the scleral zone and combined with superficial keratectomy of the corneal periphery and thorough mechanical debridement of the central cornea in their left eye. Right eye outgrowths, six of each generation, were engrafted on the ocular surface. Clinical outcomes (neovascularization, corneal clarity, and corneal fluorescein staining) were graded after 6 months. Post-mortem corneas were compared with histology, immunochemistry for p63 and Krt3, ABCG2-dependent dye exclusion, and capacity for outgrowths in explant culture.
Results
Immunohistology and western blot of the outgrowths for p63 and Krt3 indicated no differences in expression between the primary and tertiary outgrowths for these two markers of growth and differentiation. Clinically, all rabbits treated with amniotic membrane alone developed severe LSCD. Most rabbits grafted with cell outgrowths from all three outgrowth generations achieved stable (>6 months) recovery of the ocular surface. There were partial failures of grafts performed with two secondary and tertiary outgrowths. However, Kruskal–Wallis statistical analysis of the clinical scores yielded no significant difference between the three groups (p=0.524). Histology showed full anatomic recovery of grafts made with primary and tertiary outgrowths. Krt3 and p63 expression throughout the whole limbal corneal epithelium with primary or tertiary outgrowths was not distinguishable from each other. The percentage of dye-excluding cells present within this zone and the capacity of the explant epithelial outgrowth of the regenerated peripheral corneal zone were also on par with those of the donor corneas. The Krt3-negative cells that characterize the basal epithelial layer of the normal limbus could not be found in any regenerated cornea from the primary to tertiary outgrowths.
Conclusions
Our results demonstrate that in rabbits post-primary explant outgrowths retain the capacity for LSCD recovery found in primary explants.
Introduction
Loss of limbal stem cell function allows colonization of the corneal surface by the conjunctival epithelium, generally referred as limbal stem cell deficiency (LSCD) [1–3], which results in neovascularization and deficient corneal surface protection that facilitates scarring of the corneal matrix with partial or full blindness ensuing. For cases in which only one eye is affected, recovery of full vision by autologous transplantation of limbal cells obtained from the contralateral eye has achieved a high rate of success [4-7]. In the most commonly used approach to limbal epithelial cell population expansion, cells are derived by outgrowth from a small limbal biopsy of the contralateral eye on a biocompatible substratum, in particular preserved cesarean-derived human amniotic membrane (hAM). AM appears to be particularly attractive because it displays anti-inflammatory properties and in most cases fully dissolves over time on the corneal surface.
Previously, using a transparent permeable synthetic insert as growth substratum, we showed that after the initial outgrowth had developed over 2 weeks, it was possible to transfer the source biopsy in a successive manner to a new culture insert to generate multiple outgrowth generations [8]. Intriguingly, in humans and rabbits, it was observed that the late-generation outgrowths contained higher proportions of cells exhibiting ABCG2-dependent transport, which directly correlated with colony formation ability, a predictor of regenerative capacity [9]. We speculated that the ability of the extended outgrowth culture may allow the collection of a large number of cells for banking of autologous cells for repeated treatment. However, at odds with our results, a similar sequential experiment in humans concluded that clonogenic capacity was substantial only in the primary outgrowth [10]. Therefore, to directly examine the regenerative properties in late outgrowth cultures, we have now compared the regenerative capacity of grafts of contralateral limbal outgrowths from the first, second, or third generation grown over hAM on an experimental rabbit LSCD model.
Methods
Explant outgrowth culture
Unless stated otherwise, the reagents were obtained from Sigma-Aldrich (St. Louis, Mo). Amniotic membranes were obtained from cesarean sections under an informed consent protocol approved by the ethics committee of Dokuz Eylul University. All protocols were in accordance with the tenets of the Declaration of Helsinki and the ARVO Statement for Use of Animals in Research. The tissues were washed with sterile PBS ( 137 mM NaCl, 2.7 mM KCl, 10 mM Na2HPO4, 1.8 mM KH2PO4) containing antibiotics and stored at −80 °C in a 1:1 mix of Dulbecco’s modified Eagle’s medium (DMEM; Life Technologies, Istanbul Turkey) and glycerol. For culture procedures, frozen hAM was thawed, washed three times with sterile PBS containing antibiotics, cut into 4 cm ×4 cm pieces, incubated with 0.02% EDTA at 37 °C for 2 h to loosen cellular adhesion, and gently but thoroughly scraped to remove all the epithelium. The deepithelialized squares were placed in 2.5 cm custom inserts that held the amniotic membrane in a stretched, taut state. The inserts were then set in six-well plates and equilibrated overnight in the limbal explant culture medium consisting of 84% 1:1 DMEM and Ham F12 (Life Technologies) and 16% fetal bovine serum (FBS; Life Technologies) complemented with 100 U/ml Penicillin-Streptomycin and µg/ml amphotericin B.
The Animal Research Committee of Dokuz Eylul University approved the protocols described below. Under topical anesthesia, small limbal biopsies (1 mm × 2 mm) were excised from the superior limbus of the right eye of New Zealand white rabbits weighing between 2 and 3 kg. All rabbits were then subjected to a four times a day topical netilmicin antibiotic treatment for 1 week. Biopsies were split in half, and both halves were placed epithelial side up, on the center of the hAM inserts about 2.4 cm in culture area diameter, and cultured for 72 h at the air–liquid interface. After the presence of an encircling island of outgrowing cells was confirmed, and every 48 to 72 h thereafter, the culture solution was replaced with enough medium to cover the surface of the explant until the outgrowths reached the edge of the growth area. To extend the explant culture beyond the initial outgrowth round, the explants were separated from the hAM substratum using a microblade and repositioned on new hAM insert setups. When the secondary or second-generation outgrowth reached confluence, the entire process of explant transfer was repeated once more, thus generating the tertiary or third-generation outgrowth. In the initial experiments, this protocol was performed in parallel with the hAM inserts and with 0.4 μm pore, 25 mm diameter permeable Falcon inserts (Fisher Scientific, Istanbul, Turkey), to examine selected differences or similarities between the outgrowths on hAM and the synthetic culture surface. Explant outgrowths on the synthetic substratum were also performed on the limbal–peripheral biopsies excised from the control and treated eyes at the end of the regeneration period.
Limbal stem cell deficiency model and hAM-epithelial grafting
Eighteen biopsies from the contralateral donor eyes were cultured together as explants over one hAM membrane. When the resulting primary outgrowths approached confluence, typically within 10 and 13 days, six outgrowths were selected for primary outgrowth transplants. The rabbits that donated these biopsies were anesthetized with an intramuscular injection of 35 mg/kg ketamine and 5 mg/kg xylazine, the ocular surface was briefly exposed to 5% iodine–povidone/95% PBS, and the left eyes were subjected to a radical, 360° limbal peritomy using Vannas scissors and Bonn forceps as previously described [11,12]. The whole limbus with an extended dissection toward to the sclera was removed with a corneal microblade, the peripheral cornea was subjected to superficial keratectomy, and the central corneal epithelium was thoroughly scraped off from the cornea with number 15 dulled scalpel blades. The hAM membrane incorporating the approximate 20-mm-diameter outgrowths were secured to the visible edge of the bulbar conjunctiva with interrupted episcleral 8.0 absorbable Vicryl sutures (eight to 12 sutures for fixation).
Six of the 12 remaining biopsies were transferred once to hAM inserts once and the other six twice to generate secondary and tertiary outgrowths, respectively. When these outgrowths reached the 2 cm target size, the left eyes of the corresponding donor rabbits were surgically processed, and the outgrowths were sutured to the denuded limbal–corneal surfaces as described above, to assess corneal regeneration by the secondary and tertiary outgrowths, respectively. Any excess hAM was trimmed as close as possible to the sutures. Topical antibiotic and steroid drops were used four times a day for 10 days. Steroid drops were tapered and stopped after 10 days. The natural blinking action caused spontaneous removal of the biopsies included in the grafts after 1–3 days. The unused primary outgrowths from the surgeries that used secondary outgrowths and the primary and secondary outgrowths from the surgeries that used tertiary outgrowths were either discarded or used in the characterization of outgrowth with immunostaining or flow cytometry. Four additional rabbits were subjected to the same radical surgery. Three of these rabbits were treated with the hAM membrane without outgrowths, and one was immediately euthanized (intracardiac pentobarbital injection; 100 mg/kg) to characterize the extent of the surgical modification of the ocular surface.
Clinical outcome analysis
Grafted eyes were monitored with slit-lamp examination, fluorescein staining, and photography during the first postoperative week and monthly thereafter. Corneal neovascularization, corneal clarity, and corneal staining were evaluated and scored after 6 months. Scoring was done by the same examiner in masked fashion in accordance with three published criteria [13,14]: A) Neovascularization: Grade 0, no neovascularization (0 points); Grade 1, ≤2 mm from limbus (1 point); Grade 2, >2 mm from limbus (2 points). B) Corneal clarity: Grade 0, totally clear (0 points); Grade 1, mild or moderately dense opacity partially obscuring the pupil (1 point); Grade 2, severely dense opacity completely obscuring the pupil (2 points). C) Corneal fluorescein staining: Grade 0: No staining; Grade 1: <1/4 (1 point); Grade 2: 1/4–1/2 (2 points); Grade 3: >1/2 (3 points). The rabbits were euthanized after a 6-month clinical follow-up period.
Flow cytometry
Flow cytometry was used to determine cell size distribution according to the light-scattering properties and ABCG2-dependent JC1 dye exclusion, two parameters associated with corneal epithelial precursors [8]. Half of the limbal zone of each cornea was split into six segments and set in the explant culture as described above. Outgrown cells from explant cultures were fully dissociated into single cells by incubation in trypsin for 5 to 10 min. Trypsinized cells from all six inserts from a single cornea were pooled together and, after neutralization, trituration, and centrifugation, resuspended in culture medium with FBS reduced to 5%, and replated for overnight culture. After overnight incubation, the medium was complemented with 250 nM JC1 (Axxora, San Diego, CA) for 45 min. The attached cells were washed twice with PBS, released by trypsinization, spun down, resuspended in ice-cold Hanks’ balanced salt solution and 4% FBS and 1 μg/ml propidium iodide (PI) and analyzed in a Canto Flow Cytometer (BD Biosciences, San Jose, CA USA) for forward (FSC) and side (SSC) scatter and for the emissions at 530 nm (JC1 green), 585 nm (JC1 orange), and >670 nm (PI; to gate out dead cells) from a single 488 nm excitation source.
Histology and immunocytochemistry
Control and post-regeneration corneas and explant outgrowths grown on the synthetic membrane were subjected to histological examinations with hematoxylin and eosin (H&E) and immunochemistry for p63 and/or Krt3. Corneas were fixed in formalin, embedded in paraffin, and sectioned at 4 µM. Immunostaining of the corneal sections involved deparafinization and rehydration in PBS, permeabilization with 1% Triton X-100 for 10 min, blockade with 1% bovine serum albumin (BSA) for 1 h, overnight incubation at 4 °C with either mouse monoclonal antibodies (moAB) raised against p63 (clone 4A4; Biocare, Walnut Creek, CA; 20 µg/ml in 0.1% BSA) or Krt3 (clone AE5, 1 μg/ml; Abcam, Cambridge, MA), three 5-min washes with PBS, incubation for 1 h with 2 μg/ml Alexa 488-conjugated goat anti-mouse immunoglobulin G (IgG), and three 5-min washes. Sections were counterstained by incubation with 0.5 μg/ml 4',6-diamidino-2-phenylindole (DAPI) for 10 min, washed, and mounted in Vectashield (Vector Labs, Burlingame, CA). Explant outgrowths were fixed for 10 min in 4% paraformaldehyde, sectioned into angular segments, and stained in an identical manner as described above.
Western blot
Explant outgrowth cells were washed with PBS and then lysed with hot 0.1% sodium dodecyl sulfate (SDS) 10 mM Tris-HCl, pH 8.5. Protein concentration was determined using the BCA Protein Assay kit (Thermo Fisher Scientific, Rockford, IL). The lysate was complemented with 0.2 volumes of 6X sodium dodecyl sulfate (SDS) sample-loading buffer and boiled for 10 min. Equal amounts of protein in cell lysates were separated by SDS–polyacrylamide gel electrophoresis (SDS–PAGE) under reducing conditions and electrotransferred to a PDF membrane (Millipore, Billerica, MA). The membrane was blocked with 5% skim milk in PBS containing 0.1% Tween-20, incubated at 4 °C for 18 h with 1 μg/ml of either the anti-Krt3 moAB, anti-connexin 43 (Cx43) moAb (EMD Millipore, Billerica, MA) and rabbit (BioLegend, San Diego, CA) polyclonal antibodies raised against p63 sequences that are identical in humans and rabbits. After three washes, the membranes were incubated at room temperature for 1 h with the appropriate horseradish peroxidase-conjugated anti-mouse or anti-rabbit IgG Abs (Santa Cruz, Dallas, TX). Finally, the membranes were washed three times, and protein bands were detected using enhanced chemiluminescence reagent (ECL; Amersham Biosciences, Sweden). Membranes were stripped and reprobed with anti-GAPDH Moab (Santa Cruz) to confirm loading comparability.
Results
Comparison of culture on synthetic membrane versus hAM
We first performed experiments to compare the phenotypic features of cells derived from the explant outgrowths on hAM with those on synthetic permeable culture membranes, which we used before to characterize the serial explant outgrowth culture [8]. Based on the area of outgrowth as a function of time in culture, for all practical purposes there were no appreciable difference in the rate of growth between the two substrata. Nevertheless, flow cytometry analyses of outgrowth cells recovered by trypsinization and recultured overnight to allow for recovery from the enzymatic treatment showed that the cell population derived from the hAM substratum had statistically significant lower FCS and higher percentiles of JC1-excluding cells than the cells cultured on the synthetic membrane substratum (Figure 1A,B; Table 1). In addition, the main cohort of JC1-stained cells, representing cells in which the mitochondrial membrane potential (MMPT) reporter dye had reached saturation, displayed statistically significant differences in the 585/525 emission ratio (Table 1).
Figure 1.
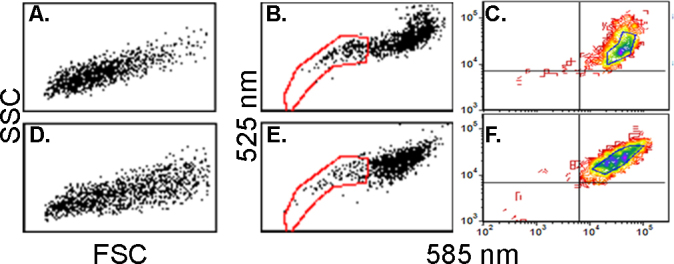
Scatter and JC1 exclusion in cells grown on hAM or Falcon substrata. A–C: Cells from explants cultured over human amniotic membrane (hAM). D–F: Cells from cultures on synthetic membrane. A and D: Scatter plots. B–F: JC1 bivariate emission dot plots. Note the distinct shape of the main cell cohort. C and F: Contour plots. The demarcated area at the center of the plot was used to determine the emission intensities and the intensity ratios described in Table 1.
Table 1. Comparison of cells properties for outgrowths performed over synthetic permeable membrane (Falcon) or preserved, de-epthelialized hAM.
| Parameter: | A. FSC | B. JC1low (%) | C. Mitochondrial membrane potential | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Substratum: |
hAM |
Synth. |
hAM |
Synth. |
hAM |
|
|
Synthetic |
|
|
| Wavelength: |
|
|
|
|
525* |
585 |
R** |
525* |
585 |
R** |
| Mean |
100 |
112.5 |
12 |
5.4 |
100 |
53.9 |
0.54 |
73.2 |
53 |
0.73 |
| ±SD |
7.1 |
8.1 |
5.5 |
1.4 |
10.8 |
3.7 |
0.03 |
7.7 |
3.7 |
0.12 |
| n= |
6 |
|
6 |
|
7 |
|
|
|
|
|
| p< | 0.01 | 0.01 | 0.0002*; 0.0001** | |||||||
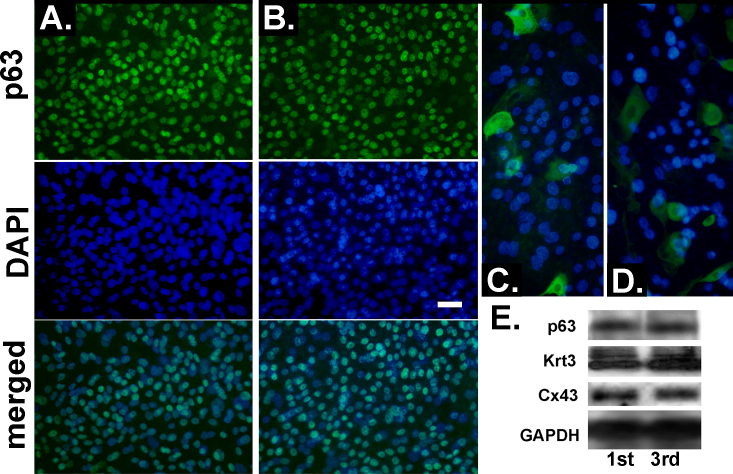
In addition to these isolated cell comparisons, to complement the previous studies on the expression of markers related to regenerative ability as a function of culture generation [8], we stained primary and tertiary outgrowths made on Falcon inserts for p63, a protein intimately linked to the ability of cells for extended proliferation [15,16] whose alpha isoform has been correlated with limbal epithelial stemness [17,18] and Krt3, a protein that undergoes acute de novo expression when limbal epithelial cells stratify and when they transition from the limbus to the cornea along the basal axis [19,20]. In the primary and tertiary cultures, more than 30% of the explant outgrowth cells displayed a high level of p63 expression (Figure 2A,B). These high levels of p63 expression need to be interpreted in the context of p63 expression in the rabbit cornea, where, unlike the case for the adult human cornea, p63 is profusely expressed throughout the limbal and corneal zone (see below). The fact that epithelial cells from the peripheral rabbit cornea have a higher clonogenic index than those in the limbus [21] may be related to this p63 distribution.
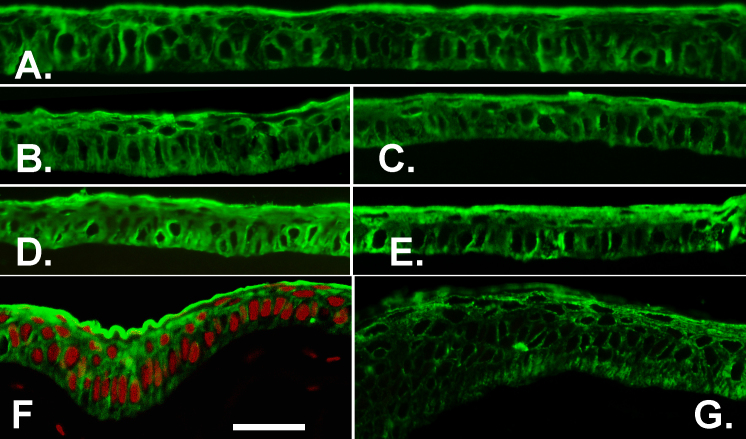
Figure 2.
Comparison of growth and differentiation markers in primary- and tertiary-generation explant outgrowths. A and B: p63 immunostaining. C and D: Krt3 immunostaining. A and C: Primary cultures. B and D: Tertiary-generation cultures. E: Western blots. Targets and culture generations are indicated. Bar equals 50 µm in A and B and 33 µm in C and D.
Substantial Krt3 levels were present only in isolated or small groups of cells in either outgrowth generation (Figure 2C,D). To establish whether, in spite of the high similarity in the immunostaining images, there were differences at the polypeptide level, the p63 and Krt3 content of three pooled primary and tertiary outgrowths were compared with western blot (Figure 3E). There were no substantial changes in the expression of these two proteins or the expression of Cx43, a gap junction protein that is not expressed in the limbal stem cells, minimally expressed throughout the limbus and highly expressed on the corneal side of the limbal–corneal demarcation [22]. The p63 blot shown was made with one of two distinct rabbit polyclonal Abs. A second polyclonal Ab yielded an equivalent result.
Figure 3.
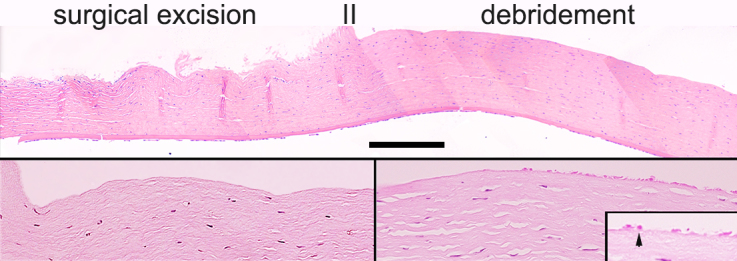
H&E stainings of the ocular surface immediately after the combined surgical and debridement treatment. A: Composite of micrographs covering the limbal and peripheral zone. The areas subjected to surgical excision and debridement are indicated. B: Higher magnification of the transition between the surgically excised and debrided areas. C: Area in the central corneal epithelium with remnants of mechanically broken basal cells (arrow in insert). Bar equals 900 μm in A, 300 μm in B and C, and 100 μm in C, insert.
Surgery and clinical graft outcomes
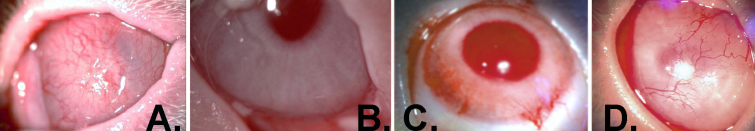
Histological examination of a cornea collected immediately after surgery indicated that the combined surgical and debridement procedure resulted in the complete removal of the limbal–corneal epithelium and the sub-epithelial, vascularized limbal zone (Figure 3). The ocular surface of all three operated rabbits grafted with amniotic membrane alone was rapidly invaded by the conjunctival epithelium. The corneas became completely vascularized and developed severe LSCD within 6 weeks (Figure 4A). All six grafts made with primary outgrowths resulted in full recovery of the ocular surface (Figure 4B). For the grafts that used secondary or tertiary outgrowths, four of each also led to complete corneal surface recovery. Two grafts of each in these series displayed certain neovascularization/opacities as annotated in Table 2. The mildly neovascularized eye from the tertiary outgrowth graft (Table 2) and the neovascularized opaque eye from the graft with a secondary outgrowth graft are shown in Figure 4C,D, respectively. Kruskal–Wallis statistical analysis of the clinical scores yielded no significant difference between the three groups (p=0.52). None of the whole or partial failures were not related to any potential physical failure at the surgical border, such as loosened sutures or folded hAM edge or infection. All donor contralateral corneas showed no visible long-term effects at the limbal excision site.
Figure 4.
Photographs of corneas 6 months after excision surgery and grafting of explant outgrowths. A: Human amniotic membrane (hAM) alone. B–D: hAM with outgrowths from the contralateral and donor eye explants. B: Grade 0 neovascularization (neo) and opacity (opc). C: Neo, 1; opc, 0. D: Neo, 2; opc, 2.
Table 2. Clinical outcome of grafts.
| Outgrowths | # Rbts | Neovascularization |
Opacity |
Staining |
||||||
|---|---|---|---|---|---|---|---|---|---|---|
| G0 | G1 | G2 | G0 | G1 | G2 | G0 | G1 | G2 | ||
| Primary |
6 |
♦ |
|
|
♦ |
|
|
♦ |
|
|
| Secondary |
4 |
♦ |
|
|
♦ |
|
|
♦ |
|
|
| 1 |
|
|
♦ |
|
|
♦ |
|
|
♦ |
|
| 1 |
|
|
♦ |
|
♦ |
|
|
|
♦ |
|
| Tertiary | 4 |
♦ |
|
|
♦ |
|
|
♦ |
|
|
| 1 |
|
♦ |
|
♦ |
|
|
|
♦ |
|
|
| 1 | ♦ | ♦ | ♦ | |||||||
Histology and immunohistochemistry analyses
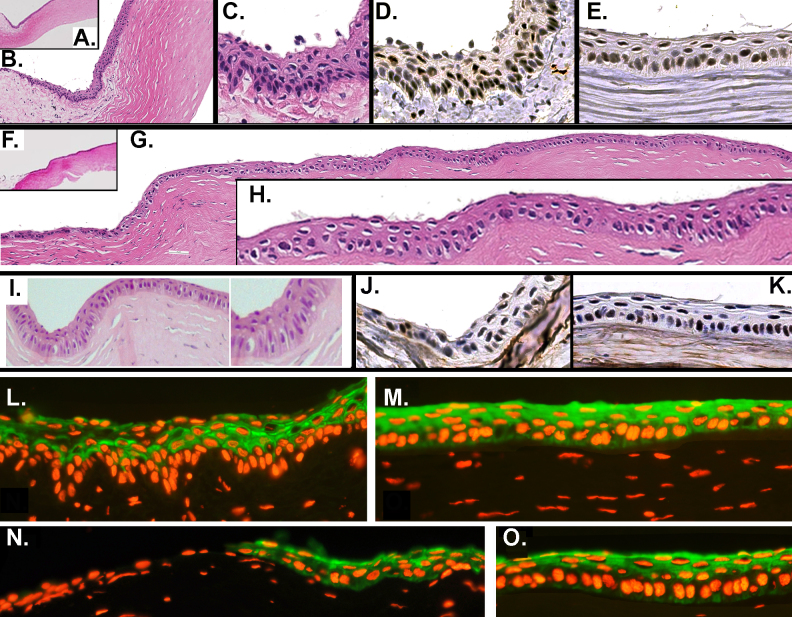
At the end of the 6-month recovery period, the rabbits were euthanized. Sections of corneas were fixed in buffered formalin and processed for H&E and immunostaining for p63 and Krt3. Figure 5 is a composite showing the H&E, p63, and Krt3 details of a control (donor) cornea (Figure 5A–E,L,M) and of two corneas regenerated using a primary outgrowth graft (Figure 5F–K,N,O). The limbal zone in the donor cornea displayed rete ridge-like epithelial formations overlying a loose sub-epithelial papilla. p63 was similarly expressed at high density throughout the limbal and central corneal epithelium (Figure 5D,E). This p63 distribution is at variance with the exclusive localization of p63 within the limbal zone in the adult human [17]. Finally, as previously shown [19], in the control all limbal basal epithelial cells lacked the Krt3 expression present in the basal cells of the central cornea (Figure 5L,M).
Figure 5.
Histological and immunochemical details of the limbal and corneal zone of the control ocular surface and ocular surfaces grafted with primary outgrowths following radical limbal excision and corneal epithelial removal. A–E, L, and M: Donor cornea. The area selected was not subjected to surgical biopsy excision. A–C: Hematoxylin and eosin (H&E) stain at three magnifications. Note the loose stroma defining the limbal papilla. D and E: p63 stain of the limbal and central corneal zone. Note that p63 is strongly expressed in the nuclei of most cells in both zones, including some suprabasal nuclei. L and M: Krt3 stain in the limbus and the central cornea. Note the pronounced limbal rete ridge appearance in the limbus and the absence of Krt3 in the basal cell layer there. F–K, N, and O: Ocular surface grafted with a primary-generation outgrowth at 6 months post-surgery. F–H: H&E stain at different magnifications in two corneas. Note the absence of a limbal papilla and rete ridges, multiple zones of engrossed epithelium with perpendicular elongated cells within the peripheral area, and the normal appearance of the central cornea (K). L and M: p63 stain of peripheral and central cornea. As in the donor eye, there is profuse expression of p63 throughout the cornea. N and O: Krt3 staining of the peripheral and central corneal zones. Note the absence of Krt3-negative cells at the conjunctival–corneal junction.
At 6 months post-grafting, the epithelium of the central cornea displayed histological features, including strata thickness and cell and nuclei size and shape, that were undistinguishable from those of the control tissue (Figure 5F–I). As expected, since the limbus had been surgically removed, the peripheral zone was devoid of a sub-epithelial loose tissue papilla. However, throughout this area, the regenerated corneal epithelia displayed multiple thickened zones, where the basal cells and their nuclei become elongated by projecting inwardly toward the stroma, reminiscent of the native limbal epithelium (Figure 5G,I). On these areas, the stroma consistently cupped inward during fixation. As in the control, p63 was profusely expressed throughout the epithelium (Figure 5J,K), but at variance with the control cornea, all basal cells within the peripheral zone corresponding to the removed limbal papilla were Krt3 positive (Figure 5N,O).
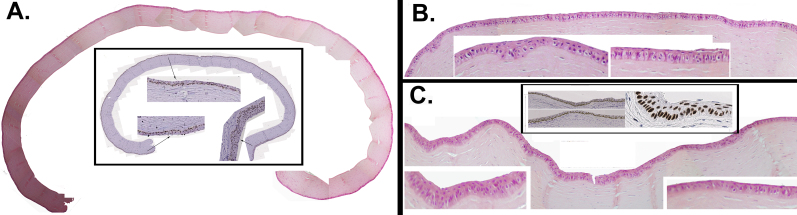
Figure 6 shows the representative histological and p63 staining features of three of the corneas that achieved complete recovery of the ocular surface after grafting with tertiary cultures. Stratification, the shape of the cell and its nucleus, and the high p63 expression throughout the whole epithelium were comparable to that of the native untreated cornea. In the periphery, the epithelium displayed increased stratification with elongated or columnar basal cells, as observed with the primary outgrowth grafts. Figure 7 shows the expression of Krt3 in the same rabbits. In the central cornea, the distribution of Krt3 in the donor (Figure 7A) and grafted eyes (Figure 7B–E) could not be distinguished from each other. In the peripheral zones, where the epithelium may resemble the native limbal epithelium or present a hyper-proliferative profile, all basal cells were always Krt3-positive (Figure 7G,F).
Figure 6.
Histological and p63 immunochemical features of four treated corneas grafted with tertiary-generation limbal explant outgrowths. Hematoxylin and eosin (H&E) composites of the full cornea (A) or large areas of it (B and C) are shown. Unframed inserts in B and C show magnified H&E details of the limbal–peripheral and corneal zone. Framed inserts show p63 staining of respective adjacent sections at arbitrary magnifications.
Figure 7.
Krt3 expression. Donor cornea in corneas regenerated with tertiary-generation explant outgrowths. A: Central epithelium of the donor cornea. B: Central epithelium of the corresponding grafted contralateral eye. C–E: Equivalent zone in another three corneas regenerated with tertiary-generation explant outgrowths. F and G: Morphology and Krt3 stain in the peripheral zone of the corneas in C and E. F: The cornea is counterstained with propidium iodide (PI). Bar=50 μm.
Cell size and JC1 exclusion
In Figure 8, the cell size and JC1-dependent outflow on cells recovered from the limbal-–peripheral zone, defined as 1.5-mm-wide biopsy spanning either side of the anatomic junction between the cornea and the conjunctiva, are compared [5]. Corneas from six rabbits, three regenerated with secondary grafts and three with tertiary grafts, were used to compare the FSC of the harvested cells from the donor and grafted eyes. In all six cases, the cell population harvested from the regenerated cornea had a lower proportion of large cells than the contralateral control cornea (Figure 8A,B). For quantitation of this difference, we set an arbitrary FCS threshold for each corneal pair such that 15% of the cells harvested from the regenerated cornea exceeded it; the percentage of control cornea-derived cells exceeding the threshold was nearly three times larger (41.6±11.2%; p<0.001) in the donor eye cells. Three of the paired specimens were stained with JC1 (two secondary, one tertiary). In all three cases, the difference in the JC1-excluding percentiles between cells from the control and treated corneas was less than 20%, and the mean ± standard deviation (SD) were 39.3±9 and 40.0±12.1, respectively.
Figure 8.
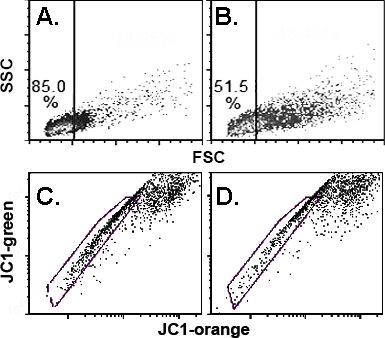
Forward scatter and JC1 exclusion of cells collected from the periphery of the contralateral donor and regenerated corneas. A and B: Forward (FSC) for regenerated and donor contralateral eyes. Density plots. C and D: Corresponding JC1 exclusion results.
Explant outgrowths of regenerated corneal epithelia
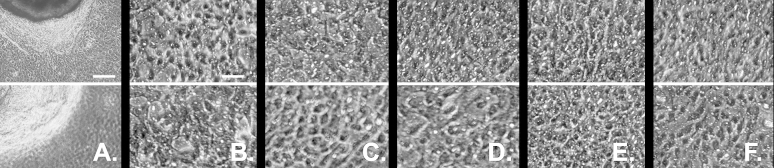
Finally, we assessed the ability of the biopsies obtained from the donor and regenerated eyes to support explant outgrowth. All biopsies yielded similar outgrowth islands and comprised cells of similar gross appearance under phase contrast examination (Figure 9). The average (n=3) outgrowth area ratios between the donor and regenerated eyes were 100:92 and 100:106 for the primary- and tertiary-based grafts, respectively.
Figure 9.
Phase contrast micrographs of outgrowths from limbal–peripheral biopsies obtained from donors and the contralateral regenerated corneas. A and B: Outgrowths from a cornea regenerated with a primary-generation explant culture (top) and from its contralateral donor eye (bottom). C–F: Equivalent micrograph pair from a secondary outgrowth-based regeneration (C) and from three tertiary outgrowth-based regenerations (D–F). The regularly distributed white puncta on these images arises from reflections of the 0.4 μm pore of the permeable substratum. The scale bars equal 250 μm in A and 50 μm in B through F.
Discussion
In a previous study, we demonstrated the cell population migrating from rabbit and human limbal explants showed an increase in the proportion of cells that exhibiting features associated with the stem cell phenotype, including ABCG2-dependent dye exclusion [8]. In more recent work, we have observed preservation of precursor cell features in rabbits for at least the first six sequential outgrowths, with this last outgrowth occurring well into the third month of continuous explant culture [23]. In this report, we demonstrate that cell outgrowth from rabbit explants after two sequential transferences to a new culture substratum, representing cells produced during the 2 weeks following a previous 20- to 25-day period of explant culture, are capable of restoring a rabbit ocular surface that has been rendered free of an anatomic limbal zone and all limbal–corneal epithelial cells. An alternative mechanism for the restoration of the ocular surface, namely, transdifferentiation of invading conjunctiva into the corneal phenotype [24,25] can be discounted. It has been thoroughly demonstrated (Kruse et al., 1990) that reports of in situ conjunctival transdifferentiation reflected, in fact, corneal recolonization of the corneal surface by small numbers of surviving limbal epithelial cells. No conjunctival-based recovery of the corneal surface was observed when the rabbit corneal surface was subjected to a radical surgical excision as we have performed in this study [26].
As for transplants, cells must be grown over a suitable biodegradable substratum, typically hAM, we first examined the impact of this substratum on the cell properties vis à vis the properties of cells from culture performed over the synthetic substratum. Consistent with studies documenting the effect of hAM on the preservation of precursor cells [27,28], the cells removed from hAM displayed features that have been linked to somatic and embryonic stem cells, including a smaller size [29,30], a higher percentile of ABCG2-dependent dye exclusion activity [9,31,32], and a lower MMPT, related to the preferential reliance of primitive cells on glycolysis [33,34]. We also expanded the phenotypic comparison between the primary and tertiary outgrowths to include three markers related to the limbal precursor or their differentiation, namely, p63, Krt3, and Cx43. These measurements failed to show any substantial differences as a result of the extension of the outgrowth period.
The clinical outcomes and the histological and biochemical comparisons of the limbal corneal epithelia 6 months after grafting of primary or tertiary explant outgrowths with the epithelium of the contralateral eye (using corneal zones not affected by biopsy excision) demonstrated the potential of secondary and tertiary outgrowths to generate stable cornea epithelial cells that have a high degree of similarity to the controls. The partial clinical failures with the post-primary outgrowths may reflect a decrease in the regenerative ability, though the outcomes were not statistically distinguishable.
In the comparison of outcomes with biochemical and functional endpoints 6 months after grafting, the high density of p63high and Krt3 expression in the central cornea and the cellular anatomy and growth rates from the biopsies from these corneas matched the features displayed by the control corneas. Anatomically, the frequent nodules of high stratification in the periphery of the recovered corneas and the anatomic resemblance of the basal cells there to the cells of limbal palisades suggest that limbal regenerative events may be active beyond the 6-month post-recovery endpoint used in this study. To our knowledge, the anatomy of the regenerated epithelium at the periphery has been described in only one previous LSCD recovery study employing full limbal-corneal lamellar removal [35]. Although the methods and protocols are different, 1 year later the recovered epithelium showed spots of epithelial thickening and downward encroachment into the stroma similar to those observed in our study.
A remarkable difference between the native and regenerated epithelia was the absence of Krt3-negative basal cells following recovery, irrespective of the outgrowth generation used. This feature suggests that critical controls of stem cells and early differentiation events have been removed or delayed by the radical limbal excision. The second difference between the donor control eye cornea and the regenerated cornea, namely, the smaller size of the cells in the latter, is difficult to interpret. If regeneration is still active at the 6-month endpoint, then the smaller cell size may be due to a higher proliferation rate during the recovery period.
In conclusion, these studies in the rabbit suggest the possibility of extending the supply of transplantable regenerative cells from small biopsies of contralateral corneas for repeated or reinforced treatment of LSCD. The applicability to human clinical practice will require further study.
Acknowledgments
Supported by grants from TUBITAK Grant 111S414 (IDD) and RO1EY14878 and Research to Prevent Blindness (JMW).
References
- 1.Tseng SCG. Concept and application of limbal stem cells. Eye (Lond) 1989;3:141–157. doi: 10.1038/eye.1989.22. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2695347&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 2.Chen JJ, Tseng SC. Abnormal corneal epithelial wound healing in partial-thickness removal of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:2219–33. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1712763&dopt=Abstract [PubMed] [Google Scholar]
- 3.Huang AJ, Tseng SC. Corneal epithelial wound healing in the absence of limbal epithelium. Invest Ophthalmol Vis Sci. 1991;32:96–105. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=1702774&dopt=Abstract [PubMed] [Google Scholar]
- 4.Tsai RJ, Li LM, Chen JK. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N Engl J Med. 2000;343:86–93. doi: 10.1056/NEJM200007133430202. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10891515&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 5.Ti S-E. David Anderson, Amel Touhami,Charles Kim, and Scheffer C. G. Tseng. Factors Affecting Outcome Following Transplantation of Ex vivo Expanded Limbal Epithelium on Amniotic Membrane for Total Limbal Deficiency in Rabbits. Invest Ophthalmol Vis Sci. 2002;43:2584–92. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=12147589&dopt=Abstract [PubMed] [Google Scholar]
- 6.Dua HS, Azuara-Blanco A. Autologous limbal transplantation in patients with unilateral corneal stem cell deficiency. Br J Ophthalmol. 2000;84:273–8. doi: 10.1136/bjo.84.3.273. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10684837&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangwan VS, Basu S, Vemuganti GK, Sejpal K, Subramaniam SV, Bandyopadhyay S, Krishnaiah S, Gaddipati S, Tiwari S, Balasubramanian D. Clinical outcomes of xeno-free autologous cultivated limbal epithelial transplantation: a 10-year study. Br J Ophthalmol. 2011;95:1525–9. doi: 10.1136/bjophthalmol-2011-300352. [DOI] [PubMed] [Google Scholar]
- 8.Selver OB, Barash A, Ahmed M, Wolosin JM. ABCG2-dependent dye exclusion activity and clonal potential in epithelial cells continuously growing for 1 month from limbal explants. Invest Ophthalmol Vis Sci. 2011;52:4330–7. doi: 10.1167/iovs.10-5897. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=21421882&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Budak MT, Alpdogan OS, Zhou M, Lavker RM, Akinci MA, Wolosin JM. Ocular surface epithelia contain ABCG2-dependent side population cells exhibiting features associated with stem cells. J Cell Sci. 2005;118:1715–24. doi: 10.1242/jcs.02279. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15811951&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Li W Hayashida Y, He H, Kuo CL, Tseng SC. The fate of limbal epithelial progenitor cells during explant culture on intact amniotic membrane. Invest Ophthalmol Vis Sci. 2007;48:605–13. doi: 10.1167/iovs.06-0514. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17251456&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Talbot M Carrier P, Giasson CJ, Deschambeault A, Guérin SL, Auger FA, Bazin R, Germain L. Autologous transplantation of rabbit limbal epithelia cultured on fibrin gels for ocular surface reconstruction. Mol Vis. 2006;12:65–75. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16479251&dopt=Abstract [PubMed] [Google Scholar]
- 12.Nakamura T Kinoshita S.Ocular surface reconstruction using cultivated mucosal epithelial stem cells. Cornea. 2003;22(Suppl):S75–80. doi: 10.1097/00003226-200310001-00011. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14703711&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 13.Ono K Yokoo S, Mimura T, Usui T, Miyata K, Araie M, Yamagami S, Amano S.Autologous transplantation of conjunctival epithelial cells cultured on amniotic membrane in a rabbit model. Mol Vis. 2007;13:1138–43. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=17653059&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]
- 14.Ang AY Chan CC, Biber JM, Holland EJ. Ocular surface stem cell transplantation rejection: incidence, characteristics, and outcomes. Cornea. 2013;32:229–36. doi: 10.1097/ICO.0b013e318255eac4. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=22668584&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 15.Yang A, Schweitzer R, Sun D, Kaghad M, Walker N, Bronson R, Tabin C, Sharpe A, Caput D, Crum C, McKeon F. p63 is essential for regenerative proliferation in limb, craniofacial and epithelial development. Nature. 1999;398:714–8. doi: 10.1038/19539. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10227294&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 16.Crum CP, McKeon FD. p63 in epithelial survival, germ cell surveillance, and neoplasia. Annu Rev Pathol. 2010;5:349–71. doi: 10.1146/annurev-pathol-121808-102117. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20078223&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 17.Pellegrini G, Dellambra E, Golisano O, Martinelli E, Fantozzi I, Bondanza S, Ponzin D, McKeon F, De Luca M. p63 identifies keratinocyte stem cells. Proc Natl Acad Sci USA. 2001;98:3156–61. doi: 10.1073/pnas.061032098. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11248048&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Di Iorio E Barbaro V, Ruzza A, Ponzin D, Pellegrini G, De Luca M. Isoforms of DeltaNp63 and the migration of ocular limbal cells in human corneal regeneration. Proc Natl Acad Sci USA. 2005;102:9523–8. doi: 10.1073/pnas.0503437102. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15983386&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schermer A, Galvin S, Sun TT. Differentiation-related expression of a major 64K corneal keratin in vivo and in culture suggests limbal location of corneal epithelial stem cells. J Cell Biol. 1986;103:49–62. doi: 10.1083/jcb.103.1.49. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2424919&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wolosin JM Xiong X, Schütte M, Stegman Z, Tieng A. Stem cells and differentiation stages in the limbo-corneal epithelium. Prog Retin Eye Res. 2000;19:223–55. doi: 10.1016/s1350-9462(99)00005-1. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=10674709&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 21.Friedrich E. Kruse and Scheffer C. G. Tseng. Growth Factors Modulate Clonal Growth and Differentiation of Cultured Rabbit Limbal and Corneal Epithelium. Invest Ophthalmol Vis Sci. 1993;34:1963–76. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=8491549&dopt=Abstract [PubMed] [Google Scholar]
- 22.Matic M, Petrov IN, Chen S, Wang C, Dimitrijevich SD, Wolosin JM. Stem cells of the corneal epithelium lack connexins and metabolite transfer capacity. Differentiation. 1997;61:251–60. doi: 10.1046/j.1432-0436.1997.6140251.x. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=9203348&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 23.Chen YT, Li W, Hayashida Y, He H, Chen SY, Tseng DY, Kheirkhah A, Tseng SC. Human amniotic epithelial cells as novel feeder layers for promoting ex vivo expansion of limbal epithelial progenitor cells. Stem Cells. 2007;25:1995–2005. doi: 10.1634/stemcells.2006-0677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zamudio A, Wang Z, Chung S-H, Wolosin JM. Inhibition of TGFβ cell signaling for limbal explant culture in serumless, defined xeno-free conditions. Exp Eye Res. 2015;145:48–57. doi: 10.1016/j.exer.2015.10.021. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26554938&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kinoshita S, Friend J, Thoft RA. removal of such cells results in corneal vascularization and conjunctivalization. Biphasic cell proliferation in transdifferentiation of conjunctival to corneal epithelium in rabbits. Invest Ophthalmol Vis Sci. 1983;24:1008–14. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=6874266&dopt=Abstract [PubMed] [Google Scholar]
- 26.Tseng SC, Farazdaghi M, Rider AA. Conjunctival transdifferentiation induced by systemic vitamin A deficiency in vascularized rabbit corneas. Invest Ophthalmol Vis Sci. 1987;28:1497–504. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2442115&dopt=Abstract [PubMed] [Google Scholar]
- 27.Kruse FE, Chen JJ, Tsai RJ, Tseng SC. Conjunctival transdifferentiation is due to the incomplete removal of limbal basal epithelium. Invest Ophthalmol Vis Sci. 1990;31:1903–13. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2211036&dopt=Abstract [PubMed] [Google Scholar]
- 28.Meller D, Pires RTF, Tseng SCG. Ex vivo preservation and expansion of human limbal epithelial stem cells on amniotic membrane cultures. Br J Ophthalmol. 2002;86:463–71. doi: 10.1136/bjo.86.4.463. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=11914219&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Barrandon Y, Green H. Cell size as a determinant of the clone-forming ability of human keratinocytes. Proc Natl Acad Sci USA. 1985;82:5390–4. doi: 10.1073/pnas.82.16.5390. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=2410922&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Romano AC, Espana EM, Yoo SH, Budak MT, Wolosin JM, And Tseng SC. Different cell sizes in human limbal and central corneal basal epithelia measured by confocal microscopy and flow cytometry. Invest Ophthalmol Vis Sci. 2003;44:5125–9. doi: 10.1167/iovs.03-0628. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=14638707&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 31.de Paiva CS, Chen Z, Corrales RM, Pflugfelder SC, Li DQ. ABCG2 transporter identifies a population of clonogenic human limbal epithelial cells. Stem Cells. 2005;23:63–73. doi: 10.1634/stemcells.2004-0093. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=15625123&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Umemoto T, Yamato M, Nishida K, Yang J, Tano Y, Okano T. Limbal epithelial side-population cells have stem cell-like properties, including quiescent state. Stem Cells. 2006;24:86–94. doi: 10.1634/stemcells.2005-0064. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=16150918&dopt=Abstract [DOI] [PubMed] [Google Scholar]
- 33.Romero-Moya D, Bueno C, Montes R, Navarro-Montero O, Iborra FJ, López LC, Martin M, Menendez P. Cord blood-derived CD34+ hematopoietic cells with low mitochondrial mass are enriched in hematopoietic repopulating stem cell function. Haematologica. 2013;98:1022–9. doi: 10.3324/haematol.2012.079244. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=23349299&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wanet A, Arnould T, Najimi M, Renard P. Connecting Mitochondria, Metabolism, and Stem Cell Fate. Stem Cells Dev. 2015;24:1957–71. doi: 10.1089/scd.2015.0117. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=26134242&dopt=Abstract [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Luengo Gimeno F, Lavigne V, Gatto S, Croxatto JO, Correa L, Gallo JE. One-year follow-up of epithelial corneal cell sheet allografts mounted on platelet poor plasma in rabbits. Mol Vis. 2009;15:2771–9. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&list_uids=20019875&dopt=Abstract [PMC free article] [PubMed] [Google Scholar]