Abstract
Purpose
To report a single institutional experience with definitive radiation therapy alone for human papillomavirus (HPV)-positive head and neck cancer
Methods and Materials
Sixty-seven patients were treated by radiation therapy alone to a median dose of 70 Gy (range, 66 to 72 Gy) for squamous cell carcinoma of the head and neck. Paraffin-embedded, formalin-fixed pre-treatment tumor tissues were used to establish HPV-positivity using standardized techniques of immunohistochemistry for p16 and polymerase chain reaction for HPV.
Results
Twenty-three patients with HPV-positive cancers were identified. With a median follow-up of 28 months (range, 6 to 85 months), the 3-year actuarial rates of overall survival, local-regional control, and distant metastasis-free survival were 83%, 90%, and 88%, respectively.
Conclusion
These findings attest to the exquisite radiosensitivity of HPV-positive head and neck cancer. The clinical outcomes observed from this selected series compare favorably to historical controls treated by more intensive chemoradiotherapy strategies.
Keywords: human papillomavirus, head and neck cancer, radiation therapy, squamous cell carcinoma, radiosensitivity
Introduction
Although evidence continues to mount that human papilomavirus (HPV)-associated squamous cell carcinoma of the head and neck represents a unique entity with distinct clinical and molecular characteristics, the optimal management for the increasing proportion of patients diagnosed with this disease is uncertain [1–3]. At present, it remains speculative whether patients with HPV-positive head and neck cancer should be treated differently than those with HPV-negative tumors. However, the increasing recognition that HPV-related head and neck cancers are exquisitely sensitive to radiation therapy has prompted investigators to question whether patients with these cancers might be over-treated and unnecessarily subjected to the toxicity of intensive treatment strategies utilizing chemoradiotherapy. This report aimed to review a single-institutional experience with the treatment of HPV-positive head and neck cancer using definitive radiation therapy alone.
Methods and Materials
Patients
This study was formally approved by all relevant Institutional Review Boards. The medical records of 67 patients treated with definitive intent for squamous cell carcinoma of the head and neck from January 2003 to December 2010 by radiation therapy alone were retrospectively reviewed. The decision not to use chemotherapy was highly individualized and made at the discretion of the physician considering such factors as disease burden and stage, performance status, medical co-morbidities, age, and patient preference. No formal institutional policy was in place during the years of the study.
Tissue analysis
The paraffin-embedded biopsy tissue of 60 of the 67 patients was retrievable from archive. Notably, HPV testing has been routinely performed at our institution since late-2008, and no attempt was made to confirm HPV status in these patients. Routine paraffin-embedded, formalin-fixed pre-treatment tumor tissues were used to establish HPV-positivity or HPV-negativity using standardized techniques of immunohistochemistry for p16 and polymerase chain reaction for HPV (Figure 1). In keeping with previous investigators’ methods, p16 overexpression was defined as tumors that displayed intense diffuse cytoplasmic and nuclear staining in greater than 70% of tumor cells using a monoclonal antibody against p16 [4]. Confirmation of HPV-positivity among tumors with p16 overexpression was then performed. Evaluation for HPV subtypes 6/11, 16/18, and 31/33 DNA using the ISH-catalyzed signal amplification method for biotinylated probes (Dako GenPoint, Carpineteria, CA) was used. Punctate hybridization signals localized to the tumor cell nuclei defined an HPV-positive tumor. All patients with HPV-positive tumors were p16-overxpression and immunoreactive for HPV, using the criteria of at least one copy per 10 cell genomes’ worth of DNA.
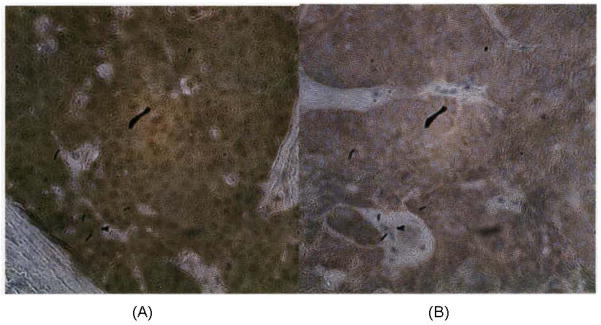
Figure 1.
Immunohistochemistry for p16 expression was determined by using monoclonal mouse antibody. (A) squamous cell carcinoma demonstrating strong and diffuse staining. (B) squamous cell carcinoma demonstrating absence of immunohistochemical staining
Follow-up and statistical analysis
Patients were asked to return for follow-up visit 2–4 weeks after completion of radiation therapy and then every 2–3 months for the first year, 4–6 months for the second year, and then annually thereafter. The endpoints analyzed were overall survival, local-regional control, and distant metastasis-free survival. Local control was judged to have been attained if there was no evidence of tumor at the primary site based on clinical and radiographic findings at follow-up. Regional failure was recorded separately if there was evidence of a cervical or supraclavicular mass distinct from the primary site. Patients who had persistent disease, either clinically or radiographically, after treatment were referred for salvage neck dissection. Failure was counted only if the patient had pathologic evidence of residual disease. Salvage of recurrences was not taken into account in the evaluation of local-regional or disease control. Patient follow-up was reported to the date last seen in clinic or to the date of expiration. All events were measured from the last day of radiation therapy.
Actuarial estimates of local-regional control, distant metastasis-free survival, and overall survival were calculated using the Kaplan-Meier method [5]. Acute and late normal tissue effects were graded according to the Radiation Therapy Oncology Group (RTOG)/European Organization for the Treatment of Cancer (EORTC) toxicity criteria [6].
Results
HPV-positive subjects
Twenty-three patients with cancers which were HPV-positive were identified. The median age was 63 years old (range, 42 to 70 years). Using the staging system of the American Joint Committee on Cancer (AJCC), six patients (26%) had stage I/II disease and 17 patients (74%) had stage III/IV disease. Distribution of T-classification was: T1 22%, T2 26%, T3 26%, and T4 26%. Eighteen patients (79%) were never smokers, defined as having smoked less than 100 cigarettes in a lifetime. Primary disease sites were as follows: tonsil (10 patients), base of tongue (9 patients), soft palate (2 patient), and supraglottic larynx (2 patients).
All HPV-positive patients had received a dose of 66 to 72 Gy (median, 70 Gy) to gross disease. Sixteen patients (70%) received intensity-modulated radiotherapy (IMRT) using a simultaneous integrated boost technique in which the primary tumor generally received a higher dose (2.12 to 2.25 Gy per fraction) compared to electively treated areas. The remaining 7 patients were treated by 3D-conformal techniques using initial opposed lateral fields with progressive cone-downs. Three of the patients treated using 3D-conformal radiotherapy received a concomitant boost, in which a smaller field encompassing gross disease was irradiated in addition to the large comprehensive field during the last 10 days of treatment.
Outcome
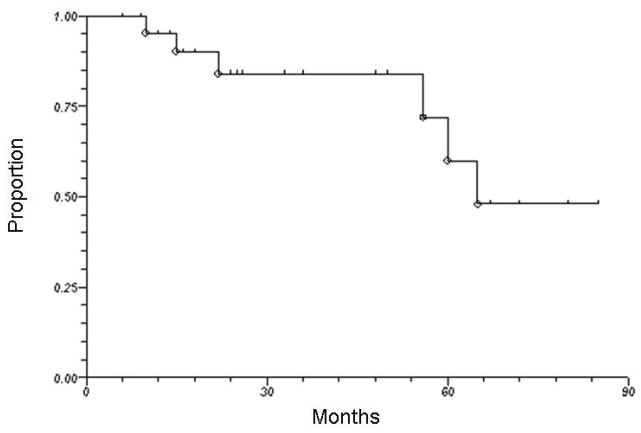
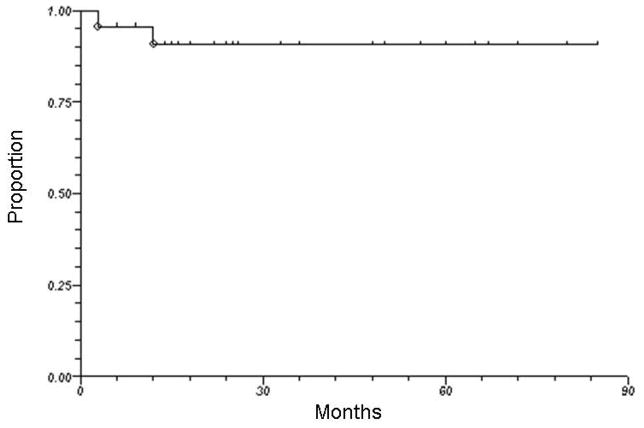
Seventeen of 23 patients are alive at the time of this analysis. With a median follow-up of 28 months (range, 6 to 85 months), the 3-year overall survival was 83%. Figure 2 illustrates overall survival for all HPV-positive patients treated by radiation therapy alone. One patient (with initial T4N2c squamous cell carcinoma of the base of tongue) had persistent pathological disease at neck dissection 3 months after completion of radiation therapy. One additional patient with initial T4N2b squamous cell carcinoma of the tonsil developed local recurrence at 12 months after completion of radiation therapy. As shown in Figure 3, the 3-year actuarial local-regional control rate was 90%. Another 3 patients developed distant metastasis (1 concurrently with local recurrence and 2 as isolated events), yielding a 3-year actuarial distant metastasis-free survival rate of 88%. The site of initial distant metastasis was the lungs in all cases. When the 2 patients with HPV-positive laryngeal cancer were excluded from the analysis, the 3-year rates of overall survival, local-regional control, and distant metastasis-free survival were 82%, 88%, and 88%, respectively.
Figure 2.
Overall survival among all patients with HPV-positive squamous cell carcinoma of the head and neck treated by radiotherapy alone
Figure 3.
Local-regional control among all patients with HPV-positive squamous cell carcinoma of the head and neck treated by radiotherapy alone
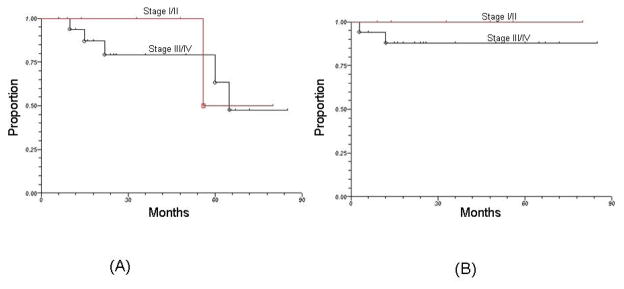
The 3-year overall survival and local-regional control rates for patients with stage I/II were 100% and 100%, respectively. For patients with stage III/IV disease, the corresponding rates were 81% and 88%, respectively. Among the 18 HPV-positive patients who were never-smokers, the 3-year rates of overall survival and local-regional control were 100% and 100%, respectively.
The most commonly reported grade 3+ acute toxicity (non-hematologic) was confluent mucositis which occurred in 11% of the patients. Other documented grade 3+ acute toxicities included moist desquamation of the skin and odynophagia/dysphagia, which generally resolved with conservative measures. No treatment-related fatalities were observed. The incidence of gastrostomy-tube dependence due to late dysphagia at 1 year after completion of treatment was 9%.
Discussion
The published literature has consistently demonstrated that patients with HPV-positive head and neck cancer have an improved prognosis compared to patients with HPV-negative tumors [7–9]. However, essentially all of these studies have included patients treated by strategies incorporating chemoradiotherapy. The present analysis, representing one of the first to report on outcomes among patients treated by radiation therapy alone for HPV-positive head and neck cancer, is thus important considering that treatment paradigms for this subset of patients remain poorly defined. While limited by its retrospective nature, our results, demonstrating the feasibility and efficacy of treating HPV-positive head and neck cancer with radiation therapy alone, are important given that the addition of chemotherapy has been shown to significantly increase acute and late toxicity [10]. Notably, the reasons why patients did not receive chemotherapy in the present study are variable and include elderly age, medical comorbidities, patient refusal, and/or lack of clinical indication.
Lassen et al similarly showed promising rates of disease control among HPV-positive head and neck cancer patients in 2 separate sub-set analysis of patients treated without chemotherapy from a previously published trial from the Danish Head and Neck Group (DAHANCA) comparing conventional versus accelerated fractionated radiation therapy [11,12]. Outcomes at 5-years among 156 patients treated by conventionally fractionated radiation therapy to doses of 66 or 68 Gy showed improved local-regional control 58% versus 28%), disease-specific survival (72% versus 34%), and overall survival (62% versus 26%) for the 35 HPV-positive patients compared to negative (as determined using p16 staining). Similarly, a separate analysis of the 794 patients treated by accelerated fractionated radiation demonstrated improved 5-year outcomes among the 179 HPV-positive patients with respect to local-regional control (69% versus 57%), disease-specific survival, and overall survival.
The mechanism of HPV-mediated radioresponse is currently unclear, and there are limited studies that have addressed this topic in the setting of head and neck cancer. The most direct explanation is that HPV infection and the subsequent interference with the normal function of p53 and pRb by the viral products E6 and E7 somehow renders the host tumor cell more susceptible to radiation-induced apoptosis. Pang et al recently showed that transfection of the E6 transcript in HPV-negative squamous cell carcinoma cell lines resulted in sensitization to radiation-induced cell death [13]. Other studies have suggested that radiation therapy enhances the host immune response to viral antigens which are expressed on the cancer [14]. Gupta et al reported that HPV-positive head and neck cancer cell lines were more radiosensitive in a series of experiments analyzing clonogenic survival; however, these results have yet to be conclusively replicated by others [15]. Investigators have also demonstrated that HPV-positive head and neck cancer cell-lines harbor distinctive molecular signatures and genomic profiles, which may be responsible for the differential response to radiation therapy [16,17]. Regardless of the underlying pathways responsible for HPV-mediated radioresponse, the results of the present study add to the growing body of literature attesting to the radiosensitivity of head and neck tumors in HPV-positive patients.
Although the bulk of the clinical data attesting to the improved prognosis of patients with HPV-positive head and neck cancer have included patients treated by radiation therapy, the heterogeneity of the treatment methods make it difficult to draw definitive conclusions. For instance, studies reporting on improved outcomes for HPV-positive tumors have used varying fractionation schemes, timing and types of chemotherapy agents, and radiation techniques [8,11,12]. Whether it is indeed the improved radiosensitivity that drives the superior survival is also uncertain since HPV-positive patients treated by surgery have also been shown to have better prognosis than their HPV-negative counterparts [9,18].
Along these lines, it remains speculative whether patients with HPV-positive head and neck cancer should be treated differently than those with HPV-negative tumors. Nonetheless, the increasing recognition that HPV-related head and neck cancers are exquisitely sensitive to radiation therapy have prompted investigators to question whether these patients derive the same amount of benefit from traditional chemoradiotherapy regimens as those with HPV-negative tumors. For instance, the Radiation Therapy Oncology Group (RTOG) is currently testing cetuximab with radiation therapy as an alternative to cisplatin-based chemoradiotherapy for HPV-positive head and neck cancer in a phase III setting. Other groups have pursued an approach of radiation dose de-escalation while continuing to use chemotherapy. The Eastern Cooperative Oncology Group (ECOG) is currently assessing dose de-escalation for HPV-positive oropharyngeal cancer patients in a phase II study with a strategy of induction chemotherapy followed by reduced-dose radiation with concurrent chemotherapy for complete responders. While data continues to emerge suggesting that treatment should be individualized for the subgroup of patients with HPV-related head and neck cancer, exactly how to do so remains uncertain. Although our results suggest that radiation therapy alone may be a feasible option for this subset of patients, it would be instructive to compare outcomes to patients treated with chemoradiotherapy to determine how the addition of chemotherapy affects the therapeutic ratio. Clearly, well-designed clinical trials analyzing the feasibility, efficacy, and toxicity of various treatment de-intensification strategies for this subgroup of patients are needed.
The limitations of this study are several-fold and relate to its retrospective nature. First, the effects of prior tobacco smoking were not accounted for among this patient population. Although 79% of the patients in this cohort were never-smokers, the inclusion of those with a smoking history may have the potential to confound results. Others have established that smoking history should be used in conjunction with HPV status to stratify patients into prognostic groups [8,19,20]. Moreover, the reasons why the HPV-positive patients were not offered chemotherapy were variable and difficult to determine with exact certainty. For instance, some of the patients had early-stage tumors or low-volume disease; many were also older and/or had medical co-morbidities, which may have placed them at higher risk for toxicity with chemotherapy; still others were not offered chemotherapy due to patient and physician preference. It should also be recognized that a small percentage of patients in this analysis had early-stage tumors, for whom the expected outcomes are excellent, possibly regardless of HPV status. Additionally, the median age for patients analyzed (63 years) is greater than that of typically reported chemoradiation trials for this population. Given the selection biases inherent in the decision to treat with and without chemotherapy, we opted not to compare the reported outcomes among patients with HPV-positive tumors with their HPV-negative counterparts.
In conclusion, clinical outcomes among patients treated by radiotherapy alone for HPV-positive head and neck cancer appear to compare favorably to those for patients treated by more intensive chemoradiotherapy approaches. Since a prospective trial analyzing radiation therapy alone for HPV-positive head and neck cancer has yet to be conducted, we believe are findings provide reasonable assurances attesting to the feasibility and efficacy of such an approach in carefully selected patients.
Figure 4.
Outcomes showing (a) overall survival and (b) local-regional control according to stage (I/II versus III/IV) among patients with HPV-positive squamous cell carcinoma of the head and neck treated by radiotherapy alone.
Footnotes
The authors indicate no potential conflicts of interest or financial disclosures.
References
- 1.Gillison ML, D’Souza G, Westra WH, et al. Distinct risk factor profiles for human papillomavirus type 16-positive and human papillomavirus type 16-negative head and neck cancers. J Natl Cancer Inst. 2008;100:407–420. doi: 10.1093/jnci/djn025. [DOI] [PubMed] [Google Scholar]
- 2.Braakhuis BJ, Snijders PJ, Keune WH, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2005;96:998–1006. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 3.Weinberger PM, Yu Z, Haffty BG, et al. Molecular classification identifies a subset of human papillomavirus--associated oropharyngeal cancers with favorable prognosis. J Clin Oncol. 2006;24:736–747. doi: 10.1200/JCO.2004.00.3335. [DOI] [PubMed] [Google Scholar]
- 4.Singhi AD, Westra WH. Comparison of human papillomavirus in situ hybridization and p16 immunohistochemistry in the detection of human papillomavirus-associated head and neck cancer based on a prospective clinical experience. Cancer. 2010;116:2166–2173. doi: 10.1002/cncr.25033. [DOI] [PubMed] [Google Scholar]
- 5.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:547–581. [Google Scholar]
- 6.Cox JD, Stetz J, Pajak TF. Toxicity criteria of the Radiation Therapy Oncology Group (RTOG) and the European Organization for Research and Treatment of Cancer (EORTC) Int J Radiat Oncol Biol Phys. 1995;31:1341–1346. doi: 10.1016/0360-3016(95)00060-C. [DOI] [PubMed] [Google Scholar]
- 7.Fakhry C, Westra WH, Li S, et al. Improved survival of patients with human papillomavirus-positive head and neck squamous cell carcinoma in a prospective clinical trial. J Natl Cancer Inst. 2008;100:261–269. doi: 10.1093/jnci/djn011. [DOI] [PubMed] [Google Scholar]
- 8.Ang KK, Harris J, Wheeler R, et al. Human papillomavirus and survival of patients with oropharyngeal cancer. N Engl J Med. 2010;363:24–35. doi: 10.1056/NEJMoa0912217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fischer CA, Zlobec I, Green E, et al. Is the improved prognosis of p16 positive oropharygneal squamous cell carcinoma dependent of the treatment modality? Int J Cancer. 2010;126:1256–1262. doi: 10.1002/ijc.24842. [DOI] [PubMed] [Google Scholar]
- 10.Machtay M, Moughan J, Trotti A, et al. Factors associated with severe late toxicity after concurrent chemoradiation for locally advanced head and neck cancer: an RTOG analysis. J Clin Oncol. 2008;26:3582–3589. doi: 10.1200/JCO.2007.14.8841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lassen P, Eriksen JG, Hamilton S, et al. Effect of HPV-associated p16 expression on response to radiotherapy and survival in squamous cell carcinoma of the head and neck. J Clin Oncol. 2009;27:1992–1998. doi: 10.1200/JCO.2008.20.2853. [DOI] [PubMed] [Google Scholar]
- 12.Lassen P, Eriksen JG, Krogdahl A, et al. The influence of HPV-associated p16-expression on accelerated fractionated radiotherapy in head and neck cancer: evaluation of the randomised DAHANCA 6&7 trial. Radiother oncol. 2011;100:49–55. doi: 10.1016/j.radonc.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 13.Pang E, Delic NC, Hong A, et al. Radiosensitization of oropharyngeal squamous cell carcinoma cells by human papillomavirus 16 oncoprotein E6*I. Int J Radiat Oncol Biol Phys. 2011;79:860–865. doi: 10.1016/j.ijrobp.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 14.Spanos WC, Nowicki P, Lee DW, et al. Immune response during therapy with cisplatin or radiation for human papillomavirus-related head and neck cancer. Arch Otolaryngol Head Neck Surg. 2009;135:1137–1146. doi: 10.1001/archoto.2009.159. [DOI] [PubMed] [Google Scholar]
- 15.Gupta AK, Lee JH, Wilke WW, et al. Radiation response in two HPV-infected head and neck cancer cell lines in comparison to non-HPV-infected cell line and relationship to signaling through AKT. Int J Radiat Oncol Biol Phys. 2009;74:928–933. doi: 10.1016/j.ijrobp.2009.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klussmann JP, Mooren JJ, Lehnen M, et al. Genetic signatures of HPV-related and unrelated oropharygneal carcinoma and their prognostic implications. Clin Cancer Res. 2009;15:1179–1786. doi: 10.1158/1078-0432.CCR-08-1463. [DOI] [PubMed] [Google Scholar]
- 17.Braakhuis BJ, Snijders PJ, Keune WH, et al. Genetic patterns in head and neck cancers that contain or lack transcriptionally active human papillomavirus. J Natl Cancer Inst. 2005;96:998–1006. doi: 10.1093/jnci/djh183. [DOI] [PubMed] [Google Scholar]
- 18.Licitra L, Perrone F, Bossi P, et al. High-risk human papillomavirus affects prognosis in patients with surgically treated oropharyngeal sqamous cell carcinoma. J Clin Oncol. 2006;24:5630–5636. doi: 10.1200/JCO.2005.04.6136. [DOI] [PubMed] [Google Scholar]
- 19.Kumar B, Cordell KG, Lee JS, et al. EGFR, p16, HPV titer, bcl-xL, and p53, sex, and smoking as indicators of response to therapy and survival in oropharyngeal cancer. J Clin Oncol. 2008;26:3128–3137. doi: 10.1200/JCO.2007.12.7662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maxwell JH, Kumar B, Feng FY, et al. Tobacco use in human papillomavirus-positive advanced oropharynx cancer patients related to increased risk of distant metastasis and tumor recurrence. Clin Cancer Res. 2010;16:1226–1235. doi: 10.1158/1078-0432.CCR-09-2350. [DOI] [PMC free article] [PubMed] [Google Scholar]