Abstract
Systemic sclerosis (SSc) vasculopathy can result in a digital ulcer (DU) and/or pulmonary arterial hypertension (PAH). We hypothesized that bedside brachial artery flow-mediated dilation (FMD) testing with duplex ultrasound could be used in SSc patients to identify features of patients at risk for DU or PAH. Thirty-eight SSc patients were compared to 52 age-matched healthy controls from the VAMC Utah Vascular Research Laboratory. Peripheral hemodynamics, arterial structure, and endothelial function were assessed by duplex ultrasound. A blood pressure cuff was applied to the forearm and 5-min ischemia was induced. Post-occlusion, brachial artery vascular reactivity (peak hyperemia/area under the curve [AUC]), shear rate, and endothelial function (FMD) were measured. SSc patients had smaller brachial artery diameters (p<0.001) and less reactive hyperemia (p<0.001), peak shear rate (p= 0.03), and brachial artery FMD (p<0.001) compared with healthy controls. Brachial artery FMD was lower (p<0.05) in SSc patients with DU. Tertile analysis suggested the 2 lower FMD tertiles (<5.40 %) had a 40–50 % chance of presenting with DU while the SSc patients with highest FMD tertile (>5.40 %) had less than 15 % chance of DU. All brachial artery FMD measurements were similar between SSc patients with and without PAH (all p>0.05). Compared to healthy controls, SSc patients had significantly smaller brachial artery diameter and blunted peripheral vascular reactivity and endothelial function. SSc patients with DU have even greater impairments in endothelial function compared to those without DU. FMD testing has clinical utility to identify SSc patients at risk for DU.
Keywords: Flow-mediated dilation, Imaging, Rheumatic diseases, Scleroderma, Specialty fields, Systemic sclerosis, Ultrasound, Vasculopathy
Introduction
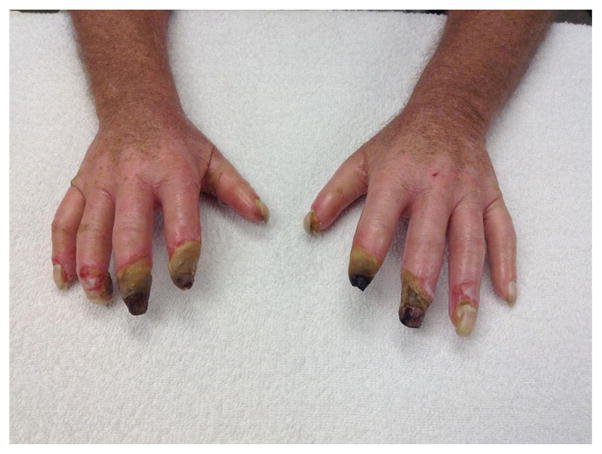
Systemic sclerosis (SSc; scleroderma) is a multi-organ system disease characterized by activation of immune cells, production of auto-antibodies, vasculopathy, and fibrosis. Although it is heterogeneous in extent of organ involvement and prognosis, it is accepted that SSc has a progressive and most often a devastating course [1]. Perhaps the most common symptom in SSc is Raynaud’s phenomenon (RP) which represents a perturbation of digital resistance artery vascular reactivity likely due to alterations in the balance between vasoconstrictor and vasodilator signaling. In its most severe form, RP can result in digital ulceration (DU) and gangrene (Fig. 1).
Fig. 1.
Raynaud’s phenomenon complicated by digital ulceration (DU) and gangrene
In SSc, RP-related impairment of tissue perfusion leads to tissue hypoxia, endothelial cell (EC) damage and dysfunction, and the promotion of vascular leak, immune activation, and fibrosis which are important for dictating the pace of vasculopathy [2, 3]. Importantly, EC damage with apoptosis coupled to insufficient compensatory repair results in the pathognomonic end-stage vascular abnormalities [4]. Over time, this abnormal perpetual perfusion results in both functional and structural disease. Unfortunately, little is known about the mechanisms that initiate RP and EC dysfunction in SSc.
A complex interaction between EC, immune cells, smooth muscle cells, extracellular matrix, and intravascular circulating factors likely contribute to the vascular reactivity, remodeling, and occlusive disease of scleroderma [5]. EC dysfunction and other hemodynamic characteristics can be assessed noninvasively in humans using the flow-mediated dilation (FMD) technique [6, 7]. This approach involves inflating a cuff on a limb (typically the upper forearm) to a supra-systolic external pressure for several minutes and measuring change in diameter and blood flow in a segment of an artery (typically the brachial artery) proximal to the occlusion following rapid deflation of the cuff [6–8]. The ischemia-evoked dilation of resistance vessels distal to the occlusion produces a marked temporary increase in blood flow (reactive hyperemia, RH) in the proximal conduit arteries that can be quantified and, in turn, causes dilation (FMD) of those proximal conduit arteries. Thus, this procedure not only assesses the ability of peripheral conduit arteries to dilate in response to the physiological stimulus of increases in intravascular shear, but also the vasodilatory ability of the peripheral resistance arteries to a brief bout of ischemia [9, 10].
Currently, noninvasive vascular measurements are not used for diagnosis or monitoring of disease activity in SSc, despite strong evidence suggesting that vasculopathy, including endothelial dysfunction and abnormal vascular reactivity, is a critical mechanism in the progression of SSc. Progressive vasculopathy results in end-stage vascular manifestations including pulmonary arterial hypertension (PAH), DU, and scleroderma renal crisis (SRC), all of which are serious causes of morbidity and mortality in this patient population [11, 12]. Thus, the objective of the current study was to utilize FMD testing in the clinical setting to evaluate the phenotype of vasculopathy in SSc at different stages of disease duration, and determine whether FMD testing has utility in predicting end-stage clinical features. Specifically, we hypothesized that bedside FMD testing with duplex ultrasound could be used in SSc patients to identify features of patients at risk for DU or PAH.
Methods
Thirty-eight patients with SSc were recruited from the University of Utah SSc Clinic. Five of those patients had repeat testing performed at a 4-month follow-up visit. Patients had either a diagnosis of SSc as accepted by the American College of Rheumatology or early SSc as described by Leroy and Medsger [13, 14]. Clinical features of the SSc patients were recorded and displayed in Table 1. Patients with SSc were divided into two groups based on the presence or absence of end-stage vasculopathy manifestations, defined by active DU, PAH, and SRC. Fifty-three age- and sex-matched healthy controls (HC) were recruited from the general population by the Utah Vascular Research Laboratory (UVRL) located at the Veterans Affairs Medical Center (VAMC) Salt Lake City Geriatric, Research, Education, and Clinical Center. Healthy control subjects did not have any evidence of vascular disease or chronic medical conditions and were not on any medications that would impact vascular function. All procedures were approved by the institutional review board, which serves as the ethics committee, of the University of Utah and Salt Lake City VAMC. Measurements were performed on prepared subjects at the University of Utah Rheumatology Clinic or VAMC UVRL according to current guidelines [6]. The nature, benefits, and risks of the study were explained to the volunteers, and their written informed consent was obtained before participation. Patients were excluded if they had used tobacco, alcohol, and/or caffeine within 12 h of testing. Only two patients were current tobacco users. There were no previous smokers who had quit. Patients that were consented for the study were acclimatized to the clinical room for 20 min. The same operators performed all assessments.
Table 1.
Characteristics of patients with SSc and healthy controls
| Scleroderma | Healthy control | |
|---|---|---|
| n | 38 | 53 |
| Female, n | 33 | 41 |
| Age, years | 56±2 | 52±3 |
| BMI, kg/m2 | 27±1 | 23±1 |
| CVD risk factor, n (%) | 10 (26) | 0 (0) |
| Medication use, n (%) | ||
| CCB | 32 (84) | 0 (0) |
| PDE5I | 9 (24) | 0 (0) |
| ERA | 6 (16) | 0 (0) |
| PCA | 2 (5) | 0 (0) |
| Immunosuppression | 11 (29) | 0 (0) |
| SSc disease duration, years | 9.5±1.5 | – |
| SSc subtype, n (%) | ||
| Early | 5 (13) | – |
| Limited | 33 (87) | – |
| Diffuse | 5 (13) | – |
| mRSS | 7.1±1.1 | – |
| End-stage vasculopathy, n (%) | ||
| DU | 14 (37) | – |
| PAH | 13 (34) | – |
| SRC | 1 (2) | – |
| Antibody presence, n (%) | ||
| ANA | 38 (100) | – |
| SCL70 | 5 (13) | – |
| Anti-centromeric | 7 (18) | – |
| RNA polymerase III | 6 (16) | – |
Data are mean±SE unless otherwise stated
BMI body mass index, CVD cardiovascular disease risk factor (hypertension, diabetes mellitus, hyperlipidemia), CCB calcium channel blocker, PDE5I phosphodiesterase inhibitor, ERA endothelin receptor antagonist, PCA prostacyclin analog, SSc scleroderma, mRSS modified Rodnan skin score, DU digital ulcer, PAH pulmonary arterial hypertension, SRC scleroderma renal crisis, ANA antinuclear antibody, SCL70 anti-Scl-70 antibody
Subject characteristics
Body mass index (BMI) was calculated from body mass and height. For patients with SSc, clinical features were measured/recorded including disease duration from start of both Raynaud’s and first non-Raynaud’s symptom and presence of DU, PAH determined by right heart catheterization, SRC, modified Rodnan skin score (mRSS), antinuclear antibody (ANA) and SSc-antibodies status (anti-centromere, anti-SCL70, and RNA polymerase III).
Brachial artery reactive hyperemia and flow-mediated dilation
Reactive hyperemia (RH) and FMD were assessed in the supine position using a duplex ultrasound (Logiq e, GE Medical Systems, Milwaukee, WI) with a linear array transducer, as previously described [6, 8, 15–20]. Briefly, the ultrasound probe was placed 3–6 cm proximal to the antecubital crease. RH was produced by inflation of a blood pressure cuff placed on the upper forearm distal to the antecubital fossa for 5 min at 250 mmHg followed by rapid deflation. Measurement of brachial artery diameter and mean blood velocity (Vmean) were obtained continuously for 30 s of rest and for 2 min after cuff deflation. FMD responses are expressed as percentage change from baseline and absolute change in diameter, per recent recommendations [6, 21]. Vmean was automatically calculated using commercially available software (Logiq e). Shear rate was calculated as follows: shear rate (s−1)=8Vmean/arterial diameter. Blood flow was calculated as follows: blood flow=Vmeanπ (arterial diameter/2)2×60. For both shear rate and blood flow, cumulative area under the curve values were integrated with the trapezoidal rule and calculated as follows: Σ{yi[x(i+1)−xi]+(1/2)[y(i+1)−yi][x(i+1)−xi]} [16, 18, 19]. RH was quantified as cumulative brachial artery blood flow for 2 min (area under the curve) after cuff occlusion. Shear normalized FMD was calculated by dividing FMD (percentage) by the cumulative shear rate area under the curve until the time of peak brachial artery vasodilation.
Data analysis
Statistical analyses were performed with SPSS (version 20, Armonk, NY). Differences between patients with SSc and healthy control subjects or between clinical groups within SSc (DU, PAH) were assessed by Student’s t-test for continuous variables (one- or two-sided determined a priori) or by chi-squared analyses for categorical variables. Pearson correlation analysis was used to assess bivariate relations between the change in %FMD and RH and the change in variables that could influence FMD. Significance was set at p<0.05, and values are presented as mean±standard error (SE). The association of digital ulcer with FMD was estimated by logistic regression using PROC Logistic in SAS (version 9.3, Cary, NC). Patients with SSc were divided into tertiles based on FMD (%). From this data, the odds ratio associated with FMD tertiles was calculated.
Results
Subject characteristics
Among the patient population, the duration of SSc from first non-Raynaud’s symptom ranged from 1–50 years (mean 18 years) and the mRSS at the time of experimental testing ranged from 0–26 (mean 14). Between patients with SSc and healthy control, there was no difference in age or sex (p>0.05); however, the patients with SSc had a greater BMI than HC (p=0.004). Among patients with SSc, 26 % had CVD risk factors, determined by the presence of treated hypertension, diabetes mellitus, and/or hyperlipidemia. Three of the five SSc patients with hyperlipidemia were on a statin drug. A significant proportion of the SSc group were taking medications with vascular effects, most notably 84 % of patients were taking calcium channel blockers for the treatment of RP. No healthy control subjects had CVD risk factors or were taking medications with vascular action (Table 1).
Baseline brachial artery and hemodynamic characteristics
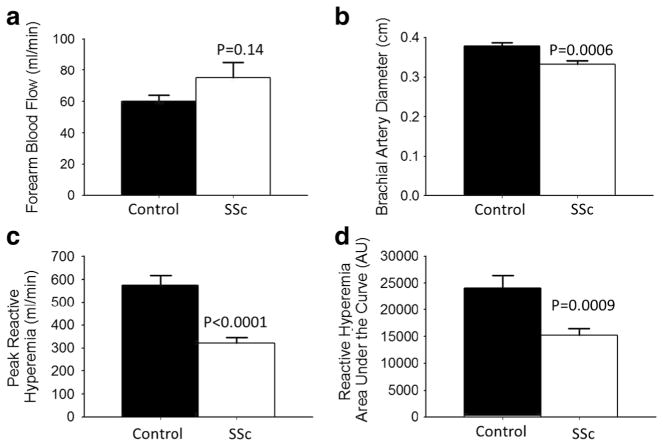
At baseline, SSc patients had similar brachial artery blood flow (p=0.14, Fig. 2a) but a smaller brachial artery diameter compared with healthy control subjects (p=0.004, Fig. 2b). In the SSc patients, a longer duration of RP was related to a smaller brachial diameter (p<0.001); however, a longer duration of SSc (defined as first non-RP symptom) was not related to baseline brachial artery size differences (p>0.05). Group differences in baseline brachial artery diameter were not affected by use of phosphodiesterase-5 inhibitors, endothelin receptor antagonists, and/or prostacyclin analogs (data not shown).
Fig. 2.
Brachial artery diameter and reactive hyperemia are lower, but baseline blood flow is similar in patients with scleroderma (SSc) compared to age-matched control subjects; a baseline forearm blood flow (ml/min) at rest prior to cuff inflation and brachial artery occlusion; b baseline brachial artery size (cm) prior to cuff inflation; c on release of the occlusion, inflow through the brachial artery is transiently increased (peak reactive hyperemia, ml/min); and d reactive hyperemia calculated as the area under the dilation curve (AU) during the vasodilation period in healthy controls and SSc patients. Values are mean ±SEM. p values denote difference from control
Peripheral reactive hyperemia and brachial artery flow-mediated dilation
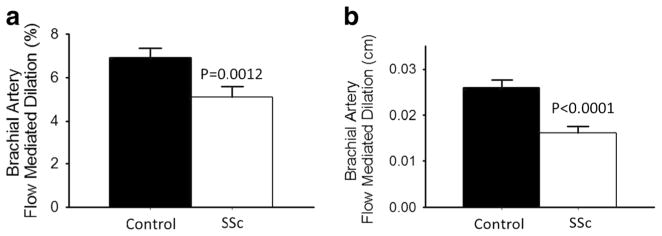
Post 5-min forearm occlusion, SSc patients had a lower peak and AUC reactive hyperemia (both p< 0.001 Fig. 2c, d) as well as peak shear rate (SSc 21,446± 1706 s−1 vs. healthy controls 27,154±1842 s−1, p= 0.03), but not AUC shear rate (SSc 32410±1992 s−1 vs. healthy controls 36,890±2227 s−1, p=0.15), compared with healthy control subjects. Absolute and percent FMD was significantly lower in SSc compared to healthy controls (Fig. 3a, b; both p≤0.001) and this difference persisted after normalizing for brachial artery shear rate (FMD %/peak shear rate: SSc 0.244±0.028 vs. healthy controls 0.320±0.031 AU, p<0.05). These group differences were not significantly influenced by medication use or other vascular risk factors. Neither age, gender, nor BMI was a significant correlate in this analysis.
Fig. 3.
Patients with scleroderma (SSc) have impaired endothelial function compared to age-matched control subjects. Endothelium-dependent dilation (brachial artery FMD); a percentage change (%); b absolute change (cm) in healthy controls and SSc patients. Values are mean±SE. p values denote difference from control
Systemic sclerosis vasculopathy population differences
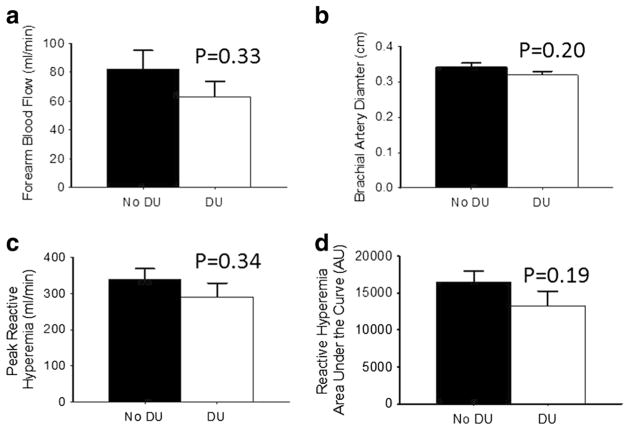
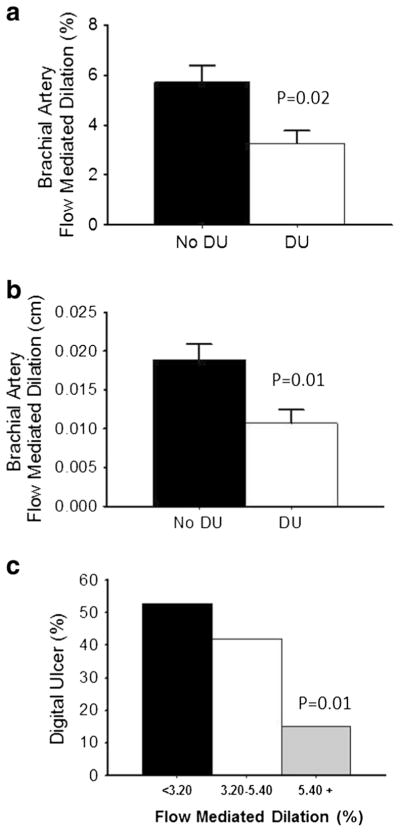
When the SSc population was divided into groups based on the presence and absence of DU, these groups did not differ for age (58±4 vs. 53±3 years, p=0.22), sex (p=0.67), BMI (26±2 vs. 27±2 kg/m2, p=0.51), or Raynaud’s and SSc disease duration (both p>0.14). Forearm blood flow and brachial artery diameter at baseline as well as reactive hyperemia and shear rate response post-occlusion were not different between patients with and without DU (Fig. 3a–d; all p>0.19). However, SSc patients with DU had lower percent and absolute FMD (Fig. 4a, b; both p<0.02) compared to SSc patients without DU. A subsequent analysis of FMD tertiles versus DU prevalence showed that SSc patients with the highest tertile of FMD (greater than 5.40 %) had significantly lower prevalence of DU (13 %) compared to patients with the lowest tertile of FMD (lower than 3.20 and 52 %; Fig. 4c; p=0.02). From these data, the odds ratio associated with FMD tertiles was calculated. The odds ratio indicates that higher FMD is protective from the development of digital ulcers, as those in the lower tertiles were at five times greater risk compared to those in the upper tertile.
Fig. 4.
Within systemic sclerosis (SSc) patients, brachial artery diameter, baseline blood flow, and reactive hyperemia are similar in patients without digital ulcerations (DU) compared to patients with DU; a baseline brachial artery size (cm) prior to cuff inflation; b baseline forearm blood flow (ml/min) prior to cuff inflation; c on release of the occlusion, inflow through the brachial artery is transiently increased (peak reactive hyperemia, ml/min); and d reactive hyperemia calculated as the area under the dilation curve (AU) during the dilation period in SSc patients without DU and with DU. Values are mean±SE. p values denote difference between patients with and without DU
SSc patients with and without PAH were of a similar age (54.9±vs. 53±3 years, p=0.30), sex (p=0.38), and both Raynaud’s and SSc disease duration (both p>0.07), but patients with PAH had a greater BMI than those without (30±2 vs. 25±1 kg/m2, p=0.02). At baseline, there was a trend for larger brachial artery diameter in SSc patients with PAH compared with those without PAH (p=0.07). Baseline blood flow, RH, and FMD were similar between patients with and without PAH (all p>0.22).
Discussion
Given the devastating course of SSc, improved descriptors and predictors of SSc vasculopathy and related clinical outcomes are of vital importance. Therefore, the aim of the current study was to utilize relatively simple, noninvasive FMD testing in the clinical setting to evaluate the phenotype of SSc vasculopathy and determine if it has utility in predicting end-stage clinical features. There are several new findings from this present study. First, an important finding of this comprehensive vascular SSc study suggests that brachial arteries of SSc patients are characterized by reductions in conduit vessel diameter, resistance artery vasoreactivity, and endothelial function compared to age-matched healthy controls. Second, SSc patients with DU demonstrated greater impairments in endothelial function as measured by FMD compared to SSc patients without DU. Additionally, SSc patients with the lowest endothelial function had a greater incidence of DU. These results suggest that the continuum of end-stage vasculopathy may differ in the SSc population and indicate that FMD testing is able to phenotype vasculopathy in this population. Furthermore, these results suggest that this noninvasive measure of endothelial function may be effective in assessing DU risk in patients with SSc.
SSc and vascular structure
While pathological changes of the microvasculature in SSc are one of the earliest and best described features of this disease, to date, there is a paucity of information regarding peripheral conduit arterial remodeling in SSc. In this study, brachial artery diameter was 12 % smaller in SSc patients compared to healthy controls (Fig. 2), despite slightly higher BMI in these individuals compared to healthy control subjects. This degree of brachial artery remodeling is greater than previous work demonstrating a smaller (8 %) and statistically insignificant reduction in brachial artery diameter in SSc patients [17]. The difference between our results is likely due to previous work having smaller number of participants (28 total subjects, patients and controls), thus perhaps being slightly underpowered to identify structural differences. Typically, remodeling of conduit arteries is due to chronic alterations in peripheral blood flow in response to activity [22]. We hypothesize that SSc patients, despite having a tendency for elevated blood flow at rest, would likely have a reduced activity in the hands and forearms which lowers total daily blood flow and therefore reduces brachial artery size. Such a hypothesis deserves more attention in future studies.
SSc and resistance artery vasoreactivity
Reactive hyperemia, an increase in peripheral blood flow due to a brief ischemic bout, has classically been utilized as a marker of peripheral resistance artery vasoreactivity [23] and is predictive of future CVD events [24, 25]. It is dependent of a multitude of vasoactive factors which include, but are not limited to, endothelial-derived nitric oxide and prostaglandins [26–28], tissue-released adenosine, and myogenic tone [29, 30]. Currently, acute vasoreactivity testing is used during right heart catheterization of SSc patients with PAH in order to screen for possible responsiveness to long-term calcium channel blocker therapy, but noninvasive testing is not a component of routine clinical visits.
We observed a severely blunted hyperemic response to ischemia in SSc patients, as demonstrated in both the peak RH response and by the AUC for the hyperemic response (Fig. 2c, d). This indicates that either vasodilation to ischemia is blunted in SSc or there are reductions in arterial network capacity in SSc versus age-matched controls. A prior study has documented a similar attenuation in peak reactive hyperemia in patients with SSc [17], and this present study extends these observations by measuring total hyperemic response, not just peak velocity. Interestingly, although this severely blunted hyperemic response was not exacerbated in SSc patients with clinical complications (DU or PAH) compared to those without, impaired RH appears to be a strong hemodynamic characteristic of patients with SSc even in the presence of targeted smooth muscle vasodilator therapies (phosphodiesterase-5 inhibitors, endothelin antagonists, and prostacyclin analogs).
SSc and endothelial function
The endothelium is a key regulator of vascular homeostasis and tone [31]. Pathophysiologic disruptions in SSc endothelial cells, smooth muscle cell, and fibroblast function may result in various changes in vascular tone favoring vasoconstriction, cellular turnover favoring proliferation, coagulant activity favoring thrombosis and release of auto-antibodies, and pro-inflammatory messengers [32]. Previous studies of endothelial dysfunction in SSc have yielded inconsistent results, with studies finding patients with SSc to have impaired FMD [17, 33–38] or similar FMD compared to healthy controls [39, 40]. These discrepancies likely resulted from underpowered experimental design, technique differences (e.g., failure to adhere to FMD standardized guidelines [20]), differences between patients and control populations (e.g., age, gender, comorbidities), and severity of clinical manifestations of SSc [17, 33–36, 39]. Importantly, past studies did not seek to determine if FMD is related to the vasculopathy clinical features in SSc or comprehensively assess the necessary variables related to brachial artery FMD (hyperemic stimulus and shear rate).
Endothelial function and clinical outcomes DU and PAH
DU is a major cause of morbidity, while PAH is a leading cause of mortality, in SSc [41]. These end-stage manifestations may represent a continuum of damage that results from increased permeability and dysregulated control of vascular tone [4]. Our results suggest that, within the SSc patient population, a measure of endothelial function, i.e., FMD, is diminished in SSc patients with DU compared to patients without DU (Fig. 4). In addition, our analysis suggests that patients with SSc that have the highest measures of endothelial function have the lowest incidence of DU (Fig. 5). In contrast, none of the hemodynamic and structural measures were different between SSc patients with and without PAH. Taken together, these data suggest that FMD testing provides a relatively simple methodology to assess endothelial dysfunction and other measures of vascular function, which may be one of the earliest measurable markers of SSc vasculopathy and therefore could be effective as an additional prognostic test of vascular risk factors in patients with SSc [42].
Fig. 5.
Augmented brachial artery flow-mediated dilation (FMD) is protective against digital ulcers in systemic sclerosis patient; a FMD percentage change (%); b absolute change (cm) in systemic sclerosis (SSc) patients without digital ulcer (DU) and with DU. c Patients with SSc divided into tertiles based on FMD (%), prevalence of DU is lowest in patients in the highest FMD tertile. Values are mean±SE. p values denote difference between patients with and without DU (a, b) or versus lowest tertile of FMD (<3.20, c)
Utility of FMD testing in SSc populations
FMD testing is a noninvasive measurement tool that provides not only an index of endothelial cell function, but also information about hemodynamic and structural properties, including reactivity, of peripheral resistance arteries in patients with SSc. Our data demonstrate that endothelial function in blunted in SSc versus healthy controls, even when accounting for blunted peripheral hyperemia and brachial artery shear stress (Fig. 2). These data support our previous histological evidence of an endothelial abnormality in patients with SSc [3].
Previous studies have demonstrated an improvement of FMD in patients with SSc after estrogen administration [43], PGE1 α-cyclodestrin [44], and bosentan use [45], suggesting the utility of FMD as a biomarker of vascular health [43] and attesting to its use for studying the effects of other SSc-related therapeutics. Furthermore, FMD has been documented to increase in patients with SSc treated with therapeutics targeting the vasculature, as well as with immunomodulators such as cyclophosphamide, suggesting that FMD may help delineate the pathogenesis of SSc vasculopathy.
Treatment of SSc DU and PAH includes smooth muscle vasodilators, encompassing calcium channel blockers, phosphodiesterase-5 inhibitors, endothelin receptor antagonists, and prostacyclin analogs. Despite frequent use of these drugs and the significant costs to insurance and patients, there are no peripheral blood flow measures currently utilized in routine clinical practice to establish the efficacy of these treatment regimens. Thus, a better understanding of the appropriate use of these medications for SSc vasculopathy is needed. The current study suggests that FMD could be used to examine patients with SSc responses to therapeutics, especially those directed to RP and those in development to target the smooth muscle and/or endothelium, to improve the course of treatment for individual patients. Furthermore, this study demonstrates that FMD is helpful for evaluating SSc patients and may allow a better understanding of vasculopathy pathogenesis. Thus, this study provides a more comprehensive assessment of endothelial function and vascular reactivity than previous reports and supports FMD testing in the clinical setting.
Limitations
This current study was not without limitations. As the current measurements were performed during a patient’s visit to the clinic, endothelium-independent dilation induced by nitroglycerin was not assessed. Nevertheless, other studies which have utilized nitroglycerin to assess smooth muscle relaxation have all revealed normal brachial artery dilation in SSc [34, 40]. Endothelial function was assessed only by one method. Other endothelial measures include distal hand warming, passive limb movement, infusion of acetylcholine, and/or use of peripheral artery tonometry (PAT), which may have better characterized RH [31, 46, 47]. These additional assessments will be important in follow-up studies to ensure the impairment in FMD seen with SSc and end-stage vasculopathy are, indeed, a result of endothelial dysfunction and not due to impaired smooth muscle reactivity. In addition, given the clinical environment, while patients were fasted and rested per guidelines, patients were not required to stop medications and the menstrual cycle phase was not controlled for, both of which could have a confounding influence on endothelial function [6]. Two of the subjects were tobacco users and while they abstained 12 h before FMD testing, constituents of cigarette smoke may contribute to endothelial dysfunction and the precise mechanisms by which tobacco leads to this altered vascular reactivity remain unclear, thus a longer period of abstinence may have been necessary [48, 49]. Additionally, we did not assess whether there was secondhand smoke exposure [50]. The heterogeneity of SSc treatment may have influenced the FMD results. There were 11 patients on immunosuppression, but due to the different types and doses of medications, we were not adequately powered to assess the effects of these therapies. Additionally, we did not check markers of inflammation on the day of testing. Furthermore, our healthy controls were leaner than patients with SSc; however, there was no correlation between BMI and FMD within our SSc or HC groups, and thus BMI was unlikely to have confounded data interpretation. Finally, we did not measure anti-endothelial cell antibodies, which have been suggested to have a pathologic role in vasculopathy [4]; such measures could provide important mechanistic insight in future studies [51].
Conclusions
Our results suggest that FMD may be a promising biomarker of SSc vasculopathy longitudinal studies. FMD is a noninvasive, objectively measured parameter that may be used to describe pathogenic processes or pharmacologic response to SSc therapeutics. This current study demonstrates that selected measures determined in FMD testing are significantly abnormal in SSc patients and may predict those SSc patients at risk of developing DU. As such, FMD testing warrants further use and validation in SSc studies of end-stage vasculopathy and with RP therapeutics.
Acknowledgments
Funding Dr. Frech was supported by the National Center for Research Resources and the National Center for Advancing Translational Sciences, National Institutes of Health, through Grant 8UL1TR000105 (formerly UL1RR025764). Dr. Donato is supported NIH Awards: AG045339, AG04029, and AG043952. Dr. Walker is supported by NIH Award AG046326. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
Disclosures None.
Contributor Information
Tracy Frech, Department of Internal Medicine, Division of Rheumatology, University of Utah, Veterans Affair Medical Center, Salt Lake City, UT, USA.
Ashley E. Walker, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA
Zachary Barrett-O’Keefe, Department of Exercise and Sport Science, University of Utah, Salt Lake City, UT, USA.
Paul N. Hopkins, Cardiovascular Genetics Research, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA
Russell S. Richardson, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA. Department of Exercise and Sport Science, University of Utah, Salt Lake City, UT, USA. Geriatric Research, Education, and Clinical Center, Veterans Affairs Medical Center, Salt Lake City, UT, USA
D. Walter Wray, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA. Department of Exercise and Sport Science, University of Utah, Salt Lake City, UT, USA. Geriatric Research, Education, and Clinical Center, Veterans Affairs Medical Center, Salt Lake City, UT, USA.
Anthony J. Donato, Email: tony.donato@utah.edu, Department of Internal Medicine, University of Utah, Salt Lake City, UT, USA, URL: http://www.tvplab.utah.edu. Department of Exercise and Sport Science, University of Utah, Salt Lake City, UT, USA. Geriatric Research, Education, and Clinical Center, Veterans Affairs Medical Center, Salt Lake City, UT, USA
References
- 1.Guiducci S, Distler O, Distler JH, Matucci-Cerinic M. Mechanisms of vascular damage in SSc—implications for vascular treatment strategies. Rheumatology (Oxford) 2008;47(Suppl 5):v18–v20. doi: 10.1093/rheumatology/ken267. [DOI] [PubMed] [Google Scholar]
- 2.Sgonc R, Gruschwitz MS, Dietrich H, Recheis H, Gershwin ME, Wick G. Endothelial cell apoptosis is a primary pathogenetic event underlying skin lesions in avian and human scleroderma. J Clin Invest. 1996;98(3):785–792. doi: 10.1172/JCI118851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frech TM, Revelo MP, Drakos SG, Murtaugh MA, Markewitz BA, Sawitzke AD, et al. Vascular leak is a central feature in the pathogenesis of systemic sclerosis. J Rheumatol. 2012;39(7):1385–1391. doi: 10.3899/jrheum.111380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kahaleh B. The microvascular endothelium in scleroderma. Rheumatology (Oxford) 2008;47(Suppl 5):v14–v15. doi: 10.1093/rheumatology/ken279. [DOI] [PubMed] [Google Scholar]
- 5.Wigley FM. Vascular disease in scleroderma. Clin Rev Allergy Immunol. 2009;36(2–3):150–175. doi: 10.1007/s12016-008-8106-x. [DOI] [PubMed] [Google Scholar]
- 6.Harris RA, Nishiyama SK, Wray DW, Richardson RS. Ultrasound assessment of flow-mediated dilation. Hypertension. 2010;55(5):1075–1085. doi: 10.1161/HYPERTENSIONAHA.110.150821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Celermajer DS, Sorensen KE, Gooch VM, Spiegelhalter DJ, Miller OI, Sullivan ID, et al. Non-invasive detection of endothelial dysfunction in children and adults at risk of atherosclerosis. Lancet. 1992;340(8828):1111–1115. doi: 10.1016/0140-6736(92)93147-f. [DOI] [PubMed] [Google Scholar]
- 8.Donato AJ, Eskurza I, Silver AE, Levy AS, Pierce GL, Gates PE, et al. Direct evidence of endothelial oxidative stress with aging in humans: relation to impaired endothelium-dependent dilation and upregulation of nuclear factor-kappaB. Circ Res. 2007;100(11):1659–1666. doi: 10.1161/01.RES.0000269183.13937.e8. [DOI] [PubMed] [Google Scholar]
- 9.Patterson GC, Whelan RF. Reactive hyperaemia in the human forearm. Clin Sci (Lond) 1955;14(2):197–211. [PubMed] [Google Scholar]
- 10.Blair DA, Glover WE, Roddie IC. The abolition of reactive and post-exercise hyperaemia in the forearm by temporary restriction of arterial inflow. J Physiol. 1959;148:648–658. doi: 10.1113/jphysiol.1959.sp006313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Trojanowska M. Cellular and molecular aspects of vascular dysfunction in systemic sclerosis. Nat Rev Rheumatol. 2010;6(8):453–460. doi: 10.1038/nrrheum.2010.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Strange G, Nash P. The manifestations of vasculopathy in systemic sclerosis and its evidence-based therapy. Int J Rheum Dis. 2009;12(3):192–206. doi: 10.1111/j.1756-185X.2009.01410.x. [DOI] [PubMed] [Google Scholar]
- 13.Preliminary criteria for the classification of systemic sclerosis (scleroderma). Subcommittee for scleroderma criteria of the American Rheumatism Association Diagnostic and Therapeutic Criteria Committee. Arthritis Rheum. 1980;23(5):581–590. doi: 10.1002/art.1780230510. [DOI] [PubMed] [Google Scholar]
- 14.LeRoy EC, Medsger TA., Jr Criteria for the classification of early systemic sclerosis. J Rheumatol. 2001;28(7):1573–1576. [PubMed] [Google Scholar]
- 15.Eskurza I, Monahan KD, Robinson JA, Seals DR. Effect of acute and chronic ascorbic acid on flow-mediated dilatation with sedentary and physically active human ageing. J Physiol. 2004;556(Pt 1):315–324. doi: 10.1113/jphysiol.2003.057042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Harris RA, Nishiyama SK, Wray DW, Tedjasaputra V, Bailey DM, Richardson RS. The effect of oral antioxidants on brachial artery flow-mediated dilation following 5 and 10 min of ischemia. Eur J Appl Physiol. 2009;107(4):445–453. doi: 10.1007/s00421-009-1147-x. [DOI] [PubMed] [Google Scholar]
- 17.Rossi P, Granel B, Marziale D, Le Mee F, Frances Y. Endothelial function and hemodynamics in systemic sclerosis. Clin Physiol Funct Imaging. 2010;30(6):453–459. doi: 10.1111/j.1475-097X.2010.00965.x. [DOI] [PubMed] [Google Scholar]
- 18.Witman MA, Fjeldstad AS, McDaniel J, Ives SJ, Zhao J, Barrett-O’Keefe Z, et al. Vascular function and the role of oxidative stress in heart failure, heart transplant, and beyond. Hypertension. 2012;60(3):659–668. doi: 10.1161/HYPERTENSIONAHA.112.193318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walker AE, Kaplon RE, Lucking SM, Russell-Nowlan MJ, Eckel RH, Seals DR. Fenofibrate improves vascular endothelial function by reducing oxidative stress while increasing endothelial nitric oxide synthase in healthy normolipidemic older adults. Hypertension. 2012;60(6):1517–1523. doi: 10.1161/HYPERTENSIONAHA.112.203661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Harris MF, Bailey L, Snowdon T, Litt J, Smith JW, Joyner B, et al. Developing the guidelines for preventive care - two decades of experience. Aust Fam Physician. 2010;39(1–2):63–65. [PubMed] [Google Scholar]
- 21.Donald AE, Halcox JP, Charakida M, Storry C, Wallace SM, Cole TJ, et al. Methodological approaches to optimize reproducibility and power in clinical studies of flow-mediated dilation. J Am Coll Cardiol. 2008;51(20):1959–1964. doi: 10.1016/j.jacc.2008.02.044. [DOI] [PubMed] [Google Scholar]
- 22.Dinenno FA, Tanaka H, Monahan KD, Clevenger CM, Eskurza I, DeSouza CA, et al. Regular endurance exercise induces expansive arterial remodelling in the trained limbs of healthy men. J Physiol. 2001;534(Pt 1):287–295. doi: 10.1111/j.1469-7793.2001.00287.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joyner MJ, Dietz NM, Shepherd JT. From Belfast to Mayo and beyond: the use and future of plethysmography to study blood flow in human limbs. J Appl Physiol (1985) 2001;91(6):2431–2441. doi: 10.1152/jappl.2001.91.6.2431. [DOI] [PubMed] [Google Scholar]
- 24.Huang AL, Silver AE, Shvenke E, Schopfer DW, Jahangir E, Titas MA, et al. Predictive value of reactive hyperemia for cardiovascular events in patients with peripheral arterial disease undergoing vascular surgery. Arterioscler Thromb Vasc Biol. 2007;27(10):2113–2119. doi: 10.1161/ATVBAHA.107.147322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lieberman EH, Gerhard MD, Uehata A, Selwyn AP, Ganz P, Yeung AC, et al. Flow-induced vasodilation of the human brachial artery is impaired in patients <40 years of age with coronary artery disease. Am J Cardiol. 1996;78(11):1210–1214. doi: 10.1016/s0002-9149(96)00597-8. [DOI] [PubMed] [Google Scholar]
- 26.Endo T, Imaizumi T, Tagawa T, Shiramoto M, Ando S, Takeshita A. Role of nitric oxide in exercise-induced vasodilation of the forearm. Circulation. 1994;90(6):2886–2890. doi: 10.1161/01.cir.90.6.2886. [DOI] [PubMed] [Google Scholar]
- 27.Takahashi T, Asano Y, Amiya E, Hatano M, Tamaki Z, Ozeki A, et al. Improvement of endothelial function in parallel with the amelioration of dry cough and dyspnea due to interstitial pneumonia by intravenous cyclophosphamide pulse therapy in patients with systemic sclerosis: a preliminary report of two cases. Mod Rheumatol. 2012;22(4):598–601. doi: 10.1007/s10165-011-0545-1. [DOI] [PubMed] [Google Scholar]
- 28.Engelke KA, Halliwill JR, Proctor DN, Dietz NM, Joyner MJ. Contribution of nitric oxide and prostaglandins to reactive hyperemia in human forearm. J Appl Physiol (1985) 1996;81(4):1807–1814. doi: 10.1152/jappl.1996.81.4.1807. [DOI] [PubMed] [Google Scholar]
- 29.Kilbom A, Wennmalm A. Endogenous prostaglandins as local regulators of blood flow in man: effect of indomethacin on reactive and functional hyperaemia. J Physiol. 1976;257(1):109–121. doi: 10.1113/jphysiol.1976.sp011358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Carlsson I, Sollevi A, Wennmalm A. The role of myogenic relaxation, adenosine and prostaglandins in human forearm reactive hyperaemia. J Physiol. 1987;389:147–161. doi: 10.1113/jphysiol.1987.sp016651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Poredos P, Jezovnik MK. Testing endothelial function and its clinical relevance. J Atheroscler Thromb. 2013;20(1):1–8. doi: 10.5551/jat.14340. [DOI] [PubMed] [Google Scholar]
- 32.Hatton N, Frech T, Smith B, Sawitzke A, Scholand MB, Markewitz B. Transforming growth factor signalling: a common pathway in pulmonary arterial hypertension and systemic sclerosis. Int J Clin Pract Suppl. 2011;172:35–43. doi: 10.1111/j.1742-1241.2011.02726.x. [DOI] [PubMed] [Google Scholar]
- 33.Lekakis JP, Papamichael CM, Vemmos CN, Voutsas AA, Stamatelopoulos SF, Moulopoulos SD. Peripheral vascular endothelial dysfunction in patients with angina pectoris and normal coronary arteriograms. J Am Coll Cardiol. 1998;31(3):541–546. doi: 10.1016/s0735-1097(97)00542-1. [DOI] [PubMed] [Google Scholar]
- 34.Cypiene A, Laucevicius A, Venalis A, Dadoniene J, Ryliskyte L, Petrulioniene Z, et al. The impact of systemic sclerosis on arterial wall stiffness parameters and endothelial function. Clin Rheumatol. 2008;27(12):1517–1522. doi: 10.1007/s10067-008-0958-1. [DOI] [PubMed] [Google Scholar]
- 35.Soltesz P, Der H, Kerekes G, Szodoray P, Szucs G, Danko K, et al. A comparative study of arterial stiffness, flow-mediated vasodilation of the brachial artery, and the thickness of the carotid artery intima-media in patients with systemic autoimmune diseases. Clin Rheumatol. 2009;28(6):655–662. doi: 10.1007/s10067-009-1118-y. [DOI] [PubMed] [Google Scholar]
- 36.Bots ML, Westerink J, Rabelink TJ, de Koning EJ. Assessment of flow-mediated vasodilatation (FMD) of the brachial artery: effects of technical aspects of the FMD measurement on the FMD response. Eur Heart J. 2005;26(4):363–368. doi: 10.1093/eurheartj/ehi017. [DOI] [PubMed] [Google Scholar]
- 37.D’Andrea A, Stisi S, Caso P, Uccio FS, Bellissimo S, Salerno G, et al. Associations between left ventricular myocardial involvement and endothelial dysfunction in systemic sclerosis: non-invasive assessment in asymptomatic patients. Echocardiography. 2007;24(6):587–597. doi: 10.1111/j.1540-8175.2007.00436.x. [DOI] [PubMed] [Google Scholar]
- 38.Rollando D, Bezante GP, Sulli A, Balbi M, Panico N, Pizzorni C, et al. Brachial artery endothelial-dependent flow-mediated dilation identifies early-stage endothelial dysfunction in systemic sclerosis and correlates with nailfold microvascular impairment. J Rheumatol. 2010;37(6):1168–1173. doi: 10.3899/jrheum.091116. [DOI] [PubMed] [Google Scholar]
- 39.Andersen GN, Mincheva-Nilsson L, Kazzam E, Nyberg G, Klintland N, Petersson AS, et al. Assessment of vascular function in systemic sclerosis: indications of the development of nitrate tolerance as a result of enhanced endothelial nitric oxide production. Arthritis Rheum. 2002;46(5):1324–1332. doi: 10.1002/art.10191. [DOI] [PubMed] [Google Scholar]
- 40.Roustit M, Simmons GH, Baguet JP, Carpentier P, Cracowski JL. Discrepancy between simultaneous digital skin microvascular and brachial artery macrovascular post-occlusive hyperemia in systemic sclerosis. J Rheumatol. 2008;35(8):1576–1583. [PMC free article] [PubMed] [Google Scholar]
- 41.Mathai SC, Hassoun PM. Pulmonary arterial hypertension in connective tissue diseases. Heart Fail Clin. 2012;8(3):413–425. doi: 10.1016/j.hfc.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Inaba Y, Chen JA, Bergmann SR. Prediction of future cardiovascular outcomes by flow-mediated vasodilatation of brachial artery: a meta-analysis. Int J Cardiovasc Imaging. 2010;26(6):631–640. doi: 10.1007/s10554-010-9616-1. [DOI] [PubMed] [Google Scholar]
- 43.Lekakis J, Mavrikakis M, Papamichael C, Papazoglou S, Economou O, Scotiniotis I, et al. Short-term estrogen administration improves abnormal endothelial function in women with systemic sclerosis and Raynaud’s phenomenon. Am Heart J. 1998;136(5):905–912. doi: 10.1016/s0002-8703(98)70137-1. [DOI] [PubMed] [Google Scholar]
- 44.Giannattasio C, Pozzi M, Gardinali M, Montemerlo E, Citterio F, Maestroni S, et al. Effects of prostaglandin E1alpha cyclodextrin [corrected] treatment on endothelial dysfunction in patients with systemic sclerosis. J Hypertens. 2007;25(4):793–797. doi: 10.1097/HJH.0b013e328032784f. [DOI] [PubMed] [Google Scholar]
- 45.Sfikakis PP, Papamichael C, Stamatelopoulos KS, Tousoulis D, Fragiadaki KG, Katsichti P, et al. Improvement of vascular endothelial function using the oral endothelin receptor antagonist bosentan in patients with systemic sclerosis. Arthritis Rheum. 2007;56(6):1985–1993. doi: 10.1002/art.22634. [DOI] [PubMed] [Google Scholar]
- 46.Mullen MJ, Kharbanda RK, Cross J, Donald AE, Taylor M, Vallance P, et al. Heterogenous nature of flow-mediated dilatation in human conduit arteries in vivo: relevance to endothelial dysfunction in hypercholesterolemia. Circ Res. 2001;88(2):145–151. doi: 10.1161/01.res.88.2.145. [DOI] [PubMed] [Google Scholar]
- 47.Trinity JD, Groot HJ, Layec G, Rossman MJ, Ives SJ, Runnels S, et al. Nitric oxide and passive limb movement: a new approach to assess vascular function. J Physiol. 2012;590(Pt 6):1413–1425. doi: 10.1113/jphysiol.2011.224741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Neunteufl T, Heher S, Kostner K, Mitulovic G, Lehr S, Khoschsorur G, et al. Contribution of nicotine to acute endothelial dysfunction in long-term smokers. J Am Coll Cardiol. 2002;39(2):251–256. doi: 10.1016/s0735-1097(01)01732-6. [DOI] [PubMed] [Google Scholar]
- 49.Frey PF, Ganz P, Hsue PY, Benowitz NL, Glantz SA, Balmes JR, et al. The exposure-dependent effects of aged secondhand smoke on endothelial function. J Am Coll Cardiol. 2012;59(21):1908–1913. doi: 10.1016/j.jacc.2012.02.025. [DOI] [PubMed] [Google Scholar]
- 50.Pinnamaneni K, Sievers RE, Sharma R, Selchau AM, Gutierrez G, Nordsieck EJ, et al. Brief exposure to secondhand smoke reversibly impairs endothelial vasodilatory function. Nicotine Tob Res. 2014;16(5):584–590. doi: 10.1093/ntr/ntt189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Castro SV, Jimenez SA. Biomarkers in systemic sclerosis. Biomark Med. 2010;4(1):133–147. doi: 10.2217/bmm.09.79. [DOI] [PMC free article] [PubMed] [Google Scholar]