Abstract
This study examined neural anticipation of monetary reward in pathological gamblers (PG) by varying the type of uncertainty associated with the reward. Ten PG and ten controls were scanned while deciding whether to accept (“bet” option, featuring high-uncertain monetary rewards) or reject (“safe” option, featuring low-certain rewards) a bet, within situations of decision-making under risk (probability of the “bet” reward is known) or ambiguity (probability of the “bet” reward is unknown). During decision under risk (as compared to ambiguity), controls exhibited activation in brain areas involved in reward processing (putamen), interoception (insula) and cognitive control (dorsolateral prefrontal cortex; middle frontal gyrus). By contrast, PG exhibited no differential brain activation as a function of the type of uncertainty associated with the “bet” option. Moreover, prior choosing of the “safe” option (as compared to “bet” choices), controls exhibited activation in the posterior insula, dorsolateral prefrontal cortex and middle frontal gyrus. By contrast, PG exhibited higher neural activation during the elaboration of “bet” choices, and in motivational-arousal areas (caudate; putamen; posterior insula). Between-groups contrasts revealed that, as compared to controls, PG showed (i) decreased neural activity in the globus pallidus for decision-making under risk, as opposed to decision under ambiguity, and (ii) increased neural activity within the putamen prior to bet choices, as opposed to safe choices. These findings suggest that (i) unlike control participants, a variation in the level of uncertainty associated with monetary rewards seems to have no significant impact on PGs' decision to gamble and (ii) PG exhibit stronger brain activation while anticipating high-uncertain monetary rewards, as compared with lower-certain rewards.
Keywords: Gambling disorder, fMRI, Uncertainty, Reward anticipation, Striatum, Insular cortex
1. Introduction
Through repetition of gambling behaviors, pathological gamblers (PG) acquire extensive experience in making complex financial decisions involving variable wins, losses, and probabilities. This is likely to bias their neurocognitive approach to decision-making. Furthermore, unlike non-problem gamblers, who shape and maintain their behaviors according to their consequences, PG continue to gamble despite the accumulation of financial losses [1]. One explanation for this stereotyped pattern of decision-making is that response output directed at gambling trigger automated and habit-like processes [2,3].
One key characteristic of habits is that, through the repetition of behaviors, it becomes increasingly estranged from variations in outcome value and reward probability [4]. In this context, addiction-related stimuli may elicit automatic, repetitive and inflexible behavioral sequences [5–7]. In other words, gambling-seeking behaviors may become persistent and ultimately insensitive to devaluation or punishment. For instance, a recent PET study highlighted that, while gambling on a slot machine, ventral striatal dopamine (the mesolimbic dopamine neurotransmitter that plays a major role in reward-driven learning) release in PG was not modulated by gambling outcome [8]. This suggests that, in PG, being embedded into gambling-related action is merely sufficient to induce dopaminergic changes independently of its outcome. Moreover, recent fMRI studies showed that PG exhibited higher activation in the brain-reward system during the pre-choice anticipation (i.e., when the subject is pondering potential options before making a decision; [9]) and the post-choice anticipation (i.e., the subject has made a decision and is awaiting the outcome; [10]) of high-uncertain monetary rewards. More specifically, as compared with low-risk decisions, before taking high-risk decisions in a quasi-realistic blackjack scenario [9], PG exhibited enhanced brain responses in the inferior frontal gyrus and lateral orbitofrontal cortex region (OFC; region involved in the integration of emotional and cognitive input; [11]) and in the medial side of the pulvinar nucleus (a relay thalamic nucleus that receives interoceptive input and in turn projects to the insular cortex all of which are brain areas associated with impulsive urges [12]). On the other hand, controls showed a significant signal increase before taking low-risk decisions, as compared to high-risk decisions. With regard to post-choice anticipation, van Holst et al. [10] have observed that, as compared to healthy controls, PG exhibited stronger activation in the ventral striatum (a region involved in reward anticipation and reward processing [13]) and the medial OFC when anticipating a large win (e.g., 5 euros) as opposed to a lower win (e.g., 1 euro). Additionally, several brain imaging studies showed that, in contrast to non-gamblers, there is evidence of a reduction of cerebral activity in the brain reward pathway during the processing of monetary gambling rewards and losses in PG [[14–16], but see [17]]. Together, these findings support the notion that PG exhibit a cue-induced signal increase toward the anticipation of high-uncertain monetary rewards. Nevertheless, a couple of recent imaging studies reported decreased neural activations during the anticipation of monetary gains in PG [18,19]. More specifically, while performing an incentive delay task (which requires an individual to react to a target stimulus presented after an incentive cue to win or to avoid losing the indicated reward), PG exhibited less frontostriatal activation than controls while anticipating monetary gains [18,19]. One possible explanation for these contradictory findings is the use of different task designs [20–23]. Indeed, experimental paradigms more closely related to gambling (e.g., a blackjack task in [9]; a guessing card game in [10]) may generate increased neural activation in PGs' brain-reward system, as compared with less gambling-related paradigms (such as the incentive delay task [22]), which may be less significant and incentive for gamblers.
The goal of the present study is to further explore the neural correlates of gambling-related choices in PG. More specifically, we aim to examine whether PGs' decision-making is modulated by the type of uncertainty associated with high monetary rewards. Indeed, if PGs' desire to gamble is triggered by the feeling that a large part of money is at stake [9,10], a variation in the type of uncertainty associated with this amount might have a low impact on their decision to gamble. In other words, the type of uncertainty should not significantly modulate risk-taking in PG. In order to test this assumption, we used an adapted version of the Card-Deck paradigm initially developed by Hsu et al. [24]. In this task, participants are asked to choose between a “safe” option, which offers a sure payoff (e.g., $9), and a “bet” option which offers larger (e.g., $25) but uncertain reward. The bet choice carried either some risk (i.e., where probability of reward is known) or some ambiguity (i.e., where probability of reward is unknown). Using this paradigm, Hsu et al. [24] reported differential brain activations according to the type of uncertainty associated with the “bet” option. More specifically, as compared with decision-making under ambiguity, explicit outcome probability during decision-making under risk heightened neural activation within brain region involved in the prediction of reward (i.e., the dorsal striatum [25]). By contrast, decision-making under ambiguity activated a vigilance-evaluative neural network (amygdala and orbitofrontal cortex activations), which suggests that ambiguous choices carry more unknown consequences, and that cognitive and behavioral resources must be mobilized in order to seek out additional information from the environment [24].
In the current study, we hypothesize that, at a behavioral level, the frequency of PGs' “bet” choices will be less modulated by the type of uncertainty (decision-making under risk versus under ambiguity), as compared to non-gambler control participants. At a neural level, we test the hypothesis that, as compared to controls, PG will exhibit less differential brain activation according to the type of uncertainty associated with the “bet” option. Moreover, based on recent findings on pre- [9] and post- [10] decision anticipation in gambling disorder, we expect that PG will exhibit higher brain activation prior taking the “bet” option, as compared to the “safe” one.
2. Methods
2.1. Participants and recruitment
Twelve pathological gamblers (PG) and twelve controls were recruited for this study. All subjects provided informed consent according to the Declaration of Helsinki. The CHU-Brugmann ethics committee approved the study. PG were recruited through advertisements in the casino complex VIAGE in Brussels, Belgium. All gamblers had to meet the criteria for DSM-IV-TR Gambling Disorder. Problem gambling severity was assessed using the South Oaks Gambling Screen (SOGS; [26]). All gambler participants scored at least a five on the SOGS, indicative of gambling disorder. Our sample of gamblers was categorized as slot machines gamblers (i.e., the gambling game reported at a higher frequency, by the gambler participants, on the SOGS). We excluded any subject who was (a) over 65 years, or (b) had any substance use disorder during the prior year before enrolling in the study. Participants were judged to be medically healthy on the basis of their medical history. The severity of problems related to substance use and medical history were also examined with items taken from the Addiction Severity Index Short Form ([27]; selection of items undertaken by S.M. and P.V.; CHU-Brugmann board-certified psychotherapists). In addition, we excluded participants who exhibited either excessive motion (i.e., >3 mm and/or >3°, or motion correlated with the task) or BOLD signal instability in a task-independent area (i.e., the occipital cortex), larger than 5%. Based in these thresholds, we excluded two pathological gamblers and two control participants who exhibited BOLD signal instabilities. Hence, our final sample consisted of ten PG and ten controls (see Table 1 for demographics and current clinical status).
Table 1.
Demographical data and current clinical status for controls and pathological gamblers.
| Controls | Gamblers | Test statistics | |
|---|---|---|---|
| n | 10 | 10 | |
| Age | 36.20 (12.95) | 34.00 (8.53) | t(19) = 0.45, p = 0.66 |
| Years of study | ≤12:70.0% | ≤12:80.0% | χ2 (1,20) = 0.27, p = 0.60 |
| >12:30.0% | >12:20.0% | ||
| Males/females | 8/2 | 8/2 | |
| Gambling frequency (day/month) | 0.69 (0.48) | 16.42 (9.53) | |
| DSM-IV | / | 7.58 (1.59) | |
| SOGS | 0.00 | 8.53 (3.48) | |
| Cig/day | 2.80 (4.73) | 4.80 (7.16) | U = 87.50, p = 0.24 |
| BDI | 3.80 (3.76) | 5.50 (4.62) | U = 34.00, p = 0.22 |
| AUDIT | 5.01 (5.04) | 6.87 (6.31) | t(19) = 0.40, p = 0.64 |
Values shown are the mean and standard deviations on each measure; SOGS, South Oaks Gambling Screen; AUDIT, Alcohol Use Disorder Identification Test; STAI-E, State version of the State-Trait Anxiety Inventory; STAI-T, Trait version of the State-Trait Anxiety Inventory; BDI, Beck Depression Inventory.
Control participants were recruited by word of mouth from the community, excluding psychiatrists, psychologists, and other personnel with previous psychological training. Based on the SOGS, none of the controls gambled frequently (see Table 1).
2.2. Clinical and neuropsychological measures
Current clinical status of depression was rated with the Beck Depression Inventory (BDI; [28]). Alcohol use was estimated through the Alcohol Use Disorder Identification Test (AUDIT; [29]). The number of cigarettes per day was also included in order to control for the effect of nicotine dependence on cognitive processing [30].
2.3. Gambling related craving
All subjects completed the Gambling Craving Scale (GACS; [31]) before and after fMRI scanning. The GACS contains three factors: anticipation (e.g., “Gambling would be fun right now”), desire (e.g., “I crave gambling right now”) and relief (e.g., “If I were gambling now, I could think more clearly”). There are nine items (three items for each of the three factors) assessed on a seven-point scale.
2.4. Paradigm and materials
The stimuli (see Fig. 1) were adapted from the Card-Deck paradigm [24]. Stimuli were presented through a computer-controlled projector, which directs visual information onto a mirror mounted inside the scanner that the participant could see through the coil's mirror. Before entering the scanner, subjects received a standardized verbal description of the task and completed a practice session. Participants were told that they would be making a series of choices between a gamble with an uncertain payoff, and a certain payoff. Importantly, participants were informed that, at the end of the experiment, the outcome of one trial (simulated by computer) would be chosen at random, and the outcome of that trial would determine their pay. The participants earned €50 for participating, plus the earning from the randomly chosen trial.
Fig. 1.

Schematic depiction of the Card-Deck paradigm. In this task, participants decided whether to take the sure payoff, or bet on either red or black card. The bet choice carried (a1) some risk (explicit probability for red and blue cards) or (a2) some ambiguity (no explicit probability for red and blue cards) (For interpretation of the color information in this figure legend, the reader is referred to the web version of the article.).
For each trial, a Card-Deck made of red and blue cards was drawn on a black screen. The total number of cards in the deck was written at the center-left of the screen. For each choice, three options were given. Two of two of those options involved to bet on either side of a binary choice gamble (i.e., red or blue card) that carried some uncertainty of winning either a positive sum or zero. The third option was the sure payoff that paid a lower-but-certain amount of money.
There were two types of conditions: ambiguous and risky. In the risky condition, the respective number of red and blue cards included in the deck was indicated. By contrast, under the ambiguous condition, these numbers were replaced by a question mark. A screenshot for one trial of the risky and the ambiguous conditions is depicted in Fig. 1.The task consisted of 60 trials (30 trials for the risky condition and 30 trials for the ambiguous condition) divided into four runs (330s) of 15 trials. The 30 ambiguous and 30 risky trials were randomly displayed throughout the four runs of 15 trials. Trials were separated by a fixation cross on a black screen. The duration of the fixation cross was randomly distributed within a 10–20 s range. Each trial ended directly after subject's choice, with a maximum allowed time of 15 s. Responses were made by pressing the button corresponding to the location of the options (left-middle-right) on the screen. Choices were made using an MRI-compatible button box. No feedback were given throughout the task.
2.5. Imaging data acquisition and preprocessing
Imaging data were obtained using a 1.5-T MRI scanner (Siemens Avanto, Siemens AG, Erlangen, Germany), with repeated single-shot echo-planar imaging: repetition time (TR) = 3.000 ms, echo time (TE) = 41 ms, flip angle (FA) = 90°, matrix size = 64 × 64, field of view (FOV) = 240 mm × 240 mm, 33 slices ordered descending and interleaved, slice thickness = 3.6 mm. A three-dimensional (3D) T1-weighted (MPRAGE) data set encompassing the whole brain was acquired to provide detailed anatomy (TR = 8 ms, TE = 2.85 ms, FA = 10°, matrix size = 256 × 256, FOV= 250mm × 250 mm, 192 slices, slice thickness = 1 mm, no gap). The functional data sets were subjected to a series of preprocessing operations: linear trend removal for excluding scanner-related signal drift, a temporal high-pass filtering applied to control for temporal frequencies, and a correction for small interscan head movements by a rigid body algorithm rotating and translating each functional volume in 3D space. The data were corrected for the difference between the scan times of the different slices. Data were smoothed in the spatial domain (kernel = 8 mm). In order to be able to compare the locations of activated brain region across participants, all anatomical as well as the functional volumes were spatially normalized [32] and the statistical maps computed were overlaid to the 3D T1-weighted scans in view to calculate Talairach coordinates for all relevant activation clusters.
2.6. Statistical analysis
Demographic and clinical data were analyzed using independent sample t-tests. Non-normally distributed data (i.e., craving scores, cigarettes/day, BDI) were analyzed using Mann-Whitney U-tests for the between-groups comparisons. Within-group differences for scores of craving at pre- and post-experiment were analyzed using Friedman's analyses of variances (ANOVAs). A repeated-measures ANOVA was used to analyze choice reaction times (RTs), with group as a between-subjects factor (PG versus controls) and type of condition (risky versus ambiguous) as a within-subjects factor. The percentage of bet choice was examined using one-sample t-tests, for each group, with level of chance (0.50) as test value. This was followed by a repeated-measures ANOVA to analyze percentage of bet versus safe choice, with group as between-subjects factor (PG versus controls) and type of condition (risky versus ambiguous) as within-subjects factor. All behavioral analyses were thresholded at α = 0.05.
Imaging analyses were performed using Brain Voyager QX (Version 2.3, Brain Innovation, Maastricht, The Netherlands). The data were modeled at the first level using a general linear model (GLM) with uncertainty (risk versus ambiguity) and decision type (bet choice versus safe choice) as regressors. These were then input into an ANOVA-based random-effect model for group analyses, comprising two within-subjects factors (uncertainty: risk versus ambiguity; decision type: bet choice versus safe choice) and one between-subjects factor (group: pathological gamblers versus controls). All analyses were generated bi-directionally using Brain Voyager's standard convention: orange/red color highlighting activation, blue color highlighting deactivation. All contrasts were generated at an uncorrected voxel p-value of 0.001, with a minimum cluster size of 30 voxels. This threshold is equivalent to p corrected <0.05 based on Monte Carlo simulations [33]. Tables for within-group activations include anatomical location and Brodmann area (as localized by Münster's T2T-Converter–http://wwwneuro03.uni-muenster.de/ger/t2tconv/), activation/deactivation, cluster size, hemisphere, coordinate of the cluster's center in the Talairach coordinate system, and mean t-value of the cluster.
3. Results
3.1. Sample characteristics
Analyses revealed that PG and controls were similar in terms of age, BDI scores, cigarettes/day, and AUDIT scores (see Table 1).
Before and after scanning, PG had significantly higher average scores of gambling-related craving than controls (except for scores on the “Relief” subscale, before scanning; see Table 2). After performing the Card-Deck experiment, gambling craving scores were increased in both groups: PGs' scores increased for each three sub-scales of the GACS (Anticipation: χ2(1) = 7.00, p = 0.008; Desire: χ2(1) = 10.00, p = 0.002; Relief: χ2(1) = 9.00, p = 0.003) whereas scores of controls increased for anticipation and desire subscales but not the relief subscale (Anticipation: χ2(1) = 10.00, p = 0.002; Desire: χ2(1) = 7.00, p = 0.008; Relief: χ2(1) = 0.33, p = 0.56).
Table 2.
Mean and standard deviations for pathological gamblers (PG) and controls on the GACS, before and after fMRI scanning.
| Controls (n = 10) | PG (n = 10) | Signifiance Mann-Whitney U | |
|---|---|---|---|
| Craving anticipation, before | 4.58 (2.02) | 14.25 (4.93) | U = 4.5, p = 0.000 |
| Craving desire, before | 5.75 (2.26) | 8.58 (3.29) | U = 32.0, p = 0.020 |
| Craving relief, before | 3.25 (0.45) | 5.41 (3.50) | U = 52.5,p = 0.18 |
| Craving anticipation, after | 6.00(2.00) | 17.67 (3.14) | U = 1.0, p = 0.000 |
| Craving desire, after | 7.50 (2.71) | 11.67 (3.84) | U = 20.5, p = 0.003 |
| Craving relief, after | 3.42 (0.51) | 8.50 (4.17) | U = 8.5, p = 0.000 |
3.2. Behavioral performance
3.2.1. Reaction time
Analyses revealed no main effect of choice type, F(1,19) = 0.35, p = 0.56, no main effect of type of condition, F(1,19) = 1.69, p = 0.21, no main effect of group, F(1,19) = 0.27, p = 0.61, and no interaction effect for RTs (estimated in milliseconds).
3.2.2. Percentage of bet choice
The mean percentage of bet choice was 40.15%. One-sample t-test showed that mean percentage of bet choice was significantly lower than chance (50.0%) (t(9) = 8.21, p < 0.0001). This indicates that controls took more safe choice than bet choices during the Card-Deck experiment. With regards to PG, the mean percentage of bet choice was 64.25%. One-sample t-test showed that mean percentage of bet choice was significantly greater than chance (50.0%) (t(9) = 16.17, p < 0.0001), indicating that PG took more bet choice than safe choices during the Card-Deck experiment. Independent sample t-test revealed that percentage of bet choice was greater in PG than in controls (t(19) = −3.87, p = 0.001).
There was a main effect of type of condition (risky versus ambiguous), F(1,19) = 6.31, p = 0.019, indicating that participants bet more under the risky condition than under the ambiguous condition. There was also a main effect of group, F(1,19) = 5.55, p = 0.027, indicating more frequent bet decisions in PG as compared with the controls. No interaction group × condition effect was observed. Additional exploratory repeated measures analyses were then undertaken separately for the control and the gambler groups. In controls, these analyses showed a significant effect of type of condition on the percentage of bet choice (F(1,9) = 9.79, p = 0.012, η2 = 0.52), indicating that controls bet more under the risky condition (M = 50.50, SD = 22.07) than under the ambiguous condition (M = 29.80, SD = 14.06). With regard to PG, within-group repeated measures analysis showed no significant effect of the type of condition on the percentage of bet choice (F(1,9) = 0.53, p = 0.48, η2 = 0.056). This indicates that PG did not bet differently under the risky (M = 67.10, SD = 14.91) and the ambiguous conditions (M = 61.40, SD = 19.83). These results are depicted in Fig. 2.
Fig. 2.

Percentage of bet choice for the risky and the ambiguous conditions in controls and pathological gamblers. Error bars are the standard errors of the mean.
3.3. Imaging results
3.3.1. Decision-making under risk versus decision-making under ambiguity
3.3.1.1. Controls
We observed increased neural activations (p < 0.001, uncorrected) in the right superior frontal gyrus, the left dorsolateral prefrontal cortex, the right middle frontal gyrus, the right putamen, and the right insular cortex (see Fig. 3 and Table 3) during decision under risk, as compared with decision under ambiguity.
Fig. 3.
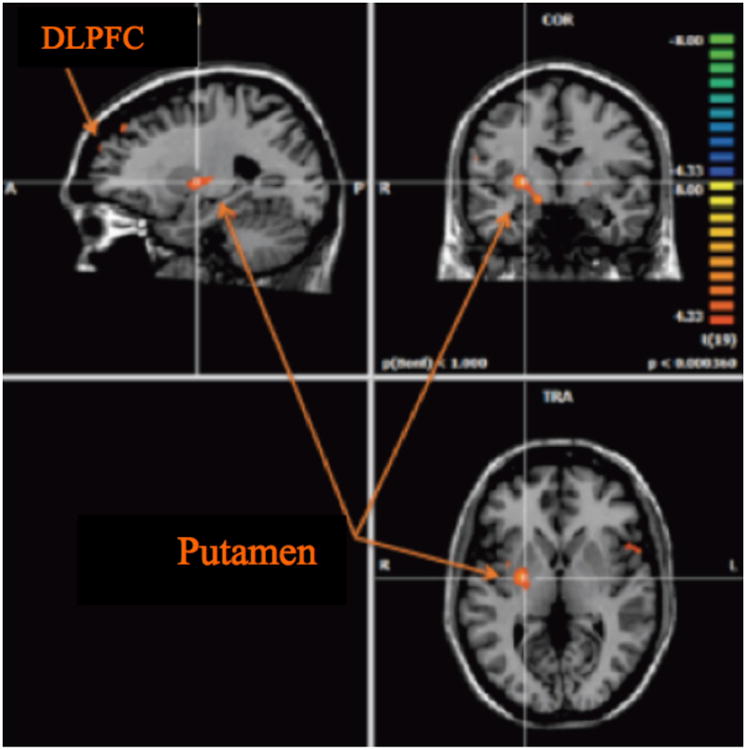
Regions activated (p < 0.001) during decision-making under risk minus decision-making under ambiguity in controls.
Table 3.
Significant brain activation (p < 0.001) within the control group (n = 10).
| Card-Deck task contrast | Regions | Left/right | Activation/deactivation | BA | k | MNI coordinates | t-value | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| x | y | z | |||||||
| Decision-making under risk minus Decision-making under ambiguity | Superior frontal gyrus | R | A | 8 | 32 | 21 | 44 | 42 | 5.07 |
| Dorsolateral prefrontal cortex | L | A | 9 | 94 | −12 | 60 | 28 | 4.98 | |
| Middle frontal gyrus | R | A | 10 | 41 | 44 | 44 | 20 | 4.94 | |
| Superior temporal gyrus | L | A | 43 | 435 | −64 | −22 | 13 | 5.77 | |
| Putamen | R | A | − | 303 | 26 | −8 | 4 | 5.27 | |
| Insular cortex | R | A | 13 | 37 | −49 | 13 | 3 | 5.05 | |
| Bet choices minus safe choices | Precentral gyrus | L | D | 4 | 108 | −37 | −27 | 56 | 4.90 |
| Superior temporal gyrus | L | D | 42 | 630 | −64 | −23 | 14 | 6.07 | |
| Posterior insular cortex | L | D | 13 | 146 | −38 | −23 | 15 | 5.26 | |
| Superior temporal gyrus | L | D | 22 | 269 | −64 | −40 | 8 | 5.28 | |
| Middle frontal gyrus | R | D | 10 | 308 | 30 | 54 | 3 | 5.20 | |
| Bet choices under risk minus Bet choices under ambiguity | Superior frontal gyrus | R | A | 8 | 45 | 1 | 34 | 51 | 3.85 |
| Dorsolateral prefrontal cortex | L | A | 9 | 298 | −40 | 3 | 23 | 4.02 | |
| Middle frontal gyrus | R | A | 10 | 232 | 30 | 58 | 9 | 3.98 | |
| Middle temporal gyrus | L | A | 21 | 194 | −44 | −28 | −7 | 4.07 | |
| Putamen | R | A | − | 195 | 16 | −11 | 7 | 3.83 | |
| Insular cortex | R | A | 13 | 179 | 42 | 15 | 10 | 3.95 | |
L, Left; R, right; A, activation; D, deactivation; BA, Brodmann area; k, voxel cluster size (each voxel = 3 mm3); MNI, Montreal Neurological Institute.
3.3.1.2. Pathological gamblers
We found that the two types of uncertainty (i.e., risk versus ambiguity) did not significantly modulate brain activation in PG.
3.3.1.3. Controls versus pathological gamblers
As compared with controls, PG showed decreased neural activity in the right globus pallidus for decision-making under risk, as opposed to decision-making under ambiguity (see Fig. 5 and Table 5).
Fig. 5.
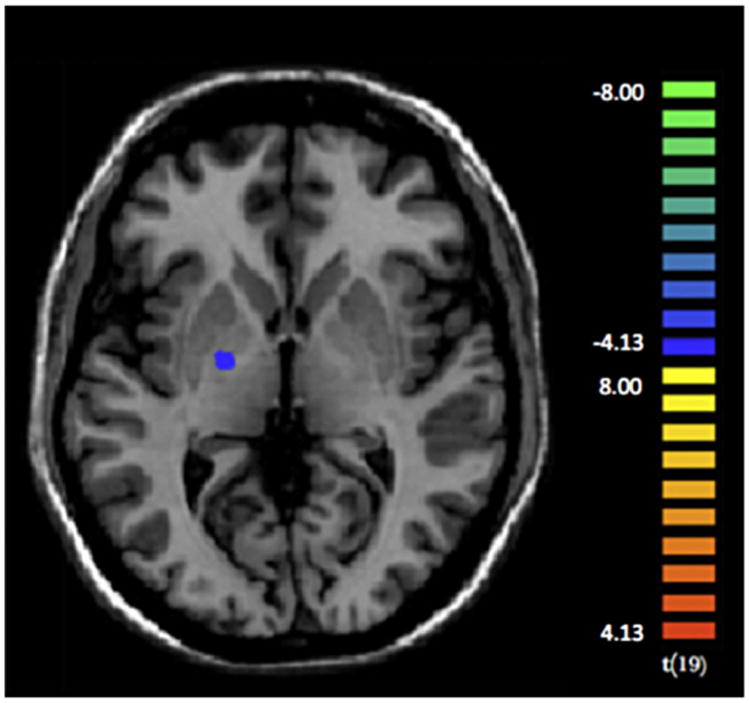
Compared to controls, pathological gamblers showed decreased activation (p < 0.001) within in the right globus pallidus, during decision-making under risk as opposed to decision-making under ambiguity.
Table 5.
Brain regions activated (p < 0.001) between pathological gamblers minus controls.
| Card-Deck task contrast | Regions | Left/right | Activation/deactivation | BA | k | MNI coordinates | t-value | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| x | y | z | |||||||
| Decision-making under risk minus Decision-making under ambiguity | Globus pallidus | R | D | − | 208 | 22 | −10 | 4 | 3.88 |
| Bet choices minus safe choices | Putamen | R | A | − | 113 | 21 | −4 | 7 | 3.94 |
3.3.2. Bet choice versus safe choice
3.3.2.1. Controls
Prior safe choices, as compared with bet decisions, we observed increased neural activations (p < .001, uncorrected) in the left posterior insular cortex and the right middle frontal gyrus (see Fig. 4 and Table 3).
Fig. 4.
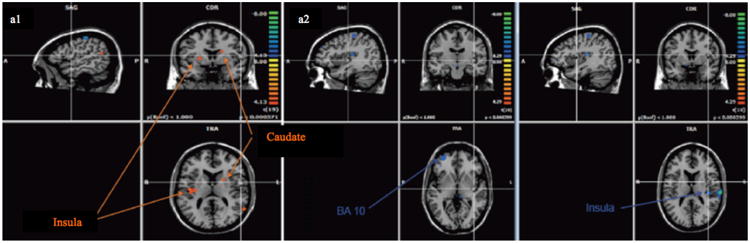
Regions activated (p < 0.001) prior bet choice minus safe choice in (a1) pathological gamblers and (a2) controls.
3.3.2.2. Pathological gamblers
Prior bet choices, as compared with safe decisions, we observed increased brain activations (p < 0.001, uncorrected) in the left caudate, the right posterior insula and the right and left putamen (see Fig. 4 and Table 4).
Table 4.
Significant brain activation (p < 0.001) within the pathological gambler group (n = 10).
| Card-Deck task contrast | Regions | Left/right | Activation/deactivation | BA | k | MNI coordinates | t-value | ||
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| x | y | z | |||||||
| Decision-making under risk minus Decision-making under ambiguity | Precentral gyrus | L | D | 4 | 916 | −43 | −30 | 53 | 5.78 |
| Precentral gyrus | R | A | 4 | 965 | 36 | −27 | 53 | 5.98 | |
| Caudate nucleus | L | A | − | 226 | −15 | −5 | 19 | 4.85 | |
| Posterior insular cortex | R | A | 13 | 53 | 37 | −22 | 15 | 4.80 | |
| Middle temporal gyrus | L | A | 39 | 143 | −58 | −57 | 13 | 4.87 | |
| Putamen | L | A | − | 65 | −22 | 6 | 10 | 4.87 | |
| Putamen | R | A | − | 290 | 28 | −16 | 7 | 4.97 | |
| Cerebellum | L | A | − | 248 | −18 | −54 | −21 | 4.85 | |
3.3.2.3. Controls versus pathological gamblers
As compared with controls, PG showed increased neural activity in the right putamen prior bet choice, as opposed to safe choice (see Fig. 6 and Table 5).
Fig. 6.
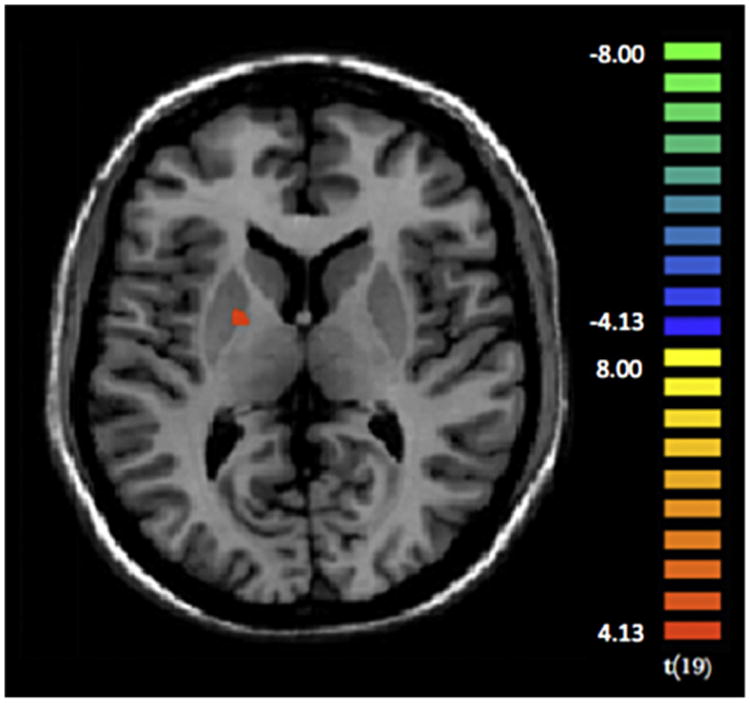
Compared to controls, pathological gamblers showed increased activation (p < 0.001) within in the right putamen, prior choosing of the bet option as opposed to the safe option.
3.4. Additional analyses
Complementary analyses showed increased neural activity (p < 0.001, uncorrected) in the control group for bet choices under risk, when compared to bet choices under ambiguity (see Table 3). These activations were observed in the same brain regions than those highlighted through the contrast:“Decision-making under risk versus decision-making under ambiguity” (see Table 3). No significant activation was observed in controls when we contrasted safe choices under risk versus safe choices under ambiguity. These results suggest that significant activations observed in controls, through the contrast: “Decision-making under risk versus decision making under ambiguity”, were due to neural activity induced by bet choices undertaken during decision-making under risk. No significant result was observed in the PG group for these two additional contrasts.
4. Discussion
In this study, we examined neural correlates of decision-making under risk and under ambiguity in a sample of individuals with gambling addiction and a sample of non-gambler control participants. We used an experimental task in which they had to choose between low-but-certain (“safe” option) versus high-but-uncertain (“bet” option) monetary rewards.
4.1. Lack of sensitivity to the type of uncertainty in pathological gamblers
In control participants, by contrast to decision-making under ambiguity, we observed higher neural activation during decision-making under risk, and within brain areas involved in the reward processing (putamen; [13]), in the perception of viscero-sensory activity (insular cortex; [34]), and in the computation of probability (dorsolateral prefrontal cortex; superior frontal gyrus; middle frontal gyrus; [35]). Hence, during the Card-Deck paradigm, controls exhibited less neural activity during decision-making under ambiguity than during decision-making under risk. These results contradict those obtained by Hsu et al. [24], who found higher brain activation within the amygdala and the orbitofrontal cortex activations during decision-making under ambiguity, as compared to decision-making under risk. Nevertheless, in contrast to control participants of Hsu et al. [24] (where no significant bet score differences were found between decision-making under ambiguity and under risk, see supporting online materials [24]), our sample of controls chose more often the “bet” option during decision-making under risk than during decision-making under ambiguity. In other words, it turns out that our current group of controls was averse to choosing the bet option during decisions made under ambiguity (i.e., ambiguity aversion; [36]), which could have lowered the mobilization of cognitive and attentional resources within these situations of decision-making. This assumption is in line with Huettel et al. [37], who found higher brain activation in individuals who exhibited a preference for uncertain rewards during decision-making under risk, as compared to decision-making under ambiguity.
Importantly, in the PG group, we observed that the type of uncertainty (i.e., risk versus ambiguity) did not trigger differential brain activation. Moreover, between-groups comparisons revealed that controls exhibited higher neural activity than PG in a brain area involved in goal-directed behavior (globus pallidus; [38]), during decision-making under risk (as opposed to decision-making under ambiguity). In addition, behavioral analyses highlighted that PG did not modify their choices according to the type of uncertainty. Taken together, these neural and behavioral findings suggest that PG were not sensitive to the variation of the type of uncertainty associated with gambling-related outcomes (i.e., the “bet” option).
4.2. Hypersensitization for “bet” choices in pathological gamblers
Compared to controls, PG chose more often the “bet” option over the “safe” option, in both situations of decision-making under risk and under ambiguity. This behavioral finding is consistent with previous studies that have highlighted a preference for alternatives featuring high-uncertain rewards in PG [39,40].
Brain imaging analyses showed that, prior to choosing the “safe” option, controls exhibited higher neural activation in brain areas related to the formation of interoceptive representation (posterior insular cortex; [34]), and in cognitive control function (dorsolateral prefrontal cortex and middle frontal gyrus; [35]), as compared to prior choosing of the “bet” option. Activation observed within these brain areas might reflect high-risk sensitivity in control participants during the elaboration of “safe” decisions. More specifically, the activation of the insular cortex prior to safe choice observed in controls is consistent with several studies on decision-making under uncertainty, which observed an activation within the insula when anticipating both monetary loss [41,42] and gain [43–46]. Other studies have shown that the insula is sensitive to risk level [47] and triggered by excessive product price when deciding on whether or not to purchase an item [48]. In other words, insular activation might play a key role in representing somatic states that can be used to simulate the potential negative consequences of an action [34,49], such as when people reject unfair offers in an economic game at substantial cost to themselves [50]. By working together with brain regions involved in high-order cognitive processes, the insula can trigger bodily states, map bodily states, and represent the relationship between physiological changes and the environmental cues that elicited them [51].
By contrast, PG exhibited higher neural activation prior to choosing the “bet” option, and within brain areas involved in reward anticipation (caudate and putamen; [13]), and in the formation of interoceptive representation (posterior insular cortex; [34]). Higher activation in motivational-arousal brain areas during reward anticipation in PG may reflect higher saliency for gambling-like choices (i.e., the “bet” option) compared to safe choices. Importantly, between-groups contrasts revealed that, as compared to controls, PG showed increased neural activity within the striatum (putamen) prior to bet choices, as opposed to safe choices. These results are in line with findings obtained by Mield et al. [10], who observed enhanced activation in motivational-arousal brain networks in PG prior to taking high-risk choices. Moreover, we observed that, during reward anticipation of uncertain choices PG failed to increase activity of brain areas involved in high-order cognitive control, such as the dorsolateral prefrontal cortex and the middle frontal gyrus. As a whole, these findings point to the persistent motivational relevance of gambling-related behaviors and cues in PG. To a broader extent, the present results are in line with recent neurocognitive models of addiction [51–56] which advance that hyperactivity in motivational-arousal brain areas toward addiction-related behavior might disable the operation of a reflective high-order cognitive control system.
Before and after scanning, PG had a significantly higher average craving for gambling score than controls. Besides, after performing the Card-Deck experiment, gambling craving scores were increased in both groups: scores of PG increased for each three subscales of the GACS, whereas scores of controls increased for the anticipation and the desire subscales but not for the relief subscale. These results are consistent with theoretical understandings advancing that states of craving in addiction are characterized by the desire to encounter pleasant experiences superimposed on a negative affective sate [57]. Nevertheless, we did not find significant correlation between gambling-related craving (baseline score and mean change) and brain activation. Thus, we cannot objectively attest that brain activations observed in the present study were involved in the increase of gambling craving score. One possible explanation for this lack of association is that gambling-related craving was assessed a few minutes after that the participant had left the scanner, rather than immediately after each choice or each block of the Card-Deck task. Another option would have been to implement a careful measurement of autonomic arousal during performance of the Card-Deck task measurement during fMRI scanning [58].
4.3. Limitations, strengths and future directions
The experimental task used in the present study was not designed to examine neural activity related to choice expected value (probability of reward for the bet option multiplied by the amount of bet/safe options ratio). In addition, the sample sizes of our groups were modest. Hence, non-significant fMRI results (e.g., risk versus ambiguity contrasts within the PG group) observed in the present study must be interpreted with caution. Nevertheless, our final sample is similar to other fMRI studies investigating decision-making in pathological gambling, with samples ranging from ten to 16 subjects per group [9,10,15]. Furthermore, our cohort of PG was selected using stringent selection criteria, resulting in a rather homogeneous cohort with no psychiatric disorders other than gambling disorder. In addition, the current sample of PG was homogenous in terms of gambling game preferences (i.e., slot machines games). Indeed, subtypes of gambling habit (e.g., slot machine gamblers versus poker players) may be associated with distinct performance on several decision-making tasks [59]. Importantly, brain imaging results reported in this study also should be interpreted with caution due to the use of an uncorrected threshold (p < 0.001). Nevertheless, a similar uncorrected threshold was used in previous studies with the same decision-making task used in the present paper [24], and others studies that used slightly different decision-making paradigms [37,60]. In addition, based on several technical papers, the use of a p < 0.001 uncorrected threshold produces a desirable balance between Types I and II error rates, especially for tasks that allow for multiple cognitive solutions to a problem (such as the Card-Deck task used in the present study) and/or variability across trials in degree or timing of cognitive processing (e.g., tasks involving emotional regulation in response to a particular stimulus) [61,62]. Furthermore, based on Monte Carlo simulations, we used a stringent cluster size of 30 voxels in order to correct for the probability of false-positive at a threshold level that is equivalent to a corrected p < 0.05. Another limitation of the present study is the absence of feedback after participants make a choice, which may have affected the monetary anticipation process. Nevertheless, similarly as in Hsu et al. [24], participants were made aware that one trial would be chosen at random after the scanning session, and that the outcome on that trial would determine their final pay. Moreover, by adopting this type of design, neural activity related to the assessment of a given decision remained uninfluenced by outcomes of previous trials (i.e., absence of between-trials collinearity effect).
5. Conclusion
In this brain imaging study, we observed that providing information on probability associated with high-uncertain monetary rewards did not moderate brain activation in a sample of individuals with gambling disorder. In addition, pathological gamblers exhibited increased striatal activation during the elaboration of “bet” choices, when compared to “safe” choices. These findings might reflect (i) a lack of sensitivity to uncertainty and (ii) a cue-induced signal increase toward the anticipation of high-uncertain monetary rewards in gambling disorder, which may be a moderator of the greatest importance to gamble “reasonably”.
Acknowledgments
This study was funded by the Belgian National Fund for Scientific Research Belgium (FC 80978, 2009–2013) and by a grant from the Belgian National Lottery to the Laboratory of Medical Psychology and Addictology, Université Libre de Bruxelles. We thank Erika Wauthia for her help in the recruitment of pathological gamblers.
Footnotes
Conflict of interest statement: All authors report no biomedical financial interests or potential conflicts of interest.
Contributor Information
Damien Brevers, Email: dbrevers@ulb.ac.be, brevers@usc.edu.
Antoine Bechara, Email: bechara@usc.edu.
Laurent Hermoye, Email: hermoye@imagilys.com.
Luisa Divano, Email: luisa.divano@chu-brugmann.be.
Charles Kornreich, Email: charles.kornreich@chu-brugmann.be.
Paul Verbanck, Email: paul.verbanck@chu-brugmann.be.
Xavier Noël, Email: xnoel@ulb.ac.be.
References
- 1.American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 5th. Arlington, VA: American Psychiatric Publishing; 2013. [Google Scholar]
- 2.Brevers D, Noël X. Pathological gambling and the loss of willpower: aneurocognitive perspective. Socioaffect Neurosci Psychol. 2013;3:21592. doi: 10.3402/snp.v3i0.21592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brewer JA, Potenza MN. The neurobiology and genetics of impulse control disorders: relationships to drug addictions. Biochem Pharmacol. 2008;75:63–75. doi: 10.1016/j.bcp.2007.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Everitt BJ, Dickinson AD, Robbins TW. The neuropsychological basis of addictive behaviour. Brain Res Rev. 2001;36:129–38. doi: 10.1016/s0165-0173(01)00088-1. [DOI] [PubMed] [Google Scholar]
- 5.Belin D, Everitt BJ. Cocaine seeking habits depend upon dopamine dependent serial connectivity linking the ventral with the dorsal striatum. Neuron. 2008;57:432–41. doi: 10.1016/j.neuron.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 6.Everitt BJ, Robbins TW. Neural systems of reinforcement for drug addiction: from actions to habits to compulsion. Nature Neurosci. 2005;8:1481–9. doi: 10.1038/nn1579. [DOI] [PubMed] [Google Scholar]
- 7.Graybiel AM. Habits, rituals and the evaluative brain. Annu Rev Neurosci. 2008;31:359–87. doi: 10.1146/annurev.neuro.29.051605.112851. [DOI] [PubMed] [Google Scholar]
- 8.Joutsa J, Johansson J, Niemelä S, Ollikainen A, Hirvonen MM, Piepponen P, et al. Mesolimbic dopamine release is linked to symptom severity in pathological gambling. NeuroImage. 2012;60:1992–9. doi: 10.1016/j.neuroimage.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 9.Miedl SF, Fehr T, Meyer G, Herrmann M. Neurobiological correlates of problem gambling in a quasi-realistic blackjack scenario as revealed by fMRI. Psychiatry Res. 2010;181:165–73. doi: 10.1016/j.pscychresns.2009.11.008. [DOI] [PubMed] [Google Scholar]
- 10.van Holst RJ, Veltman DJ, Büchel C, Van den Brink W, Goudriaan AE. Distorted expectancy coding in problem gambling: is the addictive in the anticipation? Biol Psychiatry. 2012;71:741–8. doi: 10.1016/j.biopsych.2011.12.030. [DOI] [PubMed] [Google Scholar]
- 11.Rolls ET, Grabenhorst F. The orbitofrontal cortex and beyond: from affect to decision-making. Prog Neurobiol. 2008;86:216–44. doi: 10.1016/j.pneurobio.2008.09.001. [DOI] [PubMed] [Google Scholar]
- 12.Sewards TV, Sewards MA. Representations of motivational drives in mesial cortex, medial thalamus, hypothalamus and midbrain. Brain Res Bull. 2003;61:25–49. doi: 10.1016/s0361-9230(03)00069-8. [DOI] [PubMed] [Google Scholar]
- 13.Haruno M, Kawato M. Different neural correlates of reward expectation and reward expectation error in the putamen and caudate nucleus during stimulus-action-reward association learning. J Neurophysiol. 2006;95:948–59. doi: 10.1152/jn.00382.2005. [DOI] [PubMed] [Google Scholar]
- 14.de Ruiter MB, Oosterlaan J, Veltman DJ, Van den Brink W, Goudriaan AE. Similar hyporesponsiveness of the dorsomedial prefrontal cortex in problem gamblers and heavy smokers during an inhibitory control task. Drug Alcohol Depend. 2012;121:81–9. doi: 10.1016/j.drugalcdep.2011.08.010. [DOI] [PubMed] [Google Scholar]
- 15.Reuter J, Raedler T, Rose M, Hand I, Gläscher J, Büchel C. Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neurosci. 2005;8:147–8. doi: 10.1038/nn1378. [DOI] [PubMed] [Google Scholar]
- 16.Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and non-gambling substance users during decision-making. Hum Brain Mapp. 2007;28(12):1276–86. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hewig J, Kretschmer N, Trippe RH, Hecht H, Coles MH, Holroyd CB, et al. Hypersensitivity to reward in problem gamblers. Biol Psychiatry. 2010;67:781–3. doi: 10.1016/j.biopsych.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 18.Balodis IM, Kober H, Worhunsky PD, Stevens MC, Pearlson GD, Potenza MN. Diminished fronto-striatal activity during processing of monetary rewards and losses in pathological gambling. Biol Psychiatry. 2012;71:749–57. doi: 10.1016/j.biopsych.2012.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Choi JS, Shin YC, Jung WH, Jang JH, Kang DH, Choi CH, et al. Altered brain activity during reward anticipation in pathological gambling and obsessive-compulsive disorder. PLos One. 2012;7:e45938. doi: 10.1371/journal.pone.0045938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Leyton M, Vezina P. On cue: striatal ups and downs in addictions. Biol Psychiatry. 2012;72:e21–2. doi: 10.1016/j.biopsych.2012.04.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Limbrick-Oldfield EH, van Holst RJ, Clark L. Fronto-striatal dysregulation in drug addiction and pathological gambling: consistent inconsistencies? Neuroimage Clin. 2013;2:385–93. doi: 10.1016/j.nicl.2013.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.van Holst RJ, Veltman DJ, van den Brink W, Goudriaan AE. Right on cue? Striatal reactivity in problem gamblers. Biol Psychiatry. 2012;72:e23–4. doi: 10.1016/j.biopsych.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 23.Potenza M. The neural bases of cognitive processes in gambling disorder. Trends Cogn Sci. 2014;18:429–38. doi: 10.1016/j.tics.2014.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hsu M, Bhatt M, Adolphs R, Tranel D, Camerer CF. Neural systems responding to degrees of uncertainty in human decision-making. Science. 2005;310:1680–3. doi: 10.1126/science.1115327. [DOI] [PubMed] [Google Scholar]
- 25.Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–63. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- 26.Lesieur HR, Blume SB. The South Oaks Gambling Screen (SOGS): a new instrument for the identification of pathological gamblers. Am J Psychiatry. 1987;144:1184–8. doi: 10.1176/ajp.144.9.1184. [DOI] [PubMed] [Google Scholar]
- 27.McLellan AT, Kushner H, Metzger D, Peters R, Smith I, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 28.Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–71. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- 29.Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the alcohol use disorders identification test (AUDIT): WHO collaborative project on early detection of persons with harmful alcohol consumption-II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- 30.Heishman SJ. What aspects of human performance are truly enhanced by nicotine? Addiction. 1998;93:317–20. doi: 10.1080/09652149835864. [DOI] [PubMed] [Google Scholar]
- 31.Young MM, Wohl MJ. The gambling craving scale: psychometric validation and behavioral outcomes. Psychol Addictive Behav. 2009;23:512–22. doi: 10.1037/a0015043. [DOI] [PubMed] [Google Scholar]
- 32.Talairach J, Tournoux P. Co-planar stereotactic atlas of the human brain. Stuttgart/New York: Thieme; 1988. [Google Scholar]
- 33.Cox RW. AFNI: software for analysis and visualization of functional magnetic resonance neuroimages. Comput Biomed Res. 1996;29:162–73. doi: 10.1006/cbmr.1996.0014. [DOI] [PubMed] [Google Scholar]
- 34.Craig AD. How do you feel? Interoception: the sense of the physiological condition of the body. Nature Rev Neurosci. 2002;3:655–66. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- 35.Tanji J, Hoshi E. Role of the lateral prefrontal cortex in executive behavioral control. Physiol Rev. 2008;88:37–57. doi: 10.1152/physrev.00014.2007. [DOI] [PubMed] [Google Scholar]
- 36.Ellsberg D. Risk, ambiguity, and the savage axioms. Quart J Econ. 1961;75:643–69. [Google Scholar]
- 37.Huettel SA, Stowe CJ, Gordon EM, Warner BT, Platt ML. Neural signatures of economic preferences for risk and ambiguity. Neuron. 2006;49:765–75. doi: 10.1016/j.neuron.2006.01.024. [DOI] [PubMed] [Google Scholar]
- 38.Arimura N, Nkayama Y, Yamagata T, Tanji J, Hoshi E. Involvement of the globus pallidus in behavioral goal determination and action specification. J Neurosci. 2013;33:13639–53. doi: 10.1523/JNEUROSCI.1620-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brevers D, Cleeremans A, Goudriaan AE, Bechara A, Kornreich C, Verbanck P, et al. Decision making under ambiguity but not under risk is related to problem gambling severity. Psychiatry Res. 2012;200:558–74. doi: 10.1016/j.psychres.2012.03.053. [DOI] [PubMed] [Google Scholar]
- 40.Brevers D, Cleeremans A, Bechara A, Greisen M, Kornreich C, Verbanck P, et al. Impaired self-awareness in pathological gamblers. J Gambling Stud. 2013;29:119–29. doi: 10.1007/s10899-012-9292-2. [DOI] [PubMed] [Google Scholar]
- 41.Paulus MP, Rogalsky C, Simmons A, Feinstein JS, Stein MB. Increased activation in the right insula during risk-taking decision making is related to harm avoidance and neuroticism. NeuroImage. 2003;19:1439–48. doi: 10.1016/s1053-8119(03)00251-9. [DOI] [PubMed] [Google Scholar]
- 42.Samanez-Larkin GR, Hollon NG, Carstensen LL, Knutson B. Individual differences in insular sensitivity during loss anticipation predict avoidance learning. Psychol Sci. 2008;19:320–3. doi: 10.1111/j.1467-9280.2008.02087.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Clark L, Lawrence AJ, Astley-Jones F, Gray N. Gambling near-misses enhance motivation to gamble and recruit win-related brain circuitry. Neuron. 2009;61:481–90. doi: 10.1016/j.neuron.2008.12.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Delgado MR, Nystrom LE, Fissell C, Noll DC, Fiez JA. Tracking the hemodynamic responses to reward and punishment in the striatum. J Neurophysiol. 2000;84:3072–7. doi: 10.1152/jn.2000.84.6.3072. [DOI] [PubMed] [Google Scholar]
- 45.Elliott R, Friston KJ, Dolan RJ. Dissociable neural responses in human reward systems. J Neurosci. 2000;20:6159–65. doi: 10.1523/JNEUROSCI.20-16-06159.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Izuma K, Saito DN, Sadato N. Processing of social and monetary rewards in the human striatum. Neuron. 2008;58:284–94. doi: 10.1016/j.neuron.2008.03.020. [DOI] [PubMed] [Google Scholar]
- 47.Xue G, Lu Z, Levin IP, Bechara A. The impact of prior risk experiences on subsequent risky decision-making: the role of the insula. NeuroImage. 2010;50:709–16. doi: 10.1016/j.neuroimage.2009.12.097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Knutson B, Rick S, Wimmer GE, Prelec D, Loewenstein G. Neural predictors of purchases. Neuron. 2007;53:147–56. doi: 10.1016/j.neuron.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Damasio AR. The somatic marker hypothesis and the possible functions of the prefrontal cortex. Philos Trans Royal Soc Lond. 1996;351:1413–20. doi: 10.1098/rstb.1996.0125. [DOI] [PubMed] [Google Scholar]
- 50.Sanfey AG, Rilling JK, Aronson JA, Nystrom LE, Cohen JD. The neural basis of economic decision-making in the Ultimatum Game. Science. 2003;300:1755–8. doi: 10.1126/science.1082976. [DOI] [PubMed] [Google Scholar]
- 51.Naqvi NH, Bechara A. The hidden island of addiction: the insula. Trends Neurosci. 2009;32:56–67. doi: 10.1016/j.tins.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Naqvi NH, Bechara A. The insula and drug addiction: an interoceptive view of pleasure, urges, and decision-making. Brain Struct Funct. 2010;214:435–50. doi: 10.1007/s00429-010-0268-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Naqvi NH, Bechara A. The insula: a critical neural substrate for craving and drug seeking under conflict and risk. Ann NY Acad Sci. 2014;1316:53–70. doi: 10.1111/nyas.12415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Verdejo-Garcia A, Bechara A. A somatic marker theory of addiction. Neuropharmacology. 2009;56:48–62. doi: 10.1016/j.neuropharm.2008.07.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Noel X, Brevers D, Bechara A. A neurocognitive approach to understanding the neurobiology of addiction. Curr Opin Neurobiol. 2013;23:632–8. doi: 10.1016/j.conb.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Noël X, Brevers D, Bechara A. A triadic neurocognitive approach to addiction for clinical interventions. Front Psychiatry. 2013;4:179. doi: 10.3389/fpsyt.2013.00179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.May J, Andrade J, Kavanagh DJ, Feeney GF, Gullo MJ, Statham DJ, et al. The craving experience questionnaire: a brief, theory-based measure of consummatory desire and craving. 2014;109:728–35. doi: 10.1111/add.12472. [DOI] [PubMed] [Google Scholar]
- 58.Wong SW, Xue G, Bechara A. Integrating fMRI with psychophysiological measurements in the study of decision-making. J Neurosci Psychol Econ. 2011;4:85–94. doi: 10.1037/a0023525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goudriaan AE, Oosterlaan J, de Beurs E, van den Brink W. Decision making in pathological gambling: a comparison between pathological gamblers, alcohol dependents, persons with Tourette syndrome, and normal controls. Cogn Brain Res. 2005;23:137–51. doi: 10.1016/j.cogbrainres.2005.01.017. [DOI] [PubMed] [Google Scholar]
- 60.Bach DR, Seymour B, Dolan RJ. Neural activity associated with the passive prediction of ambiguity and risk for aversive events. J Neurosci. 2009;29:1648–56. doi: 10.1523/JNEUROSCI.4578-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Liebermann MD, Cunningam WA. Type I and type II error concerns in fMRI research: re-balancing the scale. SCAN. 2009;4:423–8. doi: 10.1093/scan/nsp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Woo CW, Krishnan A, Wager D. Cluster-extent based thresholding in fMRI analyses: pitfalls and recommendations. NeuroImage. 2014;91:412–9. doi: 10.1016/j.neuroimage.2013.12.058. [DOI] [PMC free article] [PubMed] [Google Scholar]


