Abstract
Objective
Some patients with hepatitis C (HCV) starting interferon-α (IFN-α) experience depression, although many patients do not develop depressive symptoms. We have found that poor sleep is associated with increased depressive symptoms on average. It is unknown whether this association holds generally or is driven by a specific, distinct subgroup. This investigation first determined whether patterns of change in depressive symptoms form clinically meaningful, distinct sub-groups; and then tested the extent to which sleep disturbances are associated with a less favorable depression trajectory.
Method
Group-based trajectory modeling was used on 124 HCV patients who started IFN-α therapy. The Pittsburgh Sleep Quality Index (PSQI) assessed pre-treatment sleep, the Beck Depression Inventory minus the sleep question (BDI-s) assessed depression over time, and the Structured Clinical Interview for DSM-IV provided categorical diagnoses.
Results
Three distinct subgroups were found, where each subgroup shared similar patterns of depressive symptoms over time. The groups were characterized as “non-depressed”, “slow increase”, and “rapid increase”. The non-depressed subgroup (44.4%) experienced low depressive symptoms with little change over time. In comparison, all rapid increasers (11.3%) were diagnosed with a mood disorder by 12 weeks of treatment. The PSQI was strongly associated with group membership,. where the odds of developing a rapid increase was elevated 39% for every unit score increase in the PSQI compared to individuals who remained non-depressed (OR=1.39, 95%CI=1.07–1.80, adjusted for depression at baseline).
Conclusion
Only a distinct sub-population of people is notably vulnerable to a developing a rapid increase in depression symptoms during IFN-α therapy. This group may be identifiable by their markedly poor sleep prior to IFN-α therapy.
Keywords: hepatitis C virus, group-based trajectory modeling, depression, sleep quality
Major depressive disorder (MDD) is a leading cause of disability and suicide (1–5). Targets for preventive intervention include either mitigating pre-existing vulnerabilities or avoiding depression triggers such as traumatic stressors. Thankfully, most people are not vulnerable. Even when faced with medical stressors, the incidence of depression is less than 10% over several years (6–11) and less than 2% per year (12–14). The incidence of depression for newly diagnosed cancer patients is only 12% per year (15). Thus, the vast majority is not vulnerable even in the setting of severe medical stress. Similarly, the cumulative lifetime MDD prevalence of the general population is just 15–20% (16–18). We are therefore interested in identifying any modifiable resiliency factors that may be present in over 80% of the population, particularly focusing on those faced with medical co-morbidity.
One specific plausible trigger of depression is increased inflammation (19–23); and a particular instance of this is seen in patients who are given interferon-α (IFN-α) injections. IFN-α is an inflammatory cytokine that is an element of therapy for either hepatitis C (HCV) or melanoma (24–26). Inflammatory cytokines naturally elicit “acute sickness behaviors” (27) that very closely resemble MDD (28). In fact, IFN-MDD and MDD in general share similar pathophysiologies (29, 30). The depressive syndrome triggered by IFN-α (IFN-MDD) develops in a subset of HCV patients by the third month of treatment (31–39). IFN-MDD, when it occurs, has been implicated in suicide (40), discontinuation and thus inefficacy of treatment (41, 42), and overall lower quality of life (43, 44). To prevent IFN-MDD, one option is to avoid IFN-α therapy in the first place. Towards this end, new medications for HCV are now available that drastically decrease the number of HCV patients who require IFN-α (45). Nonetheless, often times more expensive medications still need to be used in combination with IFN-α rather than as monotherapies (46–49). This highlights the importance of identifying who will be vulnerable to IFN-MDD (and thus who should avoid IFN-α) and who will be resilient. Equally importantly, lessons learned from IFN-MDD may plausibly inform efforts to predict and prevent MDD in other populations. The advantage of modeling the depression development after a very specific and potent medical stressor such as IFN-α (compared with general population-based research) is that it can be done using relatively small samples for limited time periods.
To date, two general statistical methods have been employed to evaluate the development of depression during IFN-α therapy. One set uses a categorical definition of “MDD” such as from a structured interview or a retrospective chart review, or from a self-report cut-off value. The second methodological approach quantitatively examines average depression scores. However, there is another possibility that has not been used in studies of IFN-MDD. In this third approach, distinct trajectories of depressive symptoms over time can be classified using data-driven techniques such as group-based growth mixture models (50–53). These depression trajectories are defined as changes in the course of an individual’s depressive symptoms over time, with the advantage of making it possible to detect changes at both the group level and the individual level. One major advantage of this third approach is that rather than examining changes in the population-average, and rather than using a consensus categorical diagnosis, distinct sub-groups with similar trajectories are identified empirically. It is very plausible that determining patterns of depression trajectories in HCV patients can help with understanding the latent constructs that underlie differences in individual progression.
Using this type of modeling, we examined the extent to which sleep quality before starting IFN-α therapy might be an important predictor of trajectory membership. We previously found that HCV patients who report poor sleep quality prior to starting IFN-α have an increase in average depression symptoms over the course of four months of treatment (54) and are more likely to develop DSM-IV-defined IFN-MDD (34). However, it is currently unknown whether this association holds generally among the entire HCV patient population or whether it is driven by a specific, distinct and vulnerable subgroup that could be targeted for clinical intervention. This investigation therefore addressed the following two aims: first, to determine whether patterns of change in depressive symptoms in patients treated with IFN-α therapy do form distinct sub-groups; and second, to test the extent to which sleep disturbances prior to starting treatment predict the development of specific depression trajectories. This information will be clinically useful in helping to (1) determine which specific patient subgroups should avoid IFN-α therapy and (2) identify vulnerabilities that may be modifiable for prevention of IFN-MDD in those who still need to be treated with IFN-α.
Methods
Participants
Patients with HCV were recruited at the University of Pittsburgh Medical Center for Liver Diseases prior to starting treatment with pegylated interferon-alpha2 (PEG-IFN-α2a: 135 μg/week or PEG-IFN-α2b: 120 or 150 μg/week) and oral ribavirin (typically given at weight-based dose). Those who were recommended for IFN-α treatment by their hepatologist (from October 2005 and ended in August 2012) were asked to participate in a research study where they would be followed for up to four months in order to investigate the association of IFN-α and depression. All participants provided written informed consent as per protocol by the University of Pittsburgh Institutional Review Board.
We are primarily interested in risk for IFN-MDD incidence and not just prolongation or exacerbation of a pre-existing MDD episode. Therefore, exclusion criteria included an active Axis-I disorder (e.g. mood, anxiety, psychotic, impulse control, or drug/alcohol use disorders) within six months prior to starting treatment. This was assessed using the Structural Clinical Interview for DSM-IV Axis I Disorders (SCID-I) (55). Additional exclusions included a recent depressive episode in remission for less than six months, known and active neurological disease, known and active inflammatory disorders other than HCV, or taking medications such as corticosteroids, anticonvulsants, and/or antipsychotics. Other than HCV, participants had medical burden that was comparable to those in their 40s without HCV, according to the Cumulative Illness Rating Scale-Geriatric (56). Individuals with remote past histories of mood disorders were included as long as they were in sustained remission. Participants who developed major mood disorder during treatment were given a psychiatric intervention (typically consisting of an antidepressant or mood-stabilizing medication), and subsequent data was censored. Some patients discontinued IFN-α for medical reasons (n=20), and data subsequent to stopping IFN-α was censored.
Measures
Depression was measured prior to starting treatment using the Beck Depression Inventory-II (BDI) (57). BDI questionnaires were also provided to participants at weeks 2, 4, 8, 12, and 16 during treatment. The BDI consists of 21 questions (each scored 0–3), with a total range of 0–63. The BDI can be categorized into: 0–13 indicating minimal symptoms, 14–19 indicating mild symptoms, 20–28 indicating moderate symptoms, and 29–63 indicating severe symptoms (58). Because BDI scores do not always correspond to a formal DSM-IV diagnosis, individuals who scored a 15 or higher on the BDI were administered an abbreviated SCID-I in order to diagnose whether a DSM-IV mood disorder was present. In order to account for sleep as a potential risk factor, BDI minus the sleep item (BDI-s) was used in analyses. The BDI sleep item responses include: I have not experienced any change (0), I sleep somewhat less/more than usual (1), I sleep a lot less/more than usual (2), and I wake up 1–2 hours early and can’t get back to sleep (3). Self-reported sleep quality was assessed prior to starting treatment using the Pittsburgh Sleep Quality Index (PSQI) (59). The PSQI ranges from 0–21, and scores below four typically indicate no problem sleep, scores above 4 are indicative of some problems, and a score of 10 or more is observed in people with primary insomnia (59–61).
Statistical Analysis
We employed group-based trajectory modeling, using SAS TRAJ procedure, which partitions a cohort into subgroups based on repeated measurements of a variable over time and baseline risk factors, thus describing heterogeneity that may exist in populations based on longitudinal trajectories (62, 63). This was implemented to determine distinct subpopulations, where each subpopulation consists of individuals who shared a similar trajectory of depressive symptoms. Group-based trajectory modeling is data-driven, allowing an exploratory analysis of the patterns that exist within a population that may be difficult to identify a priori. The first step in model selection was determining the optimal number of distinct subgroups using the Bayesian information criterion (BIC) (64). The BIC introduces a penalty for the number of parameters added. The model with the smallest absolute BIC is chosen. To compare models, the Bayes factor provides the posterior odds of a model actually being the correct model compared with a simpler model. The log Bayes factor is approximated as being twice the change in BIC between models (63) (65). The number of distinct trajectories considered were five-, four-, three-, and two-group solutions using either linear or quadratic polynomials for each trajectory. When choosing the number of groups, the criteria were that the model had to have a significantly higher BIC than a model with a smaller number of groups and the number of individuals in each group had to be larger than 5%. After the optimal number of groups was determined, the degree of each trajectory was found by examining every permutation of linear and quadratic terms. Higher polynomial degree (i.e., >2) were not explored due to the number of subjects and time points. The BIC log Bayes factor approximation was similarly used to assess which polynomial degree for each trajectory resulted in the best model fit.
Model selection was conducted again, using group-based trajectory modeling, while adjusting for potential risk factors: sociodemographic confounders (age and sex) and PSQI. The SAS TRAJ procedure uses a multinomial modeling technique to estimate the associations between potential risk factors and group membership. Race was not included as a potential risk factor since there were a low number of non-whites in the sample. Once the best-fit model was obtained when including all risk factors, model selection was repeated including only risk factors that significantly differed among groups in order to obtain the most parsimonious model. We investigated whether the association of PSQI was independent of baseline depression symptom load by examining the association between PSQI and the group trajectories after adjusting for baseline depressive symptoms. Since PSQI was not administered repeatedly to all of these subjects during the course of treatment, we examined serial measures of the BDI seep item over time to explore the patterns of sleep disturbances across the subgroups. Descriptive statistics (mean (standard deviation) or %(n)) of baseline measures were reported by subgroup, to further illustrate similarities and differences.
Goodness of fit was assessed by examining posterior probabilities. All subjects were assigned a probability of belonging to each group given their BDI-s measurements over time and their measurements for risk factors included in the model. Each participant was then assigned the group where the posterior probability of membership was the largest. The closer the group average posterior probability is to one, the better the model fit, with the recommendation that every group have an average posterior probability that is at least 0.7 (66).
Sensitivity analyses were performed including: reducing the number of groups in the final model to further evidence for all subgroups found, examining the best model when no risk factors were included to see if consistent results were found with the literature, and dropping the last two time points measured to examine if the same final model was found regardless of the dropout rate towards the end of the study.
Results
We examined 124 HCV patients who began IFN-α therapy, did not already have a baseline DSM-IV SCID diagnosis of depression, had a pre-treatment PSQI score available, and had serial BDI-s measures. Patients were mostly male (64.5% (n=80)) and white (90.3% (n=112)) with a mean (standard deviation) age of 45.7 (12.3) years. A censored normal distribution was used to model BDI-s over time.
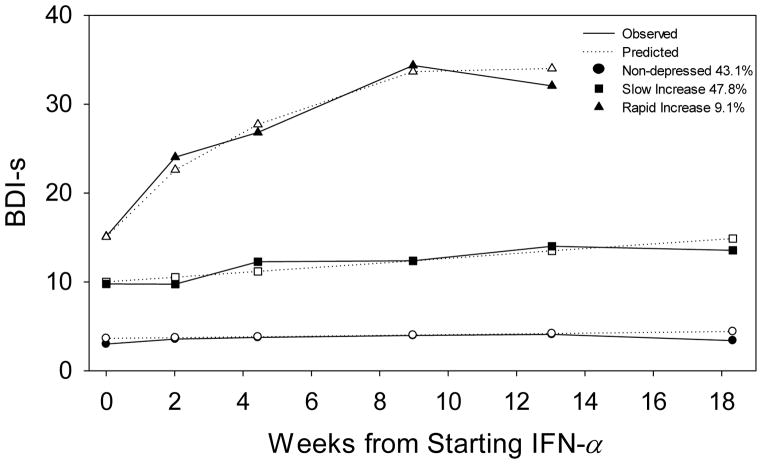
The depression trajectories among the HCV subjects undergoing IFN-α were best classified using three groups (Figure 1). The three groups were labeled as “non-depressed”, “slow increase”, and “rapid increase” (Table 1). The non-depressed and slow increase trajectories were best approximated as linear trajectories. A quadratic increase best described the rapid increase trajectory. The non-depressed group contained 44% of the sample, started out with a low overall group mean BDI-s of 2.75 at baseline, and experienced very little change over the course of treatment (Table 2). The slow increasers made up 44% of the sample, started out with an overall group mean BDI-s of 12.0 that steadily increased throughout the course of treatment, with several developing “mild” depression symptoms by week 12 of IFN-α treatment. The rapid increasers consisted of 11% of the sample. By 12 weeks into IFN-α treatment, all (100%) of the ‘rapid increasers’ were formally diagnosed with a mood disorder that necessitated antidepressant treatment. The three groups had similar average baseline age and proportions of females. There was a larger proportion of non-whites in the rapid increase subgroup and there was an increase in average baseline BDI-s and PSQI when going from the non-depressed, to slow increase, to rapid increase subgroup (Table 1).
Figure 1.
Average observed and predicted trajectories of BDI-s at baseline and during IFN-α treatment of distinct subgroups found in the sample of 124 HCV patients, while adjusting for baseline PSQI scores and baseline BDI-s
Table 1.
Baseline Descriptive Statistics by subgroup for 124 HCV patients
| Non-depressed N=55 Mean (SD) or % (n) |
Slow Increase N=56 Mean (SD) or % (n) |
Rapid Increase N=13 Mean (SD) or % (n) |
|
|---|---|---|---|
| Age | 47.1 (13.0) | 44.3 (12.0) | 45.9 (10.7) |
| Sex: Female | 40.0% (22) | 33.9% (19) | 23.1% (3) |
| Race: White | 92.7% (51) | 92.9% (52) | 69.2% (9) |
| BDI-s at Baseline | 2.65 (2.4) | 9.59 (5.6) | 17.5 (9.7) |
| PSQI at Baseline | 4.87 (3.3) | 8.11 (3.9) | 10.1 (4.4) |
Note. The three subgroups in the HCV sample had a similar average baseline age and proportion of female. There was a higher proportion of non-whites in the rapid increase subgroup, although due to the low number of non-white participants in the HCV sample we were unable to test this further. As expected, there appears to be an increase in baseline BDI-s and PSQI scores as the subgroup becomes less favorable.
Table 2.
Mean and confidence intervals of BDI-s over time by subgroup for 124 HCV patients, adjusting for baseline PSQI and BDI-s using group-based trajectory analysis
| Mean (95% CI) [n if reduced sample] | Non-depressed N=55 |
Slow Increase N=56 |
Rapid Increase N=13 |
|
|---|---|---|---|---|
| Pre IFN-α | Baseline | 2.75 (2.4, 4.9) | 9.42 (8.0, 11.4) | 17.3 (14.2, 19.8) |
| During IFN-α Treatment | Week 2 | 3.84 (2.6, 4.9) [n=32] | 9.64 (8.8, 11.8) [n=29] | 22.7 (20.1, 24.6) [n=5] |
| Month 1 | 4.08 (2.8, 5.0) [n=51] | 12.0 (9.6, 12.3) [n=42] | 25.2 (23.6, 28.8) [n=9] | |
| Month 2 | 4.03 (2.9, 5.4) [n=36] | 12.3 (10.9, 13.6) [n=36] | 31.6 (26.6, 35.8) [n=7] | |
| Month 3 | 4.33 (2.8, 5.9) [n=31] | 14.1 (11.9, 15.1) [n=21] | 32.1 (27.9, 36.4) [n=3] | |
| Month 4 | 3.56 (2.4, 6.9) [n=28] | 13.5 (12.9, 17.0) [n=22] | ------ | |
Note. BDI-s was measured prior to starting IFN-α and at week 2, and month 1, 2, 3, and 4 during IFN-α treatment in order to assess the changes in depressive symptoms. Group-based trajectory modeling was used to determine distinct subgroups in the HCV sample, where within each subgroup participants shared similar patterns of depression symptoms over times. The non-depressed subgroup of the HCV sample starts off with a low BDI-s and experiences little change throughout the course of treatment. The average BDI-s of the slow increase subgroup is categorized as minimal depression and steadily increases to mild depression by the third month of IFN-α. By the third month, all subjects in the rapid increase subgroup were diagnosed with a mood disorder and administered treatment. Sensitivity analyses were performed to ensure the rapid increase subgroup is a valid subpopulation of HCV subjects undergoing IFN-α.
Figure 1 further illustrates the difference in mean BDI-s at baseline between the three groups, although when examining subject-specific baseline BDI-s there is overlap among the three groups. Baseline depressive symptoms for the slow increasers were as low as 1 and for the rapid increasers were as low as 2. In fact, about one-third (31%) of rapid increasers start off with “minimal” depressive symptoms prior to starting IFN-α therapy. Conversely, 23% of slow increasers had baseline depressive symptoms that could be categorized as higher than minimal depression (BDI≥14). Nonetheless, baseline BDI-s was a good predictor of group membership (Table 3). Thus, we statistically adjusted for baseline BDI-s as an important covariate in subsequent analyses. Age and sex were not associated with group membership, therefore excluding them from the three-group model reported did not drastically alter the results.
Table 3.
Baseline PSQI as a risk factor of group membership for 124 HCV patients undergoing treatment using group-based trajectory analysis while adjusting for baseline BDI-s
| Reference Group: Non-depressed | |||
|---|---|---|---|
| Group | Variable | Estimate (standard error), p-value | Odds Ratio (95% Confidence Interval) |
| Slow Increase | Constant | −3.42 (0.88), p<0.001 | ---- |
| PSQI | 0.193 (0.10), p=0.05 | 1.21 (0.996, 1.48) | |
| BDI-s at baseline | 0.417 (0.11), p<0.001 | 1.52 (1.23, 1.87) | |
|
| |||
| Rapid Increase | Constant | −8.17 (1.6), p<0.001 | ---- |
| PSQI | 0.330 (0.13), p=0.01 | 1.39 (1.07, 1.80) | |
| BDI-s at baseline | 0.582 (0.12), p<0.001 | 1.79 (1.41, 2.27) | |
|
| |||
| Reference Group: Slow Increase | |||
| Group | Variable | Estimate (standard error), p-value | Odds Ratio (95% Confidence Interval) |
|
| |||
| Rapid Increase | Constant | −4.75 (1.3), p<0.001 | ---- |
| PSQI | 0.137 (0.09), p=0.14 | 1.15 (0.957, 1.38) | |
| BDI-s at baseline | 0.165 (0.06), p=0.006 | 1.18 (1.05, 1.32) | |
Note. Group-based trajectory modeling, which uses a multinomial modeling technique, was used to test the association between PSQI and group membership, while adjusting for baseline BDI-s. Worse sleep quality is associated with a less favorable depression trajectory. The association between baseline PSQI and group membership is independent of baseline BDI-s when comparing rapid increasers to non-depressed. With one unit increase in PSQI, the odds of experiencing a rapid increase in depressive symptoms compared to no change increases by 39%.
When conducting analyses without adjusting for baseline BDI-s, PSQI strongly predicted group membership. With one unit increase in PSQI, the odds of having a slow increase in depressive symptoms instead of no change (non-depressed group) increased by 31% (p<0.001) and the odds of having a rapid increase in depressive symptoms compared to no change increased by 56% (p<0.001). With one unit increase in PSQI, the odds of being a rapid increaser instead of a slow increaser went up by 19% (p=0.05). When adjusting for baseline depressive symptoms (BDI-s), the association between PSQI and group membership continued to be significant (Table 3) although attenuated. In pair-wise testing, the difference in baseline PSQI between slow increasers and the rapid increasers was no longer significant (p=0.14). However, the odds of having a rapid increase in depression symptoms compared to being in the non-depressed group still increased by 39% (p=0.01) with every unit increase in PSQI (Table 3).
Since there were a small number of rapid increasers, a sensitivity analysis was done by reducing the number of groups in the model. Consistent results were found with a two-group model: PSQI remained a significant variable influencing group membership. The two-group model was made up of a group of rapid increasers and a group of non-depressed subjects. According to the BIC log Bayes factor approximation, there was “very strong” evidence supporting a three-group model (2loge(B10)≈79.1) (63). Therefore, even though the rapid increasers in the three-group model are made up of a small number of subjects, there exists strong evidence of its validity as a subgroup in the population.
Average BDI scores for subjects who had medical side effects necessitating discontinuation of IFN-α (n=20) were 7.9 at baseline, 7.2 by week 4, and 7.5 by week 8. Interestingly of these 20, 10% (n=2) developed mild symptoms of depression before stopping treatment. Thus, discontinuation of IFN-α treatment because of medical side effects did not affect our results.
We also examined trajectories when no covariates were included, and found that a 5-group model was the best-fit. There were two groups of non-depressed subjects (rather than just one), who only differed by their average baseline BDI-s scores, but who continued to have minimal symptoms throughout the 4 months. A third group started with minimal symptoms on average that slowly worsened and by the end had mild symptoms, while a fourth group started with mild symptoms on average but ended the study with minimal symptoms. This fourth group’s baseline BDI-s was actually higher than the “rapid increasers”, but was notable for a decline in depressive symptoms during treatment. This fourth group was resilient to any further worsening after starting IFN-α. As with the three-group solution, there continued to be a unique and clearly distinct rapid-increaser group who became severely depressed by 8 weeks on IFN-α. However, this five-group model became unstable when adding covariates as risk factors. Consequently, the three-group model was preferred as the most robust characterization of the data.
We also examined the effect of data censoring towards the end of the study. The last follow-up time point was dropped and the analysis was re-run in order to determine whether the amount of censored subjects was affecting the results obtained. When doing so, a consistent model was found with only ten subjects who were re-assigned to a different group. Similar results were obtained after taking away the last two time points, but with one additional subject being re-assigned from a slow increaser to the non-depressed group. Thus, the final 3-group model was robust even when the last two values per subject were dropped.
The rapid increasers were the only group to experience an average increase in the BDI sleep item over time, indicating an increase in sleep disturbances during the course of treatment. For this single question over the first two months of treatment, the non-depressed group went from a mean (standard deviation) of 0.46 (0.75) to 0.69 (0.76), the slow increaser group went from 1.12 (1.11) to 1.05 (0.93), while the rapid increaser group went from 0.91 (1.0) to 2.5 (0.84). Unfortunately, similar prospective PSQI data in all these subjects was not available for this type of analysis. Finally, we examined whether the single sleep question on the BDI at baseline could predict subsequent symptoms. In unadjusted analysis, the sleep question did differentiate the slow increasers from the non-depressed group, but did not differentiate the rapid increasing group from the other two trajectories. Further, when controlling for baseline BDI-s, the single sleep question was no longer predictive at all, unlike the PSQI.
Discussion
Patterns of change in depressive symptoms formed three meaningful distinct subgroups of patients treated with IFN-α. PSQI strongly influenced membership into these subgroups, where worse sleep was associated with a less favorable depression trajectory – in particular, those with the least favorable trajectory. The three groups consisted of (1) a large cluster of subjects with minimal depressive symptoms who remained non-depressed throughout the course of treatment (non-depressed), (2) a second cluster of subjects who started off with slightly higher BDI values that steadily increased to a mild depression symptom load (slow increasers), and (3) a clearly distinct group (11% of the subjects) that started out with an average mild depression load that rapidly increased to severe depression by 8 weeks of treatment (rapid increasers). On average, the rapid increasers also uniquely experienced a further worsening of sleep over time. In all of the rapid increasers, interventions were necessary to address the development of a severe mood disorder. This appears to be the subgroup that is most vulnerable to the depressogenic effect of IFN-α and the one most in need of identification. Comparatively, the slow increasers appear to have a more favorable trajectory, with some demonstrating a slow decline in symptoms. But it is important to note that 79% of the slow increasers reached the point where they were administered a SCID-I. Whether some of these subjects meet a categorical “diagnosis” of depression during treatment depends on the “MDD” definition used. However, they clearly have a different trajectory of response than either the rapid increasers or the resilient non-depressed group. They tended to have mild to moderate depression symptoms -- before, during, and throughout treatment.
The non-depressed group, slow increasers, and rapid increasers all differ in their mean BDI-s at baseline; possibly suggesting that the groups can be identified simply based on the severity of depressive symptoms prior to starting IFN-α therapy. However, when examining individual subject trajectories it appears that it is not quite that simple, since individual baseline depression symptom load overlaps between all three groups. When adjusting for baseline depressive symptoms, the association between PSQI and the three trajectory groups was attenuated, but still significant, suggesting that PSQI adds additional information that is independent of baseline depression symptom load.
These results may potentially be useful to hepatology clinics, where patient’s sleep quality prior to starting treatment could necessitate a pharmacological or behavioral intervention to prevent IFN-MDD. If depression can be avoided in a patient who requires IFN-α, they could have a higher chance of being cured of HCV. Identifying the non-increasers is equally important, as this group might benefit from IFN-α therapy but have minimal psychiatric side effects. Unfortunately, using just the single sleep question from the BDI was not as capable as the PSQI in predicting trajectory. But using both baseline sleep (PSQI) and depression symptoms (BDI-s) can be informative.
The three groups were comparable in age and sex; therefore, these covariates did not influence group membership. In the final model reported, there were 4 non-whites in each of the three trajectories. This is a limitation of our study, since there are a larger proportion of non-whites in the rapid increasers than the other groups, but due to the small cell count we were unable to test whether this difference meant that race statistically influenced group membership. Another limitation of the study is that some other sociodemographic variables, such as education, were not measured.
These results might generalize to other populations. A variety of studies in other clinical contexts have found a similar percentage of highly vulnerable people. Over a 16 year period, about 11% of subjects not depressed at baseline developed an increasing trajectory of depression symptoms over time (67). Likewise, most elderly have low stable depression scores, similar to our findings (52, 53, 68), but about 10% have minimal depression at baseline that increases over time (53). Similarly, 8% of elderly have mild depressive symptoms that subsequently increase over time (52). The vast majority of people don’t develop depression after a bombing attack, but about 8% do have a rapid trajectory and develop major depression within a month (69). Also, after a cancer diagnosis, about 12% newly develop increased depression by 6 and 12 months after diagnosis (15). Most pregnant mothers likewise do not develop depression, but about 10% have sub threshold symptoms during pregnancy and subsequently develop depression post-partum (70). Likewise, in mothers of children newly diagnosed with epilepsy, only a similar minority develop depression trajectories (71). Similarly, only an equally small subset of previously un-depressed grandmothers developed depression after birth of the grandchild to their adolescent daughters (72). Again similar to our observations, three trajectories have been described in teens: low stable, high stable, and increasing (73), with only a limited percentage having increasing depression trajectories (74). Thus, across a large variety of potential depression precipitants, only about 8–12% of people are vulnerable, regardless of what triggered the depression. This estimated range might be slightly broader (75–77), but regardless, there appears to be a uniquely distinct subpopulation, identifiable with trajectory analyses, who may be ideally targeted for prevention interventions. Unfortunately, there has been limited study into what predicts trajectory of depression development. Herein, we report that poor sleep quality can predict rapid depression development. Because IFN-MDD has phenomenological resemblance to MDD diagnosis in other situations (78–81), findings in this clinical population with HCV may be applicable to other types of depression. Moreover, insomnia may be a modifiable risk factor. It is often co-morbid with medical conditions (82, 83), and can precede the future development of MDD in other clinical contexts (84–92).
Large epidemiologic studies of incidence have identified risk markers such as gender, age, cohort, family history, marital status, socioeconomic status, and stressful life events (14, 93). Whether these markers identify subpopulations with unique trajectories is less known. Previously, most other studies that examined trajectory membership focused on pre-existing depression (94, 95). As examples, an unremitting an chronic course is associated with cortisol differences (96), poor sleep (97), avoidant coping style (98), socioeconomic position (99), and childhood abuse and neglect (100). For example, childhood physical neglect predicted chronic courses of depression (101) after depression had developed in those with a breast cancer diagnosis.
In summary, we report that there appears to be a uniquely distinct subgroup of people (about 10%) who are extremely vulnerable to IFN-MDD, and who quickly develop increased depression symptoms. Inclusion in this group is strongly influenced by pre-existing poor sleep quality. Prevention of depression may rely on addressing this. Important for ongoing research on depression risk, we’ve further validated group-based trajectory analysis as a unique approach for empirically identifying vulnerable cohorts.
Acknowledgments
Source of Funding: This research is supported by NIH grants R01 MH090250 (FEL) and P30 MH090333 (CFR).
Glossary
- HCV
Hepatitis C
- IFN-α
interferon-α
- IFN-MDD
interferon-induced depression
- MDD
major depressive disorder
- PEG-IFN-α2
pegylated interferon-alpha2
- SCID-I
Structural Clinical Interview for DSM-IV Axis I Disorders
- BDI
Beck Depression Inventory
- BDI-s
Beck Depression Inventory minus the sleep item
- PSQI
Pittsburgh Sleep Quality Index
- BIC
Bayesian Information Criterion
- 2loge(B10)
Bayesian Information Criterion log Bayes factor approximation
Footnotes
Conflicts of Interest
The authors have no conflicts of interest to note.
Contributor Information
Megan M. Marron, Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
Stewart J. Anderson, Department of Biostatistics, University of Pittsburgh Graduate School of Public Health, Pittsburgh, PA, USA
Jessica Garrity, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Charles F. Reynolds, III, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
Francis E. Lotrich, Department of Psychiatry, Western Psychiatric Institute and Clinic, University of Pittsburgh School of Medicine, Pittsburgh, PA, USA
References
- 1.Insel TR, Charney DS. Research on Major Depression Strategies and Priorities. JAMA. 2003;289:3167–8. doi: 10.1001/jama.289.23.3167. [DOI] [PubMed] [Google Scholar]
- 2.Lopez AD, Murray CC. The global burden of disease, 1990–2020. Nature Medicine. 1998;4:1241–3. doi: 10.1038/3218. [DOI] [PubMed] [Google Scholar]
- 3.Panzarino PJJ. The costs of depression: direct and indirect; treatment versus nontreatment. Journal of Clinical Psychiatry. 1998;59:1–141. [PubMed] [Google Scholar]
- 4.Coyne JC, Fechner-Bates S, Schwenk TL. Prevalence, nature, and comorbidity of depressive disorders in primary care. General Hospital Psychiatry. 1994;16:267–76. doi: 10.1016/0163-8343(94)90006-x. [DOI] [PubMed] [Google Scholar]
- 5.Lawrence D, Hancock KJ, Kisely S. The gap in life expectancy from preventable physical illness in psychiatric patients in Western Australia: retrospective analysis of population based registers. British Medical Journal. 2013;346:f2539. doi: 10.1136/bmj.f2539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kim J. Depression as a psychosocial consequence of occupational injury in the US working population: findings from the medical expenditure panel survey. BMC Public Health. 2013;13:303. doi: 10.1186/1471-2458-13-303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lucas M, Mirzaei F, O’Reilly EJ, Pan A, Willett WC, Kawachi I, Koenen K, Ascherio A. Dietary intake of n-3 and n-6 fatty acids and the risk of clinical depression in women: a 10-y prospective follow-up study. American Journal of Clinical Nutrition. 2011;93:1337–43. doi: 10.3945/ajcn.111.011817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sanchez-Villegas A, Henríquez P, Figueiras A, Ortuño F, Lahortiga F, Martínez-González MA. Long chain omega-3 fatty acids intake, fish consumption and mental disorders in the SUN cohort study. European Journal of Nutrition. 2007;46:337–46. doi: 10.1007/s00394-007-0671-x. [DOI] [PubMed] [Google Scholar]
- 9.Herique A, Kahn J-P. Guidelines and reality in practical use of and compliance to antidepressants in the treatment of depression: incidence survey in Lorraine and Champagne-Ardenne. Encephale. 2009;35:73–9. doi: 10.1016/j.encep.2007.12.010. [DOI] [PubMed] [Google Scholar]
- 10.Patten SB, Lee RC. Refining estimates of major depression incidence and episode duration in Canada using a Monte Carlo Markov model. Medical Decision Making. 2004;24:351–8. doi: 10.1177/0272989X04267008. [DOI] [PubMed] [Google Scholar]
- 11.Kruijt A-W, Antypa N, Booij L, de Jong PJ, Glashouwer K, Penninx BWJH, Van der Does W. Cognitive reactivity, implicit associations, and the incidence of depression: a two-year prospective study. PLoS ONE. 2013;8:e70245. doi: 10.1371/journal.pone.0070245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Patten SB. Long-term medical conditions and major depression in a Canadian population study at waves 1 and 2. Journal of Affective Disorders. 2001;63:35–41. doi: 10.1016/s0165-0327(00)00186-5. [DOI] [PubMed] [Google Scholar]
- 13.Blazer DG. Mood disorders: Epidemiology. In: Sadock BJ, Sadock VA, editors. Comprehensive Textbook of Psychiatry. 7. Philadelphia: Lippincott Williams & Williams; 2000. pp. 1298–307. [Google Scholar]
- 14.Eaton WW, Kramer M, Anthony JC, Dryman A, Shapiro S, Locke BZ. The incidence of specific DIS/DSM-III mental disorders: Data from the NIMH Epidemiologic Catchment Area Program. Acta Psychiatrica Scandanavia. 1989;79:163. doi: 10.1111/j.1600-0447.1989.tb08584.x. [DOI] [PubMed] [Google Scholar]
- 15.Boyes AW, Girgis A, D’Este CA, Zucca AC, Lecathelinais C, Carey ML. Prevalence and predictors of the short-term trajectory of anxiety and depression in the first year after a cancer diagnosis: a population-based longitudinal study. Journal of Clinical Oncology. 2013;31:2724–9. doi: 10.1200/JCO.2012.44.7540. [DOI] [PubMed] [Google Scholar]
- 16.Green KM, Fothergill KE, Robertson JA, Zebrak KA, Banda DR, Ensminger ME. Early life predictors of adult depression in a community cohort of urban African Americans. Journal of Urban Health. 2013;90:101–15. doi: 10.1007/s11524-012-9707-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gonzalez HM, Tarraf W, Whitfield KE, Vega WA. The epidemiology of major depression and ethnicity in the United States. Journal of Psychiatric Research. 2010;44:1043–451. doi: 10.1016/j.jpsychires.2010.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patten SB. Recall bias and major depression lifetime prevalence. Social Psychiatry & Psychiatric Epidemiology. 2003;38:290–6. doi: 10.1007/s00127-003-0649-9. [DOI] [PubMed] [Google Scholar]
- 19.Gimeno D, Kivimaki M, Brunner EJ, Elovainio M, De Vogli R, Steptoe A, Kumari M, Lowe GDO, Rumley A, Marmot MG, Ferrie JE. Associations of C-reactive protein and interleukin-6 with cognitive symptoms of depression: 12-year follow-up of the Whitehall II study. Psychological Medicine. 2009;39:413–23. doi: 10.1017/S0033291708003723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Raison CL, Miller AH. Is depression an inflammatory disorder? Current Psychiatry Reports. 2011;13:467–75. doi: 10.1007/s11920-011-0232-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Miller AH, Maletic V, Raison CL. Inflammation and its discontents: the role of cytokines in the pathophysiology of major depression. Biological Psychiatry. 2009;65:732–41. doi: 10.1016/j.biopsych.2008.11.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Slopen N, Kubzansky LD, McLaughlin KA, Koenen KC. Childhood adversity and inflammatory processes in youth: a prospective study. Psychoneuroendocrinology. 2013;38:188–200. doi: 10.1016/j.psyneuen.2012.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Zuiden M, Heijnen CJ, van de Schoot R, Amarouchi K, Maas M, Vermetten E, Geuze E, Kavelaars A. Cytokine production by leukocytes of military personnel with depressive symptoms after deployment to a combat-zone: a prospective, longitudinal study. PLoS ONE. 2011;6:e29142. doi: 10.1371/journal.pone.0029142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kaufman HL, Kirkwood JM, Hodi F, Agarwala S, Amatruda T, Bines SD, Clark J, Curti B, Ernstoff MS, et al. The Society for Immunotherapy of Cancer consensus statement on tumour immunotherapy for the treatment of cutaneous melanoma. Nature Reviews Clinical Oncology. 2013;10:588–98. doi: 10.1038/nrclinonc.2013.153. [DOI] [PubMed] [Google Scholar]
- 25.Mocellin S, Lens MB, Pasquali S, Pilati P, Chiarion Sileni V. Interferon alpha for the adjuvant treatment of cutaneous melanoma. Cochrane Database of Systematic Reviews. 2013;6:CD008955. doi: 10.1002/14651858.CD008955.pub2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Tarhini AA, Gogas H, Kirkwood JM. IFN-alpha in the treatment of melanoma. Journal of Immunology. 2012;189:3789–93. doi: 10.4049/jimmunol.1290060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kent S, Bluthé R-M, Kelley KW, Dantzer R. Sickness behavior as a new target for drug development. Trends in pharmacological sciences. 1992;13:24–8. doi: 10.1016/0165-6147(92)90012-u. [DOI] [PubMed] [Google Scholar]
- 28.Charlton BG. The malaise theory of depression: major depressive disorder is sickness behavior and antidepressants are analgesic. Medical hypotheses. 2000;54:126–30. doi: 10.1054/mehy.1999.0986. [DOI] [PubMed] [Google Scholar]
- 29.Haroon E, Raison CL, Miller AH. Psychoneuroimmunology meets neuropsychopharmacology: Translational implications of the impact of inflammation on behavior. Neuropsychopharmacology. 2012;37:137–62. doi: 10.1038/npp.2011.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lotrich FE. Inflammatory cytokines, growth factors, and depression. Current Pharmaceutical Design. 2012;18:5920–35. doi: 10.2174/138161212803523680. [DOI] [PubMed] [Google Scholar]
- 31.Lotrich FE, Ferrell RE, Rabinovitz M, Pollock BG. Risk for depression during interferon-alpha treatment is affected by the serotonin transporter polymorphism. Biological Psychiatry. 2009;65:344–8. doi: 10.1016/j.biopsych.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lotrich FE, Rabinovitz F, Gironda P, Pollock BG. Depression following pegylated interferon-alpha: characteristics and vulnerability. Journal of Psychosomatic Research. 2007 doi: 10.1016/j.jpsychores.2007.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Udina M, Castellvi P, Moreno-Espana J, et al. Interferon-induced depression in chronic hepatitis C: a systematic review and meta-analysis. Journal of Clinical Psychiatry. 2012;73:1128–38. doi: 10.4088/JCP.12r07694. [DOI] [PubMed] [Google Scholar]
- 34.Franzen PL, Buysse DJ, Rabinovitz M, Pollock BG, Lotrich FE. Poor sleep quality predicts onset of either major depression or subsyndromal depression with irritability during interferon-alpha treatment. Journal of Psychiatric Research. 2009;177:240–5. doi: 10.1016/j.psychres.2009.02.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Reichenberg A, Gorman JM, Dieterich DT. Interferon-induced depression and cognitive impairment in hepatitis C virus patients: a 72 week prospective study. AIDS. 2005;19:S174–S8. doi: 10.1097/01.aids.0000192087.64432.ae. [DOI] [PubMed] [Google Scholar]
- 36.Dieperink E, Ho SB, Thuras P, Willenbring ML. A prospective study of neuropsychiatric symptoms associated with interferon-a-2b and ribavirin therapy for patients with chronic hepatitis C. Psychosomatics. 2003;44:104–12. doi: 10.1176/appi.psy.44.2.104. [DOI] [PubMed] [Google Scholar]
- 37.Hauser P, Khosla J, Aurora H, Laurin J, Kling MA, Hill J, Gulati M, Thornton AJ, Schultz RL, Valentine AD, Meyers CA, Howell CD. A prospective study of the incidence and open-label treatment of interferon-induced major depressive disorder in patients with hepatitis C. Molecular Psychiatry. 2002;7:942–7. doi: 10.1038/sj.mp.4001119. [DOI] [PubMed] [Google Scholar]
- 38.Castera L, Constant A, Henry C, Champbeniot P, Bernard PH, de Ledinghen V, Demotes-Mainard J, Couzigou P. Impact on adherence and sustained viological response of psychiatric side effects during peginterferon and ribavirin therapy for chronic hepatitis C. Alimentary Pharmacology and Therapeutics. 2006;24:1223–30. doi: 10.1111/j.1365-2036.2006.03107.x. [DOI] [PubMed] [Google Scholar]
- 39.Schaefer M, Schmidt F, Horn M, Schmid-Wendtner M-H, Volkenandt M. Depression during treatment with interferon alpha. Psychosomatics. 2004;45:176. doi: 10.1176/appi.psy.45.2.176. [DOI] [PubMed] [Google Scholar]
- 40.Janssen H, Brouwer J, van der Mast R, Schalm S. Suicide associated with alfa-interferon therapy for chronic viral hepatitis. Journal of Hepatology. 1994;21:241–3. doi: 10.1016/s0168-8278(05)80402-7. [DOI] [PubMed] [Google Scholar]
- 41.Zdilar D, Franco-Bronson K, Buchler N, Locala JA, Younossi ZM. Hepatitis C, interferon alfa, and depression. Hepatology. 2000;31:1207–11. doi: 10.1053/jhep.2000.7880. [DOI] [PubMed] [Google Scholar]
- 42.Castera L, Constant A, Henry C, Couzigou P. Psychiatric disorders during treatment of chronic hepatitis C. Gastroenterology and Clinical Biology. 2005;29:123–33. doi: 10.1016/s0399-8320(05)80714-6. [DOI] [PubMed] [Google Scholar]
- 43.Dan AA, Martin LM, Crone C, Ong JP, Farmer DW, Wise T, Robbins SC, Younossi ZM. Depression, anemia and health-related qulity of life in chronic hepatitis C. Journal of Hepatology. 2006;44:491–8. doi: 10.1016/j.jhep.2005.11.046. [DOI] [PubMed] [Google Scholar]
- 44.Younossi Z, Kallman J, Kincaid J. The effects of HCV infection and management on health-related quality of life. Hepatology. 2007;45:806–16. doi: 10.1002/hep.21565. [DOI] [PubMed] [Google Scholar]
- 45.Ilyas JA, Vierling JM. An overview of emerging therapies for the treatment of chronic hepatitis C. Medical Clinics of North America. 2014;98:17–38. doi: 10.1016/j.mcna.2013.10.011. [DOI] [PubMed] [Google Scholar]
- 46.Development JRa. FDA Advisory Committee Recommends Approval of Simeprevir for Combination Treatment of Genotype 1 Chronic Hepatitis C in Adult Patients. Oct 24, 2013. Press Release. [Google Scholar]
- 47.Sciences G. FDA Advisory Committee Supports Approval of Gilead’s Sofosbuvir for Chronic Hepatitis C Infection. Oct 25, 2013. Press Release. [Google Scholar]
- 48.Muir AJ. The Rapid Evolution of Treatment Strategies for Hepatitis C. American Journal of Gastroenterology. 2014 doi: 10.1038/ajg.2014.66. advance online. [DOI] [PubMed] [Google Scholar]
- 49.Manns MP, Pockros PJ, Norkrans G, Smith CI, Morgan TR, Haussinger D, Shiffman ML, Hadziyannis SJ, Schmidt WN, Jacobson IM, Barcena R, Schiff ER, Shaikh OS, Bacon B, Marcellin P, Deng W, Esteban-Mur R, Poynard T, Pedicone LD, Brass CA, Albrecht JK, Gordon SC. Long-term clearance of hepatitis C virus following interferon alpha-2b or peginterferon alpha-2b, alone or in combination with ribavirin. Journal of Viral Hepatitis. 2013;20:524–9. doi: 10.1111/jvh.12074. [DOI] [PubMed] [Google Scholar]
- 50.Hsu HC. Group-based trajectories of depressive symptoms and the predictors in the older population. International Journal of Geriatric Psychiatry. 2012;27:854–62. doi: 10.1002/gps.2796. [DOI] [PubMed] [Google Scholar]
- 51.Andreescu C, Chang CC, Mulsant BH, Ganguli M. Twelve-year depressive symptom trajectories and their predictors in a community sample of older adults. International Psychogeriatrics. 2008;20:221–36. doi: 10.1017/S1041610207006667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liang J, Xu X, Quinones AR, Bennett JM, Ye W. Multiple trajectories of depressive symptoms in middle and late life: racial/ethnic variations. Psychology & Aging. 2011;26:761–77. doi: 10.1037/a0023945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kuchibhatla MN, Fillenbaum GG, Hybels CF, Blazer DG. Trajectory classes of depressive symptoms in a community sample of older adults. Acta Psychiatrica Scandinavica. 2012;125:492–501. doi: 10.1111/j.1600-0447.2011.01801.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Prather A, Rabinvitz M, Pollock B, Lotrich F. Cytokine-induced depression during IFN-a treatment: the role of IL-6 and sleep quality. Brain, Behavior, & Immunity. 2009;23:1109–16. doi: 10.1016/j.bbi.2009.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.First MB, Spitzer RL, Williams JBW, et al. Structured Clinical Interview for DSM-IV (SCID-1) (Users Guide and Interview) Research Version. New York: Biometrics Research Department, New York Psychiatric Institute; 1995. [Google Scholar]
- 56.Miller MD, Paradis CF, Houck PR, Mazumdar S, Stack JA, Rifai AH, Mulsant B, Reynolds CFI. Rating chronic medical illness burden in geropsychiatric practice and research: Application of the Cumulative Illness Rating Scale. Psychiatry Research. 1992;41:237–48. doi: 10.1016/0165-1781(92)90005-n. [DOI] [PubMed] [Google Scholar]
- 57.Beck A, Steer R, Garbin M. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clinical Psychology Review. 1988;8:77–100. [Google Scholar]
- 58.Beck A, Steer R, Brown G. Manual for the Beck Depression Inventory-II. Vol. 1996 San Antonio, TX: Psychological Corporation; 1996. [Google Scholar]
- 59.Buysse DJ, Reynolds CFr, Monk TH, et al. Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatric Research. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 60.Hall M, Buysse DJ, Nowell PD, Nofzinger EA, Houck P, Reynolds CFr, Kupfer DJ. Symptoms of stress and depression as correlates of sleep in primary insomnia. Psychosomatic Medicine. 2000;62:227–30. doi: 10.1097/00006842-200003000-00014. [DOI] [PubMed] [Google Scholar]
- 61.Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test–retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. Journal of Psychosomatic Research. 2002;53:737–40. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- 62.Roeder K, Lynch KG, Nagin DS. Modeling Uncertainty in Latent Class Membership: A Case Study in Criminology. Journal of American Statistical Association. 1999;447:766–76. [Google Scholar]
- 63.Jones B, Nagin D, Roeder K. A SAS procedure based on mixture model for estimating developmental trajectories. Sociological Methods and Research. 2001;29:374–93. [Google Scholar]
- 64.Schwarz G. Estimating the Dimension of a Model. The Annals of Statistics. 1978;6:461–4. [Google Scholar]
- 65.Nagin D. Group-based modeling of development. Cambridge, Mass: Harvard University Press; 2005. [Google Scholar]
- 66.Niyonkuru C, Wagner AK, Ozawa H, Amin K, Goyal A, Fabio A. Group-based trajectory analysis applications for prognostic biomarker model development in severe TBI: a practical example. Journal of Neurotrauma. 2013;30:938–45. doi: 10.1089/neu.2012.2578. [DOI] [PubMed] [Google Scholar]
- 67.Lincoln KD, Takeuchi DT. Variation in the trajectories of depressive symptoms: results from the Americans’ Changing Lives Study. Biodemography & Social Biology. 2010;56:24–41. doi: 10.1080/19485561003709180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Li X, Wang W, Gao Q, Wu L, Luo Y, Tang Z, Guo X. The trajectories and correlation between physical limitation and depression in elderly residents of Beijing, 1992–2009. PLoS ONE. 2012;7:e42999. doi: 10.1371/journal.pone.0042999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Iguero JM, Cano-Vindel A, Iruarrizaga I, Fernandez-Berrocal P, Galea S. Trajectory and predictors of depression in a 12-month prospective study after the Madrid March 11 terrorist attacks. Journal of Psychiatric Research. 2011;45:1395–403. doi: 10.1016/j.jpsychires.2011.05.012. [DOI] [PubMed] [Google Scholar]
- 70.Christensen AL, Stuart EA, Perry DF, Le H-N. Unintended pregnancy and perinatal depression trajectories in low-income, high-risk Hispanic immigrants. Prevention Science. 2011;12:289–99. doi: 10.1007/s11121-011-0213-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ferro MA, Speechley KN. Stability of latent classes in group-based trajectory modeling of depressive symptoms in mothers of children with epilepsy: an internal validation study using a bootstrapping procedure. Social Psychiatry & Psychiatric Epidemiology. 2013;48:1077–86. doi: 10.1007/s00127-012-0622-6. [DOI] [PubMed] [Google Scholar]
- 72.Updegraff KA, Perez-Brena NJ, Umana-Taylor AJ, Jahromi LB, Harvey-Mendoza EC. Mothers’ trajectories of depressive symptoms across Mexican-origin adolescent daughters’ transition to parenthood. Journal of Family Psychology. 2013;27:376–86. doi: 10.1037/a0032909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Sabiston CM, O’Loughlin E, Brunet J, Chaiton M, Low NC, Barnett T, O’Loughlin J. Linking depression symptom trajectories in adolescence to physical activity and team sports participation in young adults. Preventive Medicine. 2013;56:95–8. doi: 10.1016/j.ypmed.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 74.Reinke WM, Eddy JM, Dishion TJ, Reid JB. Joint trajectories of symptoms of disruptive behavior problems and depressive symptoms during early adolescence and adjustment problems during emerging adulthood. Journal of Abnormal Child Psychology. 2012;40:1123–36. doi: 10.1007/s10802-012-9630-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Doyle F, McGee H, Delaney M, Motterlini N, Conroy R. Depressive vulnerabilities predict depression status and trajectories of depression over 1 year in persons with acute coronary syndrome. General Hospital Psychiatry. 2011;33:224–31. doi: 10.1016/j.genhosppsych.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 76.Kaptein KI, de Jonge P, van den Brink RH, Korf J. Course of depressive symptoms after myocardial infarction and cardiac prognosis: a latent class analysis. Psychosomatic Medicine. 2006;68:662–8. doi: 10.1097/01.psy.0000233237.79085.57. [DOI] [PubMed] [Google Scholar]
- 77.Johansson P, Lesman-Leegte I, Lundgren J, Hillege HL, Hoes A, Sanderman R, van Veldhuisen DJ, Jaarsma T. Time-course of depressive symptoms in patients with heart failure. Journal of Psychosomatic Research. 2013;74:238–43. doi: 10.1016/j.jpsychores.2012.09.019. [DOI] [PubMed] [Google Scholar]
- 78.Capuron L, Hauser P, Hinze-Selch D, Miller AH, Neveu PJ. Treatment of cytokine-induced depression. Brain Behavior and Immunology. 2002;16:575–80. doi: 10.1016/s0889-1591(02)00007-7. [DOI] [PubMed] [Google Scholar]
- 79.Donnelly S. Patient management strategies for interferon alfa-2b as adjuvant therapy of high-risk melanoma. Oncological Nursing Forum. 1998;25:921–7. [PubMed] [Google Scholar]
- 80.Malaguarnera M, Di Fazio I, Restuccia S, Pistone G, Ferlito L, Rampello L. Interferon alpha-induced depression in chronic hepatitis C patients: Comparison between different types of interferon alpha. Neuropsychobiology. 1998;37:93–7. doi: 10.1159/000026485. [DOI] [PubMed] [Google Scholar]
- 81.Trask PC, Esper P, Riba M, Redman B. Psychiatric side effects of interferon therapy: prevalence, proposed mechanisms, and future directions. Journal of Clinical Oncology. 2000;18:2316–26. doi: 10.1200/JCO.2000.18.11.2316. [DOI] [PubMed] [Google Scholar]
- 82.Taylor DJ, Mallory LJ, Lichstein KL, Durrence HH, Riedel BW, Bush AJ. Comorbidity of chronic insomnia with medical problems. Sleep. 2007;30:213–8. doi: 10.1093/sleep/30.2.213. [DOI] [PubMed] [Google Scholar]
- 83.Hayashino Y, Yamazaki S, Takegami M, Nakayama T, Sokejima S, Fukuhara S. Association between number of comorbid conditions, depression, and sleep quality using the Pittsburgh Sleep Quality Index: results from a population-based survey. Sleep Medicine. 2010;11:366–71. doi: 10.1016/j.sleep.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 84.Ford DE, Kamerow DB. Epidemiologic study of sleep disturbances and psychiatric disorders. An opportunity for prevention? Jama. 1989;262:1479–84. doi: 10.1001/jama.262.11.1479. [DOI] [PubMed] [Google Scholar]
- 85.Breslau N, Roth T, Rosenthal L, Andreski P. Sleep disturbance and psychiatric disorders: a longitudinal epidemiological study of young adults. Biol Psychiatry. 1996;39:411–8. doi: 10.1016/0006-3223(95)00188-3. [DOI] [PubMed] [Google Scholar]
- 86.Riemann D, Voderholzer U. Primary insomnia: a risk factor to develop depression? J Affect Disord. 2003;76:255–9. doi: 10.1016/s0165-0327(02)00072-1. [DOI] [PubMed] [Google Scholar]
- 87.Chang PP, Ford DE, Mead LA, Cooper-Patrick L, Klag MJ. Insomnia in young men and subsequent depression. The Johns Hopkins Precursors Study. Am J Epidemiol. 1997;146:105–14. doi: 10.1093/oxfordjournals.aje.a009241. [DOI] [PubMed] [Google Scholar]
- 88.Gregory AM, O’Connor TG. Sleep problems in childhood: a longitudinal study of developmental change and association with behavioral problems. Journal of the American Academy of Child & Adolescent Psychiatry. 2002;41:964–71. doi: 10.1097/00004583-200208000-00015. [DOI] [PubMed] [Google Scholar]
- 89.Buysse DJ, Angst J, Gamma A, Ajdacic V, Eich D, Rossler W. Prevalence, course, and comorbidity of insomnia and depression in young adults. Sleep. 2008;31:473–80. doi: 10.1093/sleep/31.4.473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Roberts RE, Roberts CR, Chen IG. Impact of insomnia on future functioning of adolescents. Journal of Psychosomatic Research. 2002;53:561–9. doi: 10.1016/s0022-3999(02)00446-4. [DOI] [PubMed] [Google Scholar]
- 91.Roane BM, Taylor DJ. Adolescent insomnia as a risk factor for early adult depression and substance abuse. Sleep. 2008;31:1351–6. [PMC free article] [PubMed] [Google Scholar]
- 92.Gregory AM, ORijsdijk FV, FLJY, et al. The direction of longitudinal associations between sleep problems and depression symptoms: a study of twins aged 8 and 10 years. Sleep. 2009;32:189–99. doi: 10.1093/sleep/32.2.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Hagnell O, Lanke J, Rorsman B, Ojesjo L. Are we entering an age of melancholy? Depressive illnesses in a prospective study epidemiological study over 25 years: The Lundby Study. Psychological Medicine. 1982:12. doi: 10.1017/s0033291700046614. [DOI] [PubMed] [Google Scholar]
- 94.Rhebergen D, Lamers F, Spijker J, de Graaf R, Beekman ATF, Penninx BWJH. Course trajectories of unipolar depressive disorders identified by latent class growth analysis. Psychological Medicine. 2012;42:1383–96. doi: 10.1017/S0033291711002509. [DOI] [PubMed] [Google Scholar]
- 95.Clapp JD, Grubaugh AL, Allen JG, Mahoney J, Oldham JM, Fowler JC, Ellis T, Elhai JD, Frueh BC. Modeling trajectory of depressive symptoms among psychiatric inpatients: a latent growth curve approach. Journal of Clinical Psychiatry. 2013;74:492–9. doi: 10.4088/JCP.12m07842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Vreeburg SA, Hoogendijk WJG, DeRijk RH, van Dyck R, Smit JH, Zitman FG, Penninx BWJH. Salivary cortisol levels and the 2-year course of depressive and anxiety disorders. Psychoneuroendocrinology. 2013;38:1494–502. doi: 10.1016/j.psyneuen.2012.12.017. [DOI] [PubMed] [Google Scholar]
- 97.van Mill JG, Vogelzangs N, van Someren EJW, Hoogendijk WJG, Penninx BWJH. Sleep duration, but not insomnia, predicts the 2-year course of depressive and anxiety disorders. Journal of Clinical Psychiatry. 2014;75:119–26. doi: 10.4088/JCP.12m08047. [DOI] [PubMed] [Google Scholar]
- 98.Cronkite RC, Woodhead EL, Finlay A, Timko C, Unger Hu K, Moos RH. Life stressors and resources and the 23-year course of depression. Journal of Affective Disorders. 2013;150:370–7. doi: 10.1016/j.jad.2013.04.022. [DOI] [PubMed] [Google Scholar]
- 99.Melchior M, Chastang J-F, Head J, Goldberg M, Zins M, Nabi H, Younes N. Socioeconomic position predicts long-term depression trajectory: a 13-year follow-up of the GAZEL cohort study. Molecular Psychiatry. 2013;18:112–21. doi: 10.1038/mp.2011.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Hovens JGFM, Giltay EJ, Wiersma JE, Spinhoven P, Penninx BWJH, Zitman FG. Impact of childhood life events and trauma on the course of depressive and anxiety disorders. Acta Psychiatrica Scandinavica. 2012;126:198–207. doi: 10.1111/j.1600-0447.2011.01828.x. [DOI] [PubMed] [Google Scholar]
- 101.Witek Janusek L, Tell D, Albuquerque K, Mathews HL. Childhood adversity increases vulnerability for behavioral symptoms and immune dysregulation in women with breast cancer. Brain, Behavior, & Immunity. 2013;30(Suppl):S149–S62. doi: 10.1016/j.bbi.2012.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]