Abstract
The amount and condition of exocrine impurities may affect the quality of islet preparations especially during culture. In this study, the objective was to determine the oxygen demandand viability of islet and acinar tissue post-isolation and whether they change disproportionately while in culture. We compare the OCR normalized to DNA (OCR/DNA, a measure of fractional viability in units nmol/min/mg DNA), and percent change in OCR and DNA recoveries between adult porcine islet and acinar tissue from the same preparation (paired) over a 6-9 days of standard culture. Paired comparisons were done to quantify differences in OCR/DNA between islet and acinar tissue from the same preparation, at specified time points during culture; the mean (± standard error) OCR/DNA was 74.0 (±11.7) units higher for acinar (vs. islet) tissue on the day of isolation (n=16, p<0.0001), but 25.7 (±9.4) units lower after 1 day (n=8, p=0.03), 56.6 (±11.5) units lower after 2 days (n=12, p=0.0004), and 65.9 (±28.7) units lower after 8 days (n=4, p=0.2) in culture. DNA and OCR recoveries decreased at different rates for acinar versus islet tissue over 6-9 days in culture (n=6). DNA recovery decreased to 24±7% for acinar and 75±8% for islets (p=0.002). Similarly, OCR recovery decreased to 16±3% for acinar and remained virtually constant for islets (p=0.005). Differences in the metabolic profile of acinarand islet tissue should be considered when culturing impure islet preparations. OCR-based measurements may help optimize pre-IT culture protocols.
Introduction
Islet transplantation (IT) remains a promising therapy for type 1 diabetics(1). Phase III clinical trials are being done to determine whether IT will become the standard of care. There is a critical need to accurately assess the identity and quality of the islet product prior to IT. The amount and condition of exocrine impurities may affect the quality of islet product especially during culture, but may also affect the success of engraftment post-IT(2-5).The main aim of this study was to determine the oxygen demand and viability of adult porcine islet and acinar tissue post-isolation and whether they change disproportionately while in culture. These data may help develop optimized culture protocols, which could be designed to maintain islet viability while they maximally reduce the amount of acinar impurities in a preparation. In addition, information on the oxygen demand of isolated islets and acinar tissue is needed for optimizing device loading in encapsulation and tissue engineering applications, in terms of oxygenation in vitro and in vivo.
Methods
Porcine procurement and preservation
Pancreata (n=16) were procured after cardiac death from adult Landrace pigs using previously described protocol(6). Each pancreas was placed on static cold storage and transported from the procurement facility to the isolation lab.
Islet isolation and culture
Islets were isolated using standard technique involving density gradient centrifugation as described previously (6). Islet and acinar tissue fractions were sampled and placed into separate silicone rubber membrane vessels (Wilson-Wolf Inc., St. Paul, MN) at densities of <4000 tissue equivalents (TEs)/cm2, or below the prescribed maximum allowable density (from the standpoint of adequate oxygenation) for tissue cultured in these vessels(7). A TE was defined as an amount of islet or acinar tissue that contains 10.4 ng DNA. Acinar and islet tissue from the same preparation were then cultured under standard conditions (37°C, no CO2) for >1 week. Islet preparations were of >90% purity by visual inspection with dithizone(DTZ) staining(8).
Oxygen consumption rate
OCR normalized to DNA content (OCR/DNA), a measure of fractional viability (in units nmol/min/mg DNA), was measured from paired aliquots of both acinar and islet tissue from the same preparations on the day of isolation (Day 0) and Days 1, 2 and 8 of culture, and the protocol has been described elsewhere in detail(3, 4, 9).
DNA and OCR Recoveries
Aliquots of acinar and islet tissue were taken on different days of culture and assessed for DNA content using a commercially-available spectrophotometric assay (Quant-IT PicoGreen dsDNA kit, Invitrogen, Grand Island, NY) and a SpectraMax M5 plate reader (Molecular Devices, Sunnyvale, CA)(10). The quantity of DNA in an aliquot was extrapolated to the entire preparation in culture based on volume. DNA content measured on Days 6-9 were normalized to the DNA content measured on Day 0 to yield a “DNA recovery”, which represented a “total tissue” recovery, since DNA is stable even in dying tissue (10). DNA recoveries measured on Days 6-9 were then multiplied by the OCR/DNA (fractional viability) to yield an “OCR recovery”, which represented the “viable tissue” recovery in the preparation. Mean percent changes in DNA and OCR recoveries were reported for acinar and islet tissue and paired comparisons were also performed for tissues derived of the same preparation.
Statistical Analysis
Normality of the data was assessed using the D'Agostino-Pearson omnibus normality test. Comparisons between means were done using the paired two-tailed t test (for normally-distributed data) or the Mann-Whitney test (for non-normally-distributed data). Statistical significance was defined by p-value < 0.05 using a 95% confidence interval. Data are presented as means ± standard error (SE). All statistical analyses were performed using either SAS Version 9.2 (SAS Institute, Inc., Cary, NC) or Graphpad Prism Version 5.03 (Graphpad Software, Inc., La Jolla, CA).
Results
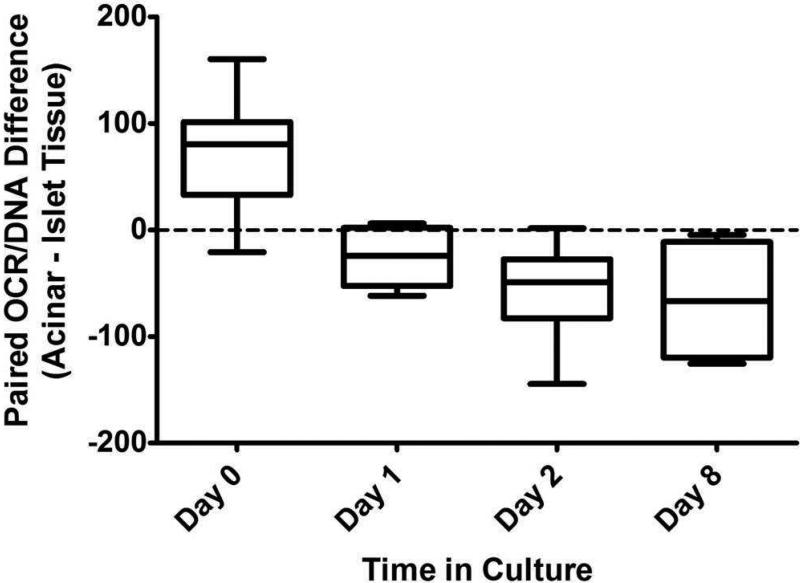
Data indicate that adult porcine islet and acinar tissue exhibit OCR of similar magnitude. However, there were differences in the OCR/DNA (fractional viability) when comparing islet and acinar tissue from the same preparation with respect to time in culture (Table 1). Mean (±SE) OCR/DNA was 74.0 (±11.7) units higher for paired acinar (vs. islet) tissue on the day of isolation (n=16, p<0.0001), but 25.7 (±9.4) units lower after 1 day (n=8, p=0.03), 56.6 (±11.5) units lower after 2 days (n=12, p=0.0004), and 65.9 (±28.7) units lower after 8 days (n=4, p=0.2) in culture (Figure 1). DNA and OCR recoveries decreased disproportionately for paired acinar (vs. islet tissue) over 6-9 days in culture (n=6); DNA recovery was only 24±7% for acinar tissue, whereas it was 76±8% for islet tissue (p=0.002). Similarly, OCR recovery was only 16±3% for acinar tissue, whereas it remained virtually unchanged at 115±7% for islet tissue (p=0.005).
Table 1.
Summary of OCR/DNA values
| Mean (± SE) OCR/DNA |
||||
|---|---|---|---|---|
| Days of culture | Paired n | Islet | Acinar | p-value |
| 0 | 16 | 167.4 (7.6) | 241.4 (10.6) | <0.0001*** |
| 1 | 8 | 204.6 (11.7) | 178.9 (7.3) | 0.03* |
| 2 | 12 | 220.5 (11.3) | 163.9 (9.8) | 0.0004** |
| 8 | 4 | 275.7 (10.0) | 209.9 (38.3) | 0.2 |
Level of statistical significance:
p<0.05
p<0.001
p<0.0001
Figure 1.
Box and whisker plot depicting the mean difference in the OCR/DNA values when comparing paired islet and acinar tissue fractions on Day 0, 1, 2 and 8 of culture. Error bars represent minimum and maximum differences. OCR/DNA is significantly higher for acinar (vs. islet) tissue on Day 0, but becomes lower with more time in culture.
Discussion
Results indicate that adult porcine acinar tissue has significantly higher OCR/DNA than islets immediately after isolation. However, the OCR/DNA of acinar tissue decreases with time in culture, whereas the opposite occurs with islet tissue. This is in concordance with acinar DNA and OCR recoveries declining significantly over approximately 1-week in culture. Collectively, the results indicate that acinar tissue is less resilient than islet tissue following isolation in culture. To our knowledge data directly comparing oxygen demand from adult porcine islets and acinar tissue originating from the same pancreas has not been previously reported. Such data is critical for designing tissue engineered constructs and in islet encapsulation applications to help define the oxygenation requirement. Finally, the data reported in this study suggest that porcine islet preparations may be further purified by prolonged culture prior to transplantation without loss of viable islet yield.
Conclusion
Adult porcine acinar tissue oxygen consumption is not lower than that of adult porcine islets originating from the same pancreas. With existing islet culture protocols the viability and recovery of acinar tissue is drastically reduced over 7 days, while viable islet recovery remains virtually unchanged. OCR-based assays may enable optimization of pre-IT culture protocols and help in the design of tissue-engineered constructs in terms of tissue loading and oxygenation requirements.
Acknowledgements
This work was supported by funding from the Iacocca Foundation, the Schott Foundation, and the Minnesota Lions Diabetes Foundation.
Abbreviations
- DNA
deoxyribonucleic acid
- DTZ
dithizone
- IT
islet transplantation
- OCR
oxygen consumption rate
- OCR/DNA
OCR normalized to DNA content (fractional viability)
- SE
standard error
- TE
tissue equivalent
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Barton FB, Rickels MR, Alejandro R, et al. Improvement in outcomes of clinical islet transplantation: 1999-2010. Diabetes Care. 2012;35(7):1436. doi: 10.2337/dc12-0063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Papas KK, Suszynski TM, Colton CK. Islet assessment for transplantation. Curr Opin Organ Transplant. 2009;14(6):674. doi: 10.1097/MOT.0b013e328332a489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Papas KK, Colton CK, Nelson RA, et al. Human islet oxygen consumption rate and DNA measurements predict diabetes reversal in nude mice. Am J Transplant. 2007;7(3):707. doi: 10.1111/j.1600-6143.2006.01655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Papas KK, Colton CK, Qipo A, et al. Prediction of marginal mass required for successful islet transplantation. J Invest Surg. 2010;23(1):28. doi: 10.3109/08941930903410825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Papas KK, Bellin MD, Sutherland DE, et al. American Transplant Congress. S5. Vol. 13. American Journal of Transplantation; Seattle, WA: 2013. Correlations of In Vitro Islet Potency Tests with Clinical Islet Auto-Transplant Outcome. p. 494. [Google Scholar]
- 6.Hering BJ, Wijkstrom M, Graham ML, et al. Prolonged diabetes reversal after intraportal xenotransplantation of wild-type porcine islets in immunosuppressed nonhuman primates. Nat Med. 2006;12(3):301. doi: 10.1038/nm1369. [DOI] [PubMed] [Google Scholar]
- 7.Papas KK, Avgoustiniatos ES, Tempelman LA, et al. High-density culture of human islets on top of silicone rubber membranes. Transplant Proc. 2005;37(8):3412. doi: 10.1016/j.transproceed.2005.09.086. [DOI] [PubMed] [Google Scholar]
- 8.Colton CK, Papas KK, Pisania A, et al. Characterization of islet preparations. In: Halberstadt C, Emerich DF, editors. Cellular Transplantation: From Laboratory to Clinic. Academic Press; Waltham, MA: 2007. p. 85. [Google Scholar]
- 9.Papas KK, Pisania A, Wu H, Weir GC, Colton CK. A stirred microchamber for oxygen consumption rate measurements with pancreatic islets. Biotechnol Bioeng. 2007;98(5):1071. doi: 10.1002/bit.21486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Suszynski TM, Wildey GM, Falde EJ, et al. The ATP/DNA ratio is a better indicator of islet cell viability than the ADP/ATP ratio. Transplant Proc. 2008;40(2):346. doi: 10.1016/j.transproceed.2008.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]