Abstract
Whole genome and whole exome sequencing technologies are being increasingly used in research. However, they have the potential to identify incidental findings (IF), findings not related to the indication of the test, raising questions regarding researchers' responsibilities toward the return of this information to participants. In this study we discuss the ethical considerations related to the return of IF to research participants, emphasizing that the type of the study matters and describing the current practice standards. There are currently no legal obligations for researchers to return IF to participants, but some viewpoints consider that researchers might have an ethical one to return IF of clinical validity and clinical utility and that are actionable. The reality is that most IF are complex to interpret, especially since they were not the indication of the test. The clinical utility often depends on the participants' preferences, which can be challenging to conciliate and relies on participants' understanding. In summary, in the context of a lack of clear guidance, researchers need to have a clear plan for the disclosure or nondisclosure of IF from genomic research, balancing their research goals and resources with the participants' rights and their duty not to harm.
Keywords: incidental findings, genetic research, return of results
Introduction
Landmark advances in science are often accompanied by ethical challenges. During the past decade, new methods for massively parallel sequencing have been developed, computational approaches have advanced, and there has been an increased availability of large public sequencing datasets. As a result, whole genome and whole exome sequencing (WGS/WES) technologies have emerged as useful tools in both research and clinical molecular diagnostics. WGS/WES facilitates the sequencing of large regions of the genome with a timely turnaround, and they are increasingly affordable.1 The potential uses for WGS/WES in medical genomics research are rapidly expanding. In the past few years, the technology has allowed the discovery of new genes and new mechanisms, unraveling the genetic cause of single gene and complex disorders where conventional sequencing methods have failed in the past.2–5 This is an exciting time for ophthalmic genomic research as these techniques are now becoming increasingly used. However, because WGS/WES are less targeted than conventional genetic testing, they generate a vast amount of genomic data well beyond what has been generated by traditional targeted genetic approaches, including the potential for incidental findings (IF). As a result, complex ethical questions arise and challenge the researchers' responsibilities regarding disclosure of these data to research participants. Although not the topic of this paper, the same ethical issues apply to genome-wide association studies, which also have the potential to identify IF.6 In this paper, we discuss the elements to consider when debating the return of genomic IF generated from WGS/WES in a research setting.
Findings from WGS/WES can be broadly classified in two categories: (1) the pertinent or primary findings that are results relevant to the indication for which the test was ordered and (2) unsolicited, secondary or incidental findings that are results that are not related to the primary indication of the test and may or may not be relevant to the patient's health (for example a variant related to cancer identified through the conduct of a study on the genetic causes of congenital glaucoma). There is presently a great deal of controversy over how IF should be handled in research: which IF, if any, should be returned to participants and how they should be returned.7–13 This debate has been described by Wolf et al.14 as a problem of translational research, when findings from research have some potential clinical utility and impact on clinical management.
The arguments in favor and against the return of IF to research participants are outlined in Table 1.9,13,15–18 When evaluating whether IF should be returned to participants, researchers need to consider the type of the study, the practice standards and ethical approvals in place, the analytical and clinical relevance of the findings, and the participant's preferences in relation to return of results with specific reference to the research consent documents.
Table 1.
Ethical Principles in Favor or Against the Disclosure of Incidental Findings to Research Participants

Research Versus Clinical Settings
The context in which the return of individual genomic research results is discussed does matter.19,20 The distinction between research and clinical care is important because the underlying key principles are different. The goal in research is to generate data for a communal benefit, whereas in clinical care the individual patient's needs and benefits prevail. As a result, the rights and duties of the individuals implicated are different.21–23 However, the boundary between clinical and research settings can be blurred, especially when the research participants are patients and when the researcher could also be their clinician, making it harder to distinguish the responsibilities of each person.16,22 This is a complex area, and the distinctions are often poorly understood by patients and health care workers generally. Even within the research context, there are nuances, depending on the circumstances, including the type of WGS/WES performed, and the social context in which they take place.19 Researchers' obligations toward participants are defined by the consent form and the protocol approved by institutional review boards (IRBs) or their equivalents, the overriding duty to protect participants from harm, and the respect of privacy and confidentiality.24 It is suggested that rather than a one-size fits all, a case-by-case (or disease-by-disease) approach is required regarding factors such as degree of vulnerability of the study cohort, depth of researcher/participant relationship, and degree of participant dependence.20
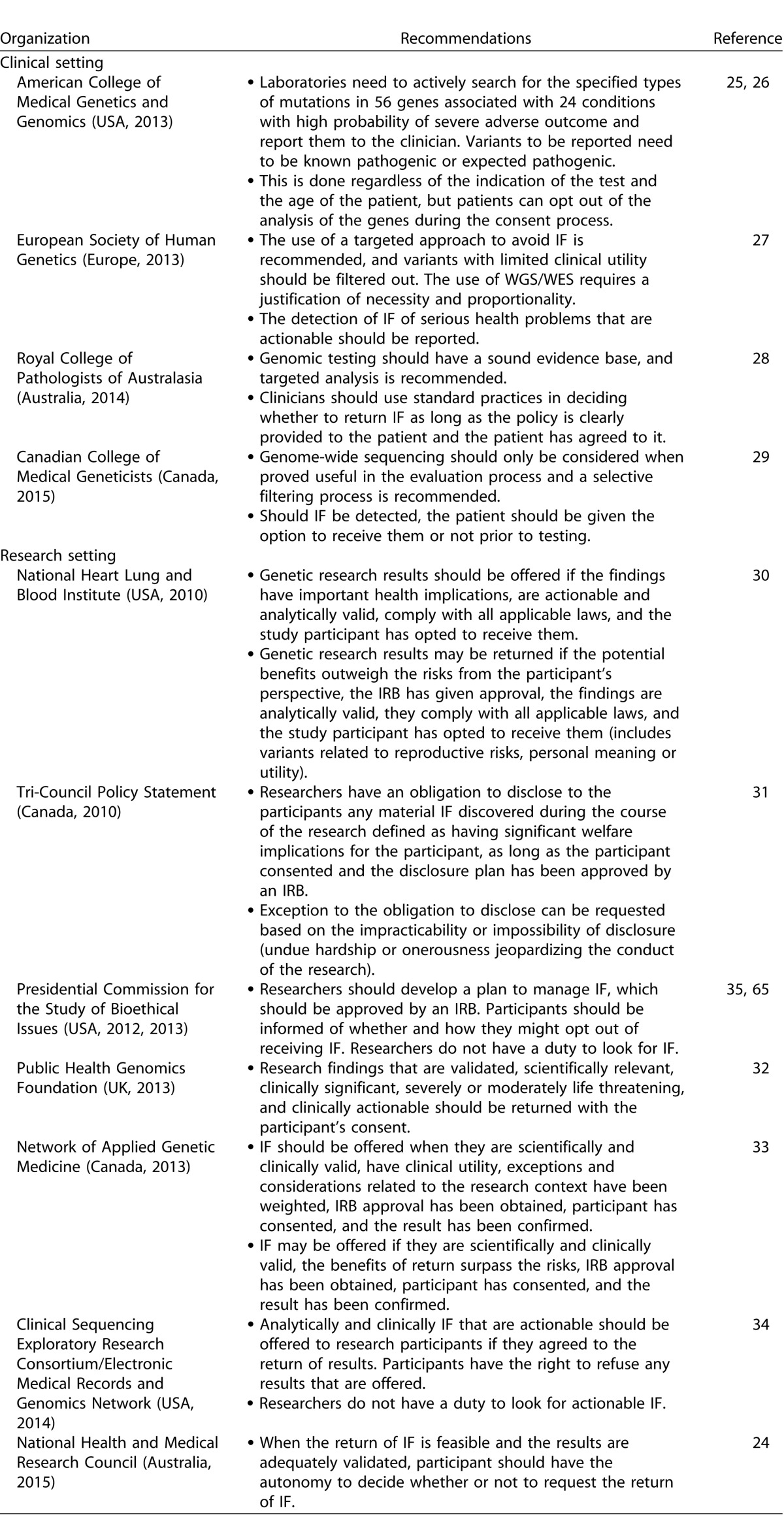
Existing Recommendations
Several recommendations have been published regarding the return of IF in both clinical and research settings (Table 2). In the clinical setting, on one side of the spectrum, the American College of Medical Genetics and Genomics (ACMG) published a statement advocating for opportunistic screening and recommended that variants from a list of 56 genes associated with 24 disorders with high penetrance and clinical actionability be actively looked for and returned, regardless of the age of the patient.25,26 In the wake of vocal criticism of its position, the ACMG revised its recommendations to allow patients to opt out of the analysis of medically actionable genes when undergoing WGS/WES.26 On the other end of the spectrum, the European Society of Human Genetics, the Canadian College of Medical Geneticists, and the Royal College of Pathologists of Australasia encouraged caution and recommended a targeted approach to the clinical question to avoid the detection of IF.27–29 In the research setting, the guidelines vary from defining which IF should or may be returned30–34 to recommendations that do not advocate for or refrain from looking for IF, but frames how IF should be returned if feasible.24,35,36
Table 2.
Published Guidelines for the Report of Genomic Results in a Clinical and a Research Setting
Return of Incidental Genetic Findings in the Context of Eye Diseases
Although still disputed, there is a viewpoint that even if researchers have no legal obligation, they could have an ethical obligation to return genomic variants that are of clinical validity (the variant is known to be associated with a particular disease), have clinical utility (the likelihood of a positive health outcome), and are actionable (medical actions can be taken to decrease the risk).8,13,30,34,37,38 As an example, clinical validity would be low for genetic variants associated with macular degeneration because of the weak correlation between specific genotypes and visual outcome,39 but would it be higher for disease-causing variants in the MYOC gene associated with glaucoma and high penetrance.40 Similarly, retinitis pigmentosa (RP) disease-causing variants would currently be of limited clinical utility due to the lack of available treatments. This may change with the advent of gene therapy for retinal dystrophies. Predictive genetic testing for RP family members is a controversial topic.41 There is some evidence that taking high doses of vitamin A supplements may slow the progression of RP.42 A patient may be symptomatic of RP, having nyctalopia, but not be diagnosed with RP. Genetic testing would then alert them to their symptoms to justify further diagnostic testing with visual fields, an electroretinogram, and dark adaptation. Finally, diagnosis of RP in young adults helps ensures safety with driving and allows reproductive choices before they have children. Genetic variants known to cause retinoblastoma or choroidal melanoma would have a stronger clinical utility based on their actionability and the importance of early diagnosis. Information related to reproductive or personal utility has received much less consensus for disclosure. Overall, the consensus reached refers to situations where the potential benefits outweigh the potential harm for the participant and the findings reach a relevant threshold of validity and medical significance.43
Despite the endorsement of clinical validity, clinical utility, and actionability for the return of IF, the definition of each criterion has been relatively inconsistent and is based on a range of different interpretations.43 The reality is that many IF are actually of unknown or dubious significance and therefore not interpretable. Additionally, the meaning of a pathogenic variant can differ between different family members. Across the world it is generally accepted that children should not be tested for adult-onset conditions unless there is an immediate medical benefit. When research involving children discovers results related to adult predisposition conditions that can be clinically relevant to the parents well before it will have a clinical impact on the child, the question has been raised whether these results should be disclosed to the parents.44 Finally, an area that has received little discussion is the lack of empirical evidence regarding the clinical utility of most IF in routine testing. Most data on disease-causing variants have been collected using cohorts of affected individuals, which can result in an overestimation of the penetrance and expressivity45 and limit the extrapolation to low-risk populations. The sensitivity and specificity of any genetic test is only as strong as the indication for the test. Along the same lines, the US Preventive Services Task Force has recommended against routine genetic testing for BRCA-related cancer.46 Overall, researchers need to think about how the information can be used for patients' better health and the potential to do more clinical harm than good.
Prevalence of IF
Undoubtedly, WGS/WES will discover clinically actionable variants in research participants. The ACMG statement anticipated medically relevant IF in 1% of sequencing reports.25 Based on a mathematical model and using the ACMG list, Ding et al.47 predicted IF in 2.7% of screened participants. Two recent studies reported pathogenic variants from the ACMG list among 0.9% to 1.7% of individuals,48,49 while others have reported prevalence of up to 12% for variants of various clinical utility.50–53 The difference between the studies can be attributed to the cohort selection, the pathogenicity classification criteria of variants, and the inclusion of conditions and genes based on the definition of clinical utility. When including variants associated with carrier status of newborn diseases, risk factors for macular degeneration, and drug response, Tabor et al.54 demonstrated that every exome would contain variants of potential clinical utility. Furthermore, the prevalence of clinically actionable findings is expected to increase in the future with the improved accuracy of variant annotation of genomic databases, better understanding of the genetics of diseases, and development of therapies.
Practical Considerations in the Return of Research Results
Additional factors for the potential return of IF to research participants must be considered. Most research laboratories are not accredited to report findings that could be used in clinical management. The analytical validity of genetic variants identified through WGS/WES in a research setting is not reliable or robust enough to be reported. Validation in an accredited laboratory and assessment for clinical validity and significance by competent and accredited professionals has been strongly advocated for disclosure.24,30,32–34 Researchers often have a lack of expertise for results or conditions that are outside the scope of their research. As a result, posttest counseling and medical follow-up needs to be provided by trained professionals. Many have argued that the requirements for the return of IF take substantial time, effort, and resources that would put an unsustainable burden on the research enterprise and move resources away from the primary research.15,16,55,56 Substantial resources are required for each of these steps, and current research funding is typically not allocated to conduct this activity. One study suggested a framework by which the clinical setting would take care of those steps, ensuring the distinction between research and clinical care remains.57 However, this option would move the burden to the clinical setting, which would equally struggle to sustain this workload.
Participants' Perspectives and Understanding
All guidelines recognize that the participants' preferences need to be taken into account. Whenever possible, participants should be informed of the possibility of return of IF and the potential risks and benefits, and they should be able to opt out of its return.8,24,30,33,34,58 Participants' familial, cultural, and religious beliefs also need to be acknowledged. Different models of consent59,60 and dynamic return of results61,62 have been proposed to address the complexity of the return of IF. To give informed consent for every eventuality is impossible, and studies have shown that categorizing the results potentially returned facilitates the process.38,63
Respecting participants' preferences can also pose some challenges. In some situations, further investigations of the participant and his or her family, necessitating recontact, can be required to ascertain the pathogenicity of a variant, making it difficult to respect an individual's wishes to learn only about clinically significant variants.64 Historically, IF were not always addressed properly in consent forms, which creates issues for disclosure. Published guidelines have discussed whether the absence of reference to IF disclosure in the consent form would prevent their return and to what extent researchers can respect participants' wishes of not knowing IF of clinical significance.24,30,34,65 Consultation with IRBs has been advised in these situations.
Most studies evaluating the intention to receive results among research participants66–71 or the general public in hypothetical scenarios18,67,72–74 have shown that the majority wish to receive results, regardless of the clinical validity and utility. However, previous studies have often shown that patients who expressed interest in obtaining results do not always get tested, and even though the uptake of genetic testing is higher for conditions with preventive measures, it is still lower than expected based on intentions.75–77 Moreover, individuals make different choices depending on what is at stake and on the framing of the options, emphasizing the difficulty of explaining the complexity and uncertainty of research findings.78 The issues surrounding IF are complex and take time to explain and process. Tabor et al. evaluated a protocol for obtaining informed consent for WGS in two families that was nine pages long and took 2 to 3 hours.79 Although both families complained about the length of time and the complexity of the process, they both recognized the extent of the scope of information that needed to be covered in order for them to make informed decisions regarding the return of IF. Few studies have reported what patients really understood of the actual impact of reporting or evaluated their experience of receiving IF and the potential psychological harm.80 More empirical data are needed on the actual benefits or harm of receiving IF and the true understanding of participants in regard to IF.
Researchers' Perspectives
Genetic professionals and researchers are generally supportive of the disclosure of actionable IF but are usually less so with results pertaining to untreatable conditions, adult-onset conditions for pediatric participants, or variants with lower clinical validity and utility.56,74,81–84 Surveys among researchers showed that although the majority are in favor of returning highly penetrant, clinically actionable results, they also feel that it would be a burden on researchers.74,82
Integrating the opinions of both stakeholders and participants is vital in developing an effective plan for the return of IF, but the discrepancies between what results researchers and participants believe should be disclosed might pose a challenge in balancing the integrity of participant autonomy with researcher's decisions. Increasingly, particularly in light of the growing discourse supporting disclosure, there is need to ensure that participants' expectations are carefully managed during the informed consent process and through clear information in the information sheet and consent form as to what, if any, results will or may be returned.
Incidental Genetic Findings: A Duty to Find and Recontact?
If there is a duty for researchers to report IF, some have questioned whether there could also be a duty to actively look for IF since researchers have access to the genomic data. Studies so far have concluded that researchers do not have an obligation to look for IF.34,56,65 The rationale is that it would blur the distinction between research and clinical care, create clinical responsibilities for researchers, and accentuate therapeutic misconception—the notion that research will benefit individuals.22 Similarly, Gliwa et al.55 concluded that at present, although there could be benefits for participants, and researchers are in a unique position to access these data, the burden on the research is too extensive for researchers to actively look for IF. However, they argued that in the future, if the analysis process becomes more efficient and if WGS/WES are not yet implemented as a standard of care in clinical care, researchers could face an obligation to look for IF.
Similarly, knowledge about disease associations will evolve over time, and variants are likely to be interpreted differently.85 This raises the issue of a potential duty to recontact research participants in the light of new information. The question of recontact could also apply to IF related to adult-onset conditions identified in children. Most guidelines recommend that researchers do not have to return IF beyond the termination of research funding.30,34 Indeed, even in the clinical setting it is recognized that there must be limits on the duty to recontact in the context of WGS/WES given the vast amount of data potentially available.23 The preferable approach is to explain to patients the fast-moving nature of this area and put the onus on them to recontact in the future if they want to find out if any new information has come to light.
The Importance of Implementing a Disclosure Plan
In the context of a lack of clear policies, researchers need to implement a plan for managing genomic data.30,31,33,34,65 The plan should describe the type of results that could be disclosed, the modalities of communication (who would disclose results, to whom, when, how), and what should be discussed during the consent process. Different frameworks for the return of results have been proposed in the literature: policy of no disclosure, disclosure of IF of clinical utility and actionability only, disclosure of all IF, return of all genomic data without interpretation, and participant decides which IF would be returned.8,13,16,32,37 Obviously, the frameworks providing more autonomy to participants also put additional burden on the research infrastructure. Another suggested approach has been to apply filters during the analysis stage to hide unwanted results to minimize the potential for IF.27,86 This strategy has the benefit of limiting IF of potential clinical utility and minimizing the burden on the research infrastructure. Ultimately, the feasibility, cost, and consequences of each approach need to be balanced. Finally, IRBs oversee research involving human subjects. They are in a unique position to provide valuable insight in reviewing the disclosure plan to research participants and participate in the development of policies and guidelines.8,30,87
Conclusion
In summary, there is a lack of definite guidance regarding the return of personal genomic research results. At present, there is no legal obligation for researchers to return IF from WGS/WES, but the emerging view is that there might be an ethical one. However, many have raised concerns about the impact such obligation would have, and the feasibility of such return is debated, with many arguing that the burden on the research infrastructure would be too significant. In any case, adopting a plan for the return of IF needs to take into account the nature of the research, the relationship between the researcher and the participants, the nature of the informed consent, and the duty to do no harm. Ultimately, even in the case of an ethical obligation, the decision is at the researcher's discretion, with the support of IRBs, recognizing that the participants' rights need to be balanced with the research goals. There is an evolving need to develop stronger frameworks and guidance to assist researchers in clarifying their responsibilities toward the management and return of IF, particularly in the view that the genetic landscape is continuously expanding.
Acknowledgments
Disclosure: E. Souzeau, None; K.P. Burdon, None; D.A. Mackey, None; A.W. Hewitt, None; R. Savarirayan, None; M. Otlowski, None; J.E. Craig, None
References
- 1. Biesecker LG,, Green RC. Diagnostic clinical genome and exome sequencing. N Engl J Med. 2014; 371: 1170. [DOI] [PubMed] [Google Scholar]
- 2. Bamshad MJ,, Ng SB,, Bigham AW,, et al. Exome sequencing as a tool for Mendelian disease gene discovery. Nat Rev Genet. 2011; 12: 745–755. [DOI] [PubMed] [Google Scholar]
- 3. Gilissen C,, Hoischen A,, Brunner HG,, Veltman JA. Unlocking Mendelian disease using exome sequencing. Genome Biol. 2011; 12: 228–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzaga-Jauregui C,, Lupski JR,, Gibbs RA. Human genome sequencing in health and disease. Annu Rev Med. 2012; 63: 35–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Zhang X. Exome sequencing greatly expedites the progressive research of Mendelian diseases. Front Med. 2014; 8: 42–57. [DOI] [PubMed] [Google Scholar]
- 6. Gharahkhani P,, Burdon KP,, Hewitt AW,, et al. Accurate imputation-based screening of Gln368Ter myocilin variant in primary open-angle glaucoma. Invest Ophthalmol Vis Sci. 2015; 56: 5087–5093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Pinxten W,, Howard HC. Ethical issues raised by whole genome sequencing. Best Pract Res Clin Gastroenterol. 2014; 28: 269–279. [DOI] [PubMed] [Google Scholar]
- 8. Wolf SM,, Lawrenz FP,, Nelson CA,, et al. Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics. 2008; 36: 219–248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Christenhusz GM,, Devriendt K,, Dierickx K. To tell or not to tell? A systematic review of ethical reflections on incidental findings arising in genetics contexts. Eur J Hum Genet. 2013; 21: 248–255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Shkedi-Rafid S,, Dheensa S,, Crawford G,, Fenwick A,, Lucassen A. Defining and managing incidental findings in genetic and genomic practice. J Med Genet. 2014; 51: 715–723. [DOI] [PubMed] [Google Scholar]
- 11. McEwen JE,, Boyer JT,, Sun KY. Evolving approaches to the ethical management of genomic data. Trends Genet. 2013; 29: 375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Tabor HK,, Berkman BE,, Hull SC,, Bamshad MJ. Genomics really gets personal: how exome and whole genome sequencing challenge the ethical framework of human genetics research. Am J Med Genet A. 2011; 155A: 2916–2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Pike ER,, Rothenberg KH,, Berkman BE. Finding fault? Exploring legal duties to return incidental findings in genomic research. Georgetown Law J. 2014; 102: 795–843. [PMC free article] [PubMed] [Google Scholar]
- 14. Wolf SM. Return of individual research results and incidental findings: facing the challenges of translational science. Annu Rev Genomics Hum Genet. 2013; 14: 557–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bredenoord AL,, Kroes HY,, Cuppen E,, Parker M,, van Delden JJ. Disclosure of individual genetic data to research participants: the debate reconsidered. Trends Genet. 2011; 27: 41–47. [DOI] [PubMed] [Google Scholar]
- 16. Hallowell N,, Hall A,, Alberg C,, Zimmern R. Revealing the results of whole-genome sequencing and whole-exome sequencing in research and clinical investigations: some ethical issues. J Med Ethics. 2015; 41: 317–321. [DOI] [PubMed] [Google Scholar]
- 17. McGuire AL,, Lupski JR. Personal genome research: what should the participant be told? Trends Genet. 2010; 26: 199–201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Murphy J,, Scott J,, Kaufman D,, Geller G,, LeRoy L,, Hudson K. Public expectations for return of results from large-cohort genetic research. Am J Bioeth. 2008; 8: 36–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Blasimme A,, Soulier A,, Julia S,, Leonard S,, Cambon-Thomsen A. Disclosing results to genomic research participants: differences that matter. Am J Bioeth. 2012; 12: 20–22. [DOI] [PubMed] [Google Scholar]
- 20. Beskow LM,, Burke W. Offering individual genetic research results: context matters. Sci Transl Med. 2010; 2:38cm20. [DOI] [PMC free article] [PubMed]
- 21. Evans JP,, Rothschild BB. Return of results: not that complicated? Genet Med. 2012; 14: 358–360. [DOI] [PubMed] [Google Scholar]
- 22. Berkman BE,, Hull SC,, Eckstein L. The unintended implications of blurring the line between research and clinical care in a genomic age. Per Med. 2014; 11: 285–295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Clayton EW,, McGuire AL. The legal risks of returning results of genomics research. Genet Med. 2012; 14: 473–477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. National Health Medical Research Council; Principles for the Translation of ‘Omics'-Based Tests from Discovery to Health Care. Canberra, Australia: National Health and Medical Research Council; 2015. Available at https://www.nhmrc.gov.au/guidelines‐publications/g10. Accessed January 28, 2016. [Google Scholar]
- 25. Green RC,, Berg JS,, Grody WW,, et al. ACMG recommendations for reporting of incidental findings in clinical exome and genome sequencing. Genet Med. 2013; 15: 565–574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. ACMG policy statement: updated recommendations regarding analysis and reporting of secondary findings in clinical genome-scale sequencing. Genet Med. 2014; 17: 68–69. [DOI] [PubMed] [Google Scholar]
- 27. van El CG,, Cornel MC,, Borry P,, et al. Whole-genome sequencing in health care. Recommendations of the European Society of Human Genetics. Eur J Hum Genet. 2013; 21: 580–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Royal College of Pathologists of Australasia. Implementation of Massively Parallel Sequencing. Location: Royal College of Pathologists of Australasia; 2014. Available at https://www.rcpa.edu.au/getattachment/7d264a73‐938f‐45b5‐912f‐272872661aaa/Massively-Parallel-Sequencing-Implementation. Accessed January 28, 2016.
- 29. Boycott K,, Hartley T,, Adam S,, et al. The clinical application of genome-wide sequencing for monogenic diseases in Canada: Position Statement of the Canadian College of Medical Geneticists. J Med Genet. 2015; 52: 431–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Fabsitz RR,, McGuire A,, Sharp RR,, et al. Ethical and practical guidelines for reporting genetic research results to study participants: updated guidelines from a National Heart, Lung, and Blood Institute working group. Circ Cardiovasc Genet. 2010; 3: 574–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Canadian Institutes of Health Research, National Sciences and Engineering Research Council of Canada, and Social Sciences and Humanities Research Council of Canada. Tri-Council Policy Statement: Ethical Conduct for Research Involving Humans. 2014. Available at http://www.pre.ethics.gc.ca/eng/policy-politique/initiatives/tcps2-eptc2/Default/. Accessed January 15, 2016.
- 32. Public Health Genomics Foundation. Managing incidental and pertinent findings from WGS in the 100,000 Genome Project. 2013. Available at http://www.phgfoundation.org/reports/13799. Accessed January 15, 2016.
- 33. Senecal K,, Rahimzadeh V,, Knoppers BM,, et al. Statement of principles on the return of research results and incidental findings in paediatric research: a multi-site consultative process. Genome. 2015; 58: 541–548. [DOI] [PubMed] [Google Scholar]
- 34. Jarvik GP,, Amendola LM,, Berg JS,, et al. Return of genomic results to research participants: the floor, the ceiling, and the choices in between. Am J Hum Genet. 2014; 94: 818–826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Presidential Commission for the Study of Bioethical Issues. Privacy and Progress in Whole Genome Sequencing. Washington, DC: Presidential Commission for the Study of Bioethical Issues; 2012. Available at http://bioethics.gov/node/764. Accessed January 28, 2016.
- 36. Weiner C. Anticipate and communicate: ethical management of incidental and secondary findings in the clinical research, and direct-to-consumer contexts (December 2013 report of the Presidential Commission for the Study of Bioethical Issues). Am J Epidemiol. 2014; 180: 562–564. [DOI] [PubMed] [Google Scholar]
- 37. Ravitsky V,, Wilfond BS. Disclosing individual genetic results to research participants. Am J Bioeth. 2006; 6: 8–17. [DOI] [PubMed] [Google Scholar]
- 38. Berg JS,, Khoury MJ,, Evans JP. Deploying whole genome sequencing in clinical practice and public health: meeting the challenge one bin at a time. Genet Med. 2011; 13: 499–504. [DOI] [PubMed] [Google Scholar]
- 39. Stone EM. Genetic testing for age-related macular degeneration: not indicated now. JAMA Ophthalmol. 2015; 133: 598–600. [DOI] [PubMed] [Google Scholar]
- 40. Craig JE,, Baird PN,, Healey DL,, et al. Evidence for genetic heterogeneity within eight glaucoma families, with the GLC1A Gln368STOP mutation being an important phenotypic modifier. Ophthalmology. 2001; 108: 1607–1620. [DOI] [PubMed] [Google Scholar]
- 41. Mackey DA,, Heon E,, Webster AR. Predictive DNA testing in ophthalmology. Br J Ophthalmol. 2003; 87: 633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Rayapudi S,, Schwartz SG,, Wang X,, Chavis P. Vitamin A and fish oils for retinitis pigmentosa. Cochrane Database Syst Rev. 2013; 12:CD008428. [DOI] [PMC free article] [PubMed]
- 43. Eckstein L,, Garrett JR,, Berkman BE. A framework for analyzing the ethics of disclosing genetic research findings. J Law Med Ethics. 2014; 42: 190–207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Knoppers BM,, Avard D,, Senecal K,, Zawati MH. Return of whole-genome sequencing results in paediatric research: a statement of the P3G international paediatrics platform. Eur J Hum Genet. 2014; 22: 3–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Begg CB. On the use of familial aggregation in population-based case probands for calculating penetrance. J Natl Cancer Inst. 2002; 94: 1221–1226. [DOI] [PubMed] [Google Scholar]
- 46. Moyer VA. Risk assessment genetic counseling, and genetic testing for BRCA-related cancer in women: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014; 160: 271–281. [DOI] [PubMed] [Google Scholar]
- 47. Ding LE,, Burnett L,, Chesher D. The impact of reporting incidental findings from exome and whole-genome sequencing: predicted frequencies based on modeling. Genet Med. 2015; 17: 197–204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Amendola LM,, Dorschner MO,, Robertson PD,, et al. Actionable exomic incidental findings in 6503 participants: challenges of variant classification. Genome Res. 2015; 25: 305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jurgens J,, Ling H,, Hetrick K,, et al. Assessment of incidental findings in 232 whole-exome sequences from the Baylor-Hopkins Center for Mendelian Genomics. Genet Med. 2015; 17: 782–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Xue Y,, Chen Y,, Ayub Q,, et al. Deleterious- and disease-allele prevalence in healthy individuals: insights from current predictions, mutation databases, and population-scale resequencing. Am J Hum Genet. 2012; 91: 1022–1032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Yang Y,, Muzny DM,, Reid JG,, et al. Clinical whole-exome sequencing for the diagnosis of Mendelian disorders. N Engl J Med. 2013; 369: 1502–1511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cassa CA,, Savage SK,, Taylor PL,, Green RC,, McGuire AL,, Mandl KD. Disclosing pathogenic genetic variants to research participants: quantifying an emerging ethical responsibility. Genome Res. 2012; 22: 421–428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Lawrence L,, Sincan M,, Markello T,, et al. The implications of familial incidental findings from exome sequencing: the NIH Undiagnosed Diseases Program experience. Genet Med. 2014; 16: 741–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Tabor HK,, Auer PL,, Jamal SM,, et al. Pathogenic variants for Mendelian and complex traits in exomes of 6,517 European and African Americans: implications for the return of incidental results. Am J Hum Genet. 2014; 95: 183–193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Gliwa C,, Berkman BE. Do researchers have an obligation to actively look for genetic incidental findings? Am J Bioeth. 2013; 13: 32–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Middleton A,, Morley KI,, Bragin E,, et al. No expectation to share incidental findings in genomic research. Lancet. 2014; 385: 1289–1290. [DOI] [PubMed] [Google Scholar]
- 57. Kaye J,, Hurles M,, Griffin H,, et al. Managing clinically significant findings in research: the UK10K example. Eur J Hum Genet. 2014; 22: 1100–1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Rotimi CN,, Marshall PA. Tailoring the process of informed consent in genetic and genomic research. Genome Med. 2010; 2: 20–26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Biesecker LG,, Mullikin JC,, Facio FM,, et al. The ClinSeq Project: piloting large-scale genome sequencing for research in genomic medicine. Genome Res. 2009; 19: 1665–1674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Appelbaum PS,, Parens E,, Waldman CR,, et al. Models of consent to return of incidental findings in genomic research. Hastings Cent Rep. 2014; 44: 22–32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Yu JH,, Jamal SM,, Tabor HK,, Bamshad MJ. Self-guided management of exome and whole-genome sequencing results: changing the results return model. Genet Med. 2013; 15: 684–690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Kaye J,, Curren L,, Anderson N,, et al. From patients to partners: participant-centric initiatives in biomedical research. Nat Rev Genet. 2012; 13: 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Henderson GE,, Wolf SM,, Kuczynski KJ,, et al. The challenge of informed consent and return of results in translational genomics: empirical analysis and recommendations. J Law Med Ethics. 2014; 42: 344–355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Crawford G,, Foulds N,, Fenwick A,, Hallowell N,, Lucassen A. Genetic medicine and incidental findings: it is more complicated than deciding whether to disclose or not. Genet Med. 2013; 15: 896–899. [DOI] [PubMed] [Google Scholar]
- 65. Presidential Commission for the Study of Bioethical Issues; Ethical Management of Incidental and Secondary Findings in the Clinical Research, and Direct-to-Consumer Contexts. Washington, DC: Presidential Commission for the Study of Bioethical Issues; 2013. Available at http://bioethics.gov/node/3183. Accessed January 28, 2016. [DOI] [PubMed] [Google Scholar]
- 66. Facio FM,, Sapp JC,, Linn A,, Biesecker LG. Approaches to informed consent for hypothesis-testing and hypothesis-generating clinical genomics research. BMC Med Genomics. 2012; 5: 45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Townsend A,, Adam S,, Birch PH,, Lohn Z,, Rousseau F,, Friedman JM. “I want to know what's in Pandora's Box”: comparing stakeholder perspectives on incidental findings in clinical whole genomic sequencing. Am J Med Genet A. 2012; 158A: 2519–2525. [DOI] [PubMed] [Google Scholar]
- 68. Shahmirzadi L,, Chao EC,, Palmaer E,, Parra MC,, Tang S,, Gonzalez KD. Patient decisions for disclosure of secondary findings among the first 200 individuals undergoing clinical diagnostic exome sequencing. Genet Med. 2014; 16: 395–399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Bergner AL,, Bollinger J,, Raraigh KS,, et al. Informed consent for exome sequencing research in families with genetic disease: the emerging issue of incidental findings. Am J Med Genet A. 2014; 164A: 2745–2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Jelsig AM,, Qvist N,, Brusgaard K,, Ousager LB. Research participants in NGS studies want to know about incidental findings. Eur J Hum Genet. 2015; 23: 1423–1426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Kaphingst KA,, Ivanovich J,, Biesecker BB,, et al. Preferences for return of incidental findings from genome sequencing among women diagnosed with breast cancer at a young age. Clin Genet. 2015; doi: http://dx.doi.org/10.1111/cge.12597. [DOI] [PMC free article] [PubMed]
- 72. Bollinger JM,, Scott J,, Dvoskin R,, Kaufman D. Public preferences regarding the return of individual genetic research results: findings from a qualitative focus group study. Genet Med. 2012; 14: 451–457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Daack-Hirsch S,, Driessnack M,, Hanish A,, et al. ‘Information is information': a public perspective on incidental findings in clinical and research genome-based testing. Clin Genet. 2013; 84: 11–18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Middleton A,, Morley KI,, Bragin E,, et al. Attitudes of nearly 7000 health professionals, genomic researchers and publics toward the return of incidental results from sequencing research. Eur J Hum Genet. 2015; doi: http://dx.doi.org/10.1038/ejhg.2015.58. [DOI] [PMC free article] [PubMed]
- 75. Bernhardt C,, Schwan AM,, Kraus P,, Epplen JT,, Kunstmann E. Decreasing uptake of predictive testing for Huntington's disease in a German centre: 12 years' experience (1993–2004). Eur J Hum Genet. 2009; 17: 295–300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76. Miller EM,, Wang Y,, Ware SM. Uptake of cardiac screening and genetic testing among hypertrophic and dilated cardiomyopathy families. J Genet Couns. 2013; 22: 258–267. [DOI] [PubMed] [Google Scholar]
- 77. Brooks L,, Lennard F,, Shenton A,, et al. BRCA1/2 predictive testing: a study of uptake in two centres. Eur J Hum Genet. 2004; 12: 654–662. [DOI] [PubMed] [Google Scholar]
- 78. Viberg J,, Segerdahl P,, Langenskiold S,, Hansson MG. Freedom of choice about incidental findings can frustrate participants' true preferences. Bioethics. 2015. ; doi: http://dx.doi.org/10.1111/bioe.12160. [DOI] [PubMed]
- 79. Tabor HK,, Stock J,, Brazg T,, et al. Informed consent for whole genome sequencing: a qualitative analysis of participant expectations and perceptions of risks, benefits, and harms. Am J Med Genet A. 2012; 158A: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Johns AL,, Miller DK,, Simpson SH,, et al. Returning individual research results for genome sequences of pancreatic cancer. Genome Med. 2014; 6: 42–49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Green RC,, Berg JS,, Berry GT,, et al. Exploring concordance and discordance for return of incidental findings from clinical sequencing. Genet Med. 2012; 14: 405–410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Klitzman R,, Appelbaum PS,, Fyer A,, et al. Researchers' views on return of incidental genomic research results: qualitative and quantitative findings. Genet Med. 2013; 15: 888–895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Lohn Z,, Adam S,, Birch P,, Townsend A,, Friedman J. Genetics professionals' perspectives on reporting incidental findings from clinical genome-wide sequencing. Am J Med Genet A. 2013; 161A: 542–549. [DOI] [PubMed] [Google Scholar]
- 84. Brandt DS,, Shinkunas L,, Hillis SL,, et al. A closer look at the recommended criteria for disclosing genetic results: perspectives of medical genetic specialists, genomic researchers, and institutional review board chairs. J Genet Couns. 2013; 22: 544–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Donegan RK,, Hill SE,, Freeman DM,, et al. Structural basis for misfolding in myocilin-associated glaucoma. Hum Mol Genet. 2015; 24: 2111–2124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Christenhusz GM,, Devriendt K,, Vermeesch J,, Dierickx K. Why genomics shouldn't get too personal: in favor of filters: re: invited comment by Tabor Holly K,, et al. in American Journal of Medical Genetics Part A Volume 155. Am J Med Genet A. 2012; 158A: 2641–2642. [DOI] [PubMed]
- 87. Simon CM,, Williams JK,, Shinkunas L,, Brandt D,, Daack-Hirsch S,, Driessnack M. Informed consent and genomic incidental findings: IRB chair perspectives. J Empir Res Hum Res Ethics. 2011; 6: 53–67. [DOI] [PMC free article] [PubMed] [Google Scholar]


