Abstract
Purpose
The subretinal layer between apical retinal pigment epithelium (RPE) and the apices of the photoreceptor outer segments is important to aging and degenerative pathogenesis, but current protocols do not provide intact horizontal images of this retinal space. Thus, an RPE/retina whole mount staining protocol was developed to observe integral subretinal regions.
Methods
RPE/retina whole mounts were stained instead of separated retina or RPE whole mounts. Hydrogen peroxide (H2O2) treatment was applied in different conditions of concentration, time, and temperature for the bleaching of RPE and choroidal melanocyte pigmentation in the pigmented RPE/retina whole mounts before antibody staining.
Results
An RPE/retina whole mount staining protocol provided better morphology of the photoreceptor outer segment than current retina whole mount. For the pigmented eyes, 10% H2O2 pretreatment effectively bleached melanin at 55°C less than 2 hours, or at 4°C within 7 days, without significant effect on immunolabeling efficacy of most antibodies tested. Actually, 55°C bleaching improved immunolabeling intensities compared to the nonbleaching control. This melanin bleaching RPE/retina protocol was applied further to observe macrophage/microglia extending from the sclera to outer plexiform layer in the CX3CR1+/GFP retina.
Conclusions
The pigment bleaching RPE/retina whole mount allowed integral horizontal imaging between choroid to photoreceptor layers, which could not be accomplished with existing methods of separated retina or RPE whole mount. Under these procedures, antigenicity of most antibodies also was well preserved.
Translational Relevance
This efficient protocol provides a tool to observe an integral view of subretinal structures including macrophage/microglia and transplanted cells, and further allows study of the interrelationship between the choroid and photoreceptor in models of aging, disease, and retinal degeneration.
Keywords: RPE, retina whole mount; melanin bleaching; subretina
Introduction
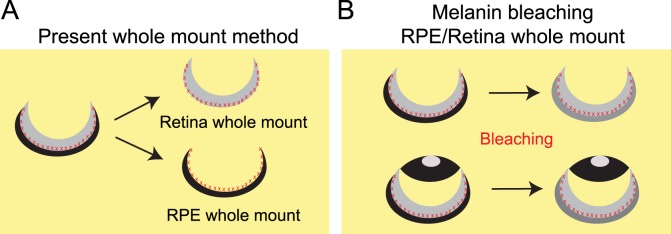
The subretinal space, the area between the apices of the photoreceptor outer segments and the apical retinal pigment epithelium (RPE), is essential to the functional relationship between photoreceptors and RPE. However, it is difficult to image this region in the whole retina because in the existing protocol, the retina tissue is separated from sclerochoroid/RPE, and each side of the retina and RPE is used for the following whole mount staining procedures (Fig. 1A). Therefore, we developed a new technique that allows imaging of intact three-dimensional (3D) morphology of the subretinal space in RPE/retina whole mount.
Figure 1.
Diagram showing the current protocol of whole mount and a new method of pigment bleaching RPE/retina whole mount to observe intact subretinal space. (A) In current protocols, whole mount staining is performed with separated tissues of RPE and retina, which damages the subretinal region (indicated by red Xs) between RPE and retina. (B) A melanin bleaching procedure is applied to the whole eyeball or posterior eyeball, to observe the intact subretinal region.
Imaging of the subretinal space is important to understand immune function and to visualize the integration of transplanted cells in the retina. For an example, aging and degenerative disease conditions affect the healthy functions of the subretinal space and disturb its integral structure. In particular, retinal microglia and/or macrophages migrate from the outer plexiform layer (OPL) and choroid vessels, respectively, into the subretinal space under the retinal inflammatory condition.1,2 Moreover, subretinal transplanted therapeutic cells become integrated into the host retinas.3,4 Therefore, it is desirable to maintain the integral subretina between the RPE and retinal tissue while we image its structure.
Recently, CLARITY nervous tissue imaging has been developed and still is being optimized, depending on tissue type, to increase the depth for intact biological image acquisition, and improve antibody staining applications.5,6 The CLARITY technique is easily applicable for whole RPE/retina tissue imaging because the retina is more transparent than brain tissue, and it also is thin; an adult mouse eye is less than 150 μm in total thickness, including the sclerochoroid, RPE, and retina. However, one of the major problems in eye tissue that limits RPE/retina whole imaging is the presence of pigmented cells in the RPE and choroid that hinder light transmission. In this study, we first assessed the possible image acquisition of the apices of the photoreceptor outer segments from RPE/retina whole mounts using nonpigmented eyes, and further adapted a melanin bleaching procedure using whole RPE/retina tissues (Fig. 1B) to enable the acquisition of intact retinal morphology from RPE to inner retinal tissue in pigmented eyes.
Methods
Tissue Processing and Immunohistochemistry
Wild type (WT) CD1, C57BL/6J, and CX3CR1+/GFP 2 mice were used. All studies adhered to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee of the National Eye Institute. For whole mount pigment bleaching and immunostaining, mouse eyes were enucleated, fixed in 4% paraformaldehyde PBS for 30 minutes to 4 hours, and the anterior segments were removed before or after melanin bleaching. For whole mount staining, primary and secondary antibodies were treated at 4°C for 3 and 2 days, respectively. For 10 μm cryosectioned retina tissues, primary and secondary antibodies were treated at room temperature for 1.5 hours and 30 minutes, respectively. The primary antibodies used were rabbit anti-cone arrestin (CAR; Millipore, Billerica, MA), calbindin (Calbiochem, La Jolla, CA), protein kinase C alpha (PKCα; Sigma-Aldrich, St. Louis, MO), and ionized calcium-binding adapter molecule 1 (Iba-1; Wako, Richmond, VA), chicken anti-S-opsin, and green fluorescent protein (GFP; Millipore), rat anti-CD11b (Serotec, Oxford, England, UK), mouse anti-glutamine synthase (GS; Millipore), and ezrin (Thermo Scientific, Rockford, IL). The relevant secondary antibodies conjugated with Alexa Fluor 488 or 555 (Life Technologies, Carlsbad, CA) were used at a dilution of 1:1000. Bovine serum albumin (1%) and 0.2% or 0.5% Triton X-100 in PBS was used for blocking and antibody treatment. All experiments were repeated separately, at least twice, using multiple retinas.
Mounting on Slide
RPE/retina whole tissues were mounted on glass slides with the sclerochoroid abutted against the coverslip, and retina whole mounts were applied with the photoreceptor layer facing the coverslip.
Tissue Melanin Bleaching
The hydrogen peroxide (H2O2) method7 was applied for melanin bleaching in sclerochoroid/RPE/retina whole tissue of pigmented eyes. First, whole eyeballs were fixed and the cornea and lens were removed before or after H2O2 bleaching. Then, 3% or 10% H2O2 PBS solution was tested at 55°C for 30 minutes to 2.5 hours and at 4°C for several days.
Microscopy
Specimen slides were imaged using a Zeiss 700 confocal microscope (Carl Zeiss Meditec, Jena, Germany). Z stack images were captured at 0.5 or 1 μm thickness. 3D images were reconstructed using Volocity software (Perkin-Elmer, Waltham, MA).
Results
RPE/Retina Whole Mount Imaging in Nonpigmented Mouse Eye
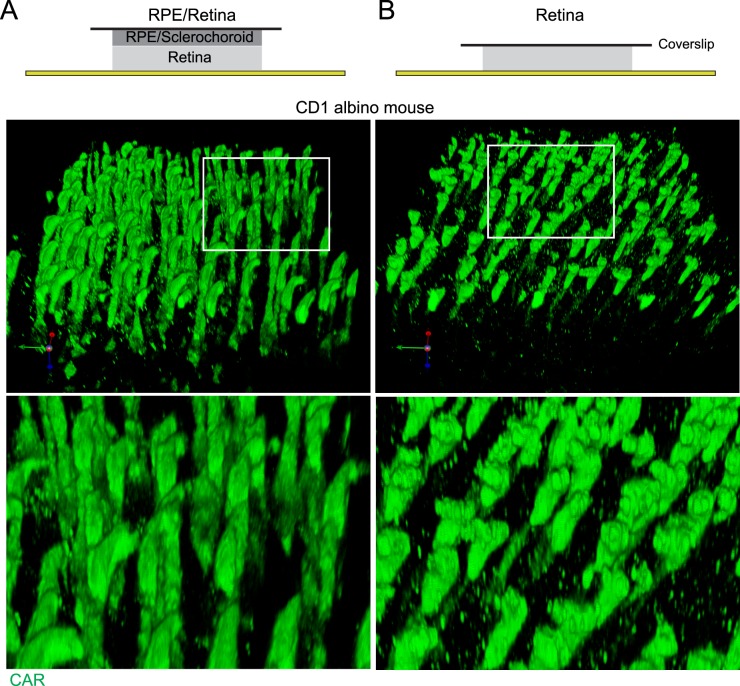
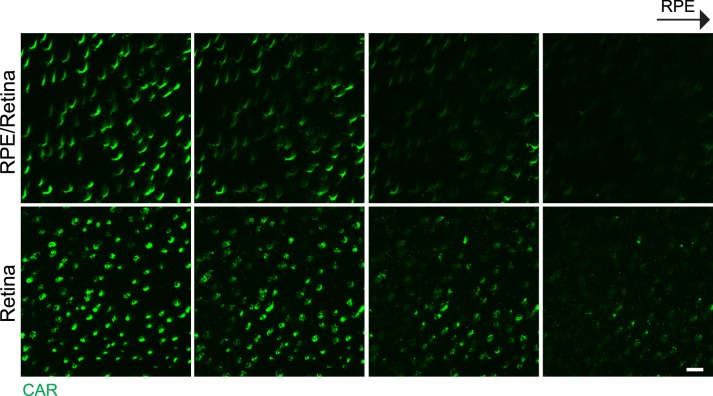
Nonpigmented CD1 mouse eyes (1 month) were first tested for acquisition of subretinal space images using RPE/retina whole mount staining. Images of the apices of photoreceptor outer segments were compared between RPE/retina whole mount and separated retina whole mount (Figs. 2, 3). Our results showed more intact morphology of the apices of photoreceptor outer segments stained with CAR antibody in RPE/retina whole mount than retina whole mount only. The apices of the photoreceptor outer segments in the 3D image of RPE/retina whole mount showed sharp terminals (Fig. 2A), but the 3D reconstruction of the photoreceptor outer segment in retina whole mount exhibited blunted ends with bumps and knobs (Fig. 2B). Each horizontal z image also showed sharp ends of the photoreceptor outer segment in RPE/retina whole mount, but blunt ends of photoreceptor outer segment in retina whole mount (Fig. 3).
Figure 2.
Comparison of photoreceptor outer segments in 3D reconstructions of RPE/retina whole mount or retina whole mount, of CD1 albino mouse. Whole mounts of 1-month-old CD1 mouse eye ([A] RPE/retina, [B] retina) were stained for CAR (green). Confocal images of whole mounts were 3D reconstructed using Volocity software (z thickness, 0.5 μm).
Figure 3.
Comparison of apices of photoreceptor outer segments in horizontal images of RPE/retina whole mount and retina whole mount of CD1 albino mouse eye. Confocal z images taken from RPE/retina whole mount (top) and retina whole mount (bottom), showing 1 μm thickness intervals toward RPE. Scale bar: 10 μm.
RPE/Retina Whole Mount Imaging in Pigmented Mouse Eye After Melanin Bleaching
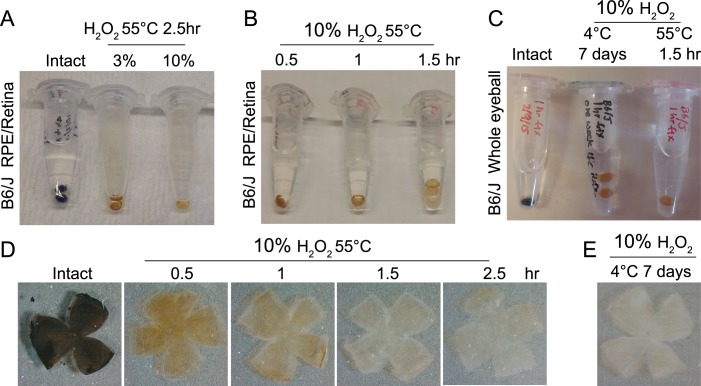
RPE/retina whole mount immunostainings of C57BL/6J mouse eyes were performed after H2O2 bleaching (Figs. 4, 5). Melanin bleaching in RPE/sclerochoroid tissues depended on H2O2 concentration (Fig. 4A) and treatment time (Figs. 4B, 4D). Treatment with 10% H2O2 at 55°C displayed a sufficient melanin bleaching effect within 1.5 hours using RPE/retina whole tissues (Figs. 4B, 4D) and whole eyeballs (Fig. 4C). Whole eyeballs showed similar whitening at 7 days after 4°C H2O2 treatment as well (Figs. 4C, 4E).
Figure 4.
Optimization of melanin bleaching conditions for C57BL/6J pigmented mouse eye. (A) Effect of different H2O2 concentrations (3% or 10%) at 55°C for 2.5 hours. (B) Effect of different treatment times (0.5, 1.0, or 1.5 hours) on 10% H2O2 bleaching at 55°C. (C) 4°C and 55°C H2O2 bleaching using whole eyeballs. (D) 55°C, 10% H2O2 bleached whole RPE/retina from C57BL/6J eyes shown at different treatment times. (E) 4°C H2O2 bleached whole RPE/retina from a C57BL/6J mouse.
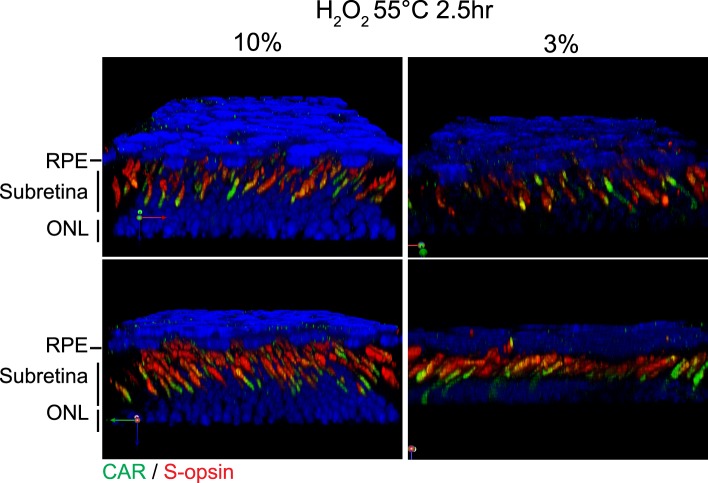
Figure 5.
Immunostaining and imaging using bleached C57BL/6J RPE/retina whole mounts. Confocal images of 10% and 3% H2O2 bleached RPE/retina whole mounts were 3D reconstructed using Volocity software. Images were acquired and adjusted in the same manner.
Treatment with 10% H2O2 (2.5 hours) presented strong immunostaining of CAR and S-opsin from choroid to photoreceptor layers compared to 3% H2O2 treatment (2.5 hours) under the same acquisition conditions for confocal imaging (Fig. 5). For the following experiments of this study, 10% H2O2 treatment of 1.5 hours at 55°C or of 7 days at 4°C was applied. However, the H2O2 treatment and tissue fixation time should be optimized according to the staining method, pigmentation and subretinal conditions, which could be different depending on species, age, and pathological states.
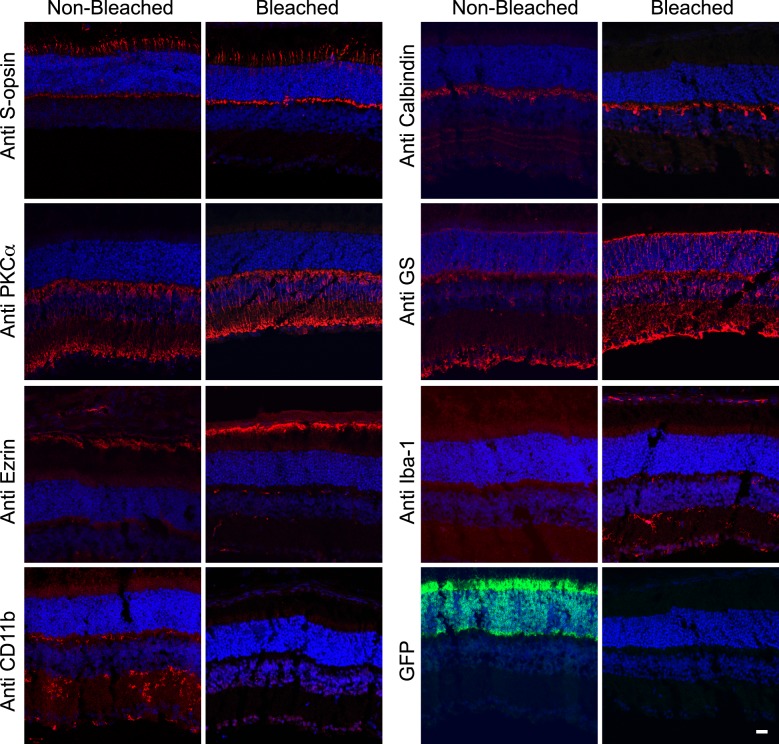
Comparison of Immunolabeling Intensities Between Bleached and Nonbleached Retinal Tissues
Although it is expected that H2O2 treatment does not harm antigenicity in immunohistochemical procedures,7 we compared the immunostaining intensities of several antibodies in bleached and nonbleached retinal tissues (Fig. 6). S-opsin antibody was used to label the outer segments of cone photoreceptors, calbindin, and PKCα for horizontal and rod bipolar cells, respectively, GS for Müller glia, ezrin for RPE apical microvilli, and Iba-1 and CD11b for microglia. Most of all the tested antibodies exhibited brighter intensity in 55°C bleached retina than nonbleached retina. The warm condition of the bleaching method is considered to have antigen retrieval effect because 4°C bleached retina did not show the improved intensities (not shown) compared to the nonbleached retina. However, immunoreactivity for CD11b was abolished. Also, endogenous GFP signals were completely eliminated in the 10% H2O2 bleached tissue.
Figure 6.
Comparison of antibody labeling intensities in nonbleached and bleached vertical sectioned-retinas. Antibody staining in intact and 55°C 10% H2O2 bleached (1.5 hours) cryosectioned vertical retinas. First and second antibodies were treated at room temperature for 1.5 and 0.5 hours, respectively. Scale bar: 20 μm.
Machrophage/Microglia From Sclerochoroid to OPL in Retina of CX3CR1+/GFP Mice
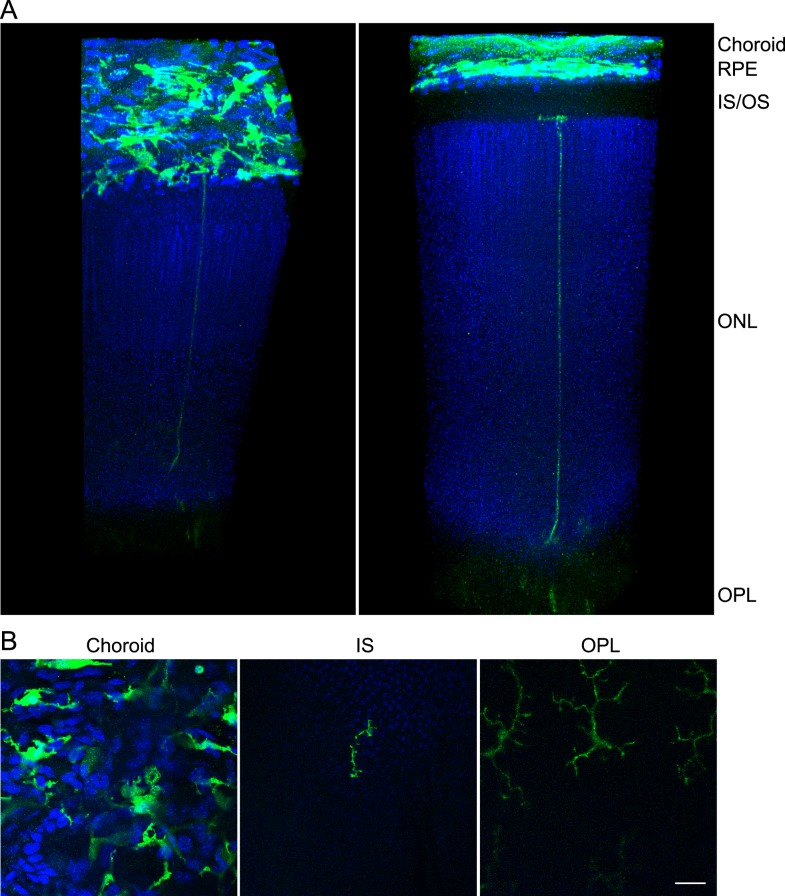
This melanin bleaching RPE/retina whole mount protocol was applied to observe macrophage/microglia extending from the sclerochoroid to the OPL of 2-month-old CX3CR1+/GFP mice with C57BL/6J background. Our observation confirmed successful application of 10% H2O2 pretreatment of 1.5 hours at 55°C or of 7 days at 4°C, before GFP staining. GFP positive macrophages in the choroid layer, ramified pseudopodia of microglia in the photoreceptor inner segment layer, and even microglia in OPL were observed in pigment bleaching RPE/retina whole mount (Fig. 7).
Figure 7.
GFP cells extending from sclerochoroid to OPL in retina of CX3CR1+/GFP mouse. (A) Confocal images from sclerochoroid to OPL of retina were reconstructed in a 3D view using Volocity software. RPE/retina whole mounts of 2-month-old CX3CR1+/GFP mice were immunostained with GFP antibody after 10% H2O2 pretreatment at 55°C for 1.5 hours or 4°C for 7 days. These representative images were taken from 55°C H2O2 pretreatment. Left is the tilted view of right panel to better display the horizontal plane of the sclerochoroid. (B) Original confocal images. From a total of 1190 z stack images, the 58th, 138th, and 993rd single images were taken for the presentation of CX3CR1+/GFP macrophage/microglia in choroid, IS, and OPL. IS/OS, inner and outer segments of photoreceptors. Scale bar: 20 μm.
Discussion
In this study, we report a successful, adapted imaging method of pigment bleaching RPE/retina whole mount staining using hydrogen peroxide pretreatment. This simple, but efficient protocol let us observe 3D retinal structure from the choroid to photoreceptor layers, and allows us to understand the relationship between choroid and photoreceptors because we no longer separate sclerochoroid/RPE and retina for the whole mount staining.
The H2O2 pretreatment yields good imaging of whole RPE/retina staining within 7 days at 4°C, and within 1.5 hours at 55°C, indicating that the H2O2 melanin bleaching protocol is safe and fast. H2O2 melanin bleaching exhibits, as good, or better, immunostaining intensities against most immunogens, but any signals against CD11b, which is a membrane protein, were not detected. Thus, H2O2 treatment for melanin bleaching seems to affect and/or destroy some cell surface epitopes, as already reported in a blocking procedure of endogenous peroxidase activity.8 However, it would be worthy to note that GFP immunoreactivity is maintained after H2O2 melanin bleaching treatment although endogenous GFP signals disappear. Therefore, careful optimization may be required for immunostaining depending on the tissues and antibodies used.
The pigment bleaching RPE/retina whole mount protocol reported will contribute to the better understanding of how disease and degeneration affect subretinal regions, and also allow for the observation of therapeutic subretinal transplanted cells in a 3D context between apical RPE and the apices of outer segment layer of the retina. The H2O2 pretreatment also might be applied to observe the choroid alone and/or RPE more clearly, as well as whole retina of pigmented eyes. Importantly, this protocol can be adapted with the recently developed CLARITY technique5 to scan whole eyes from the top of the sclerochoroid to the bottom of the retinal tissue.
Acknowledgments
The authors thank Robert N. Fariss and Anand Swaroop for advice and support, Wei Wong for CX3XR1-GFP retina, and Gail Seabold for manuscript editing.
Supported by the Intramural Research Program of the National Eye Institute.
Disclosure: S.-Y. Kim, None; J. Assawachananont, None
References
- 1. Kim SY,, Yang HJ,, Chang YS,, et al. Deletion of aryl hydrocarbon receptor AHR in mice leads to subretinal accumulation of microglia and RPE atrophy. Invest Ophthalmol Vis Sci. 2014; 55: 6031–6040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Damani MR,, Zhao L,, Fontainhas AM,, Amaral J,, Fariss RN,, Wong WT. Age-related alterations in the dynamic behavior of microglia. Aging Cell. 2011; 10: 263–276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Eberle D,, Kurth T,, Santos-Ferreira T,, Wilson J,, Corbeil D,, Ader M. Outer segment formation of transplanted photoreceptor precursor cells. PloS One. 2012; 7: e46305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Gonzalez-Cordero A,, West EL,, Pearson RA,, et al. Photoreceptor precursors derived from three-dimensional embryonic stem cell cultures integrate and mature within adult degenerate retina. Nat Biotechnol. 2013. ; 31: 741–747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Chung K,, Deisseroth K. CLARITY for mapping the nervous system. Nat Methods. 2013; 10: 508–513. [DOI] [PubMed] [Google Scholar]
- 6. Poguzhelskaya E,, Artamonov D,, Bolshakova A,, Vlasova O,, Bezprozvanny I. Simplified method to perform CLARITY imaging. Mo Neurodegen. 2014; 9: 19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Momose M,, Ota H,, Hayama M. Re-evaluation of melanin bleaching using warm diluted hydrogen peroxide for histopathological analysis. Path Int. 2011; 61: 345–350. [DOI] [PubMed] [Google Scholar]
- 8. Cheng TM,, Mao SJ,, Lai ST,, et al. Haemoglobin-induced oxidative stress is associated with both endogenous peroxidase activity and H2O2 generation from polyunsaturated fatty acids. Free Radic Res. 2011. ; 45: 303–316. [DOI] [PubMed] [Google Scholar]