Abstract
IMPORTANCE
Many preschool children develop recurrent, severe episodes of lower respiratory tract illness (LRTI). Although viral infections are often present, bacteria may also contribute to illness pathogenesis. Strategies that effectively attenuate such episodes are needed.
OBJECTIVE
To evaluate if early administration of azithromycin, started prior to the onset of severe LRTI symptoms, in preschool children with recurrent severe LRTIs can prevent the progression of these episodes.
DESIGN, SETTING, AND PARTICIPANTS
A randomized, double-blind, placebo-controlled, parallel-group trial conducted across 9 academic US medical centers in the National Heart, Lung, and Blood Institute’s AsthmaNet network, with enrollment starting in April 2011 and follow-up complete by December 2014. Participants were 607 children aged 12 through 71 months with histories of recurrent, severe LRTIs and minimal day-to-day impairment.
INTERVENTION
Participants were randomly assigned to receive azithromycin (12 mg/kg/d for 5 days; n = 307) or matching placebo (n = 300), started early during each predefined RTI (child’s signs or symptoms prior to development of LRTI), based on individualized action plans, over a 12-through 18-month period.
MAIN OUTCOMES AND MEASURES
The primary outcome measure was the number of RTIs not progressing to a severe LRTI, measured at the level of the RTI, that would in clinical practice trigger the prescription of oral corticosteroids. Presence of azithromycin-resistant organisms in oropharyngeal samples, along with adverse events, were among the secondary outcome measures.
RESULTS
A total of 937 treated RTIs (azithromycin group, 473; placebo group, 464) were experienced by 443 children (azithromycin group, 223; placebo group, 220), including 92 severe LRTIs (azithromycin group, 35; placebo group, 57). Azithromycin significantly reduced the risk of progressing to severe LRTI relative to placebo (hazard ratio, 0.64 [95% CI, 0.41-0.98], P = .04; absolute risk for first RTI: 0.05 for azithromycin, 0.08 for placebo; risk difference, 0.03 [95% CI, 0.00-0.06]). Induction of azithromycin-resistant organisms and adverse events were infrequently observed.
CONCLUSIONS AND RELEVANCE
Among young children with histories of recurrent severe LRTIs, the use of azithromycin early during an apparent RTI compared with placebo reduced the likelihood of severe LRTI. More information is needed on the development of antibiotic-resistant pathogens with this strategy.
Acute episodes of severe lower respiratory tract illness (LRTI) are common among preschoolers, and up to 14% to 26% of preschoolers present with recurrent wheezing during the first 6 years of life.1,2 These severe episodes are often associated with substantial morbidity, resulting in unscheduled visits to physician offices, urgent care, and emergency departments. Many of these young children are diagnosed with asthma, and among them, 20.9% seek emergency department care and 6.5% are hospitalized each year.3 Thus, identification of novel treatment approaches that attenuate the severity of these recurrent episodes would provide substantial benefit to preschool children with recurrent severe LRTI.
The etiology of these acute episodes has not been completely elucidated. Although initial reports showed frequent detection of respiratory viruses in nasopharyngeal secretions obtained during episodes of wheezing in preschoolers,4,5 bacteria are also often present during such episodes.6 In children with asthma aged 4 through 12 years, isolation of Streptococcus pneumoniae or Moraxella catarrhalis from nasal samples also containing rhinovirus was associated with increased likelihood of having an asthma exacerbation.7 The ketolide antibiotic telithromycin, when started within the first 24 hours of acute asthma episodes, significantly improved symptom scores and lung function relative to placebo and unrelated to bacteriologic status,8 suggesting a mechanism unrelated to direct antimicrobial action. These findings are compatible with the decrease in neutrophilic inflammation observed in patients with severe asthma treated with the macrolide clarithromycin9 and higher neutrophil counts and rates of isolation of pathogenic bacteria in bronchoalveolar lavage fluid in recurrently wheezing preschool children.10 The primary chemoattractant for neutrophils is interleukin 8 (IL-8), and it has been shown that a polymorphism in the IL-8 gene, rs4073, modulates IL-8 production.11
Based upon these findings, we conducted a randomized clinical trial of early administration of the macrolide azithromycin, started early in the course of an RTI and prior to the onset of severe LRTI symptoms, in preschool children with recurrent severe LRTIs to determine if this intervention can safely prevent the progression of such episodes.
Methods
Participants
The full protocol and statistical analysis plan are available in Supplement 1. The institutional review board at each center approved and monitored the study. Parents or guardians provided written informed consent. Participants received compensation for time and travel expenses. Details about inclusion and exclusion criteria are provided in Supplement 2. Briefly, eligible participants were children aged 12 through 71 months with recurrent severe wheezing in the context of clinically significant LRTIs that required systemic corticosteroids, an unscheduled physician office visit, an urgent or emergency department visit, or hospitalization. Exclusion criteria included more than 4 courses of systemic corticosteroids or more than 1 hospitalization in the past 12 months, or use of long-term controllers for asthma for more than 8 months in the past 12 months. These criteria excluded children with more severe disease who require daily controller medication. Children receiving monotherapy with asthma controllers (either low-dose inhaled corticosteroids or montelukast) at enrollment were eligible but had their controller discontinued upon study entry, consistent with recommendations for step-down therapy. Children with significant symptomatic asthma and those with inadequate adherence to diary card completion (<80% of days) during the 2 to 4 week run-in period (defined in Supplement 2) were also excluded. Race was assessed by parent/guardian report, using National Institutes of Health race/ethnicity reporting standards and categories.
A participant was classified as having a positive modified asthma predictive index (API) if the participant had experienced at least 4 wheezing episodes in the past year and had 1 major criterion (physician-diagnosed atopic dermatitis, parental history of asthma, or allergic sensitization to ≥1 aeroallergen) or 2 minor criteria (wheezing unrelated to colds, blood eosinophils ≥4%, or allergic sensitization to milk, eggs, or peanuts).12
Study Design and Treatment
The study was a double-blind, parallel-group trial (eFigure 1 in Supplement 2), in which participants were randomized in a 1:1 ratio to receive either oral azithromycin (12 mg/kg once daily for 5 days) or matching placebo. Parents or guardians were provided with an individualized care plan, developed with the study team, that instructed starting study therapy as soon as participants developed the symptoms or signs that parents or guardians defined as the child’s usual starting point before the development of a severe LRTI.13-15 During RTIs, all participants received albuterol inhalation treatments 4 times daily while awake for the first 48 hours as well as whenever needed at any time during the RTI.13-15
Computer-generated randomization was implemented via a secure web-based randomization module at the Data Coordinating Center and stratified by clinical center and age (12-41 months or 42-71 months), with treatment assignments made in random permuted blocks of size 4.
The trial began in April 2011 with a follow-up period of 52 weeks, and with study treatment used during a maximum of 3 treated RTIs not progressing to severe LRTI. In June 2012, based upon a prespecified interval assessment that demonstrated a lower than expected RTI rate, the follow-up period was extended to 78 weeks for those participating in the study at that time (n = 164) or enrolled thereafter (n = 292) and allowed for use of study treatment during a maximum of 4 treated RTIs.
Antibiotic Resistance, Viral Detection, and IL-8 rs4073 Genotyping
To assess the effect of azithromycin therapy on colonization with antimicrobial-resistant bacteria, screening cultures were performed on deep oropharyngeal swab samples (see eMethods in Supplement 2) from participants at a single site (St Louis) at randomization (n = 86) and at study completion at least 14 days after the final dose of study medication (n = 81). Samples were inoculated onto sheep’s blood agar containing 2 μg/mL of azithromycin (Remel) incubated at 35°C in 5% carbon dioxide, and evaluated after 18 to 24 hours. The absence or presence of normal upper respiratory tract flora was assessed, and pathogenic organisms were isolated and identified. Susceptibility testing was performed on pathogenic bacteria using disk diffusion for azithromycin, erythromycin, clindamycin, clarithromycin, and cefoxitin (to assess for Staphylococcus aureus).
Nasal secretions were collected by direct “nasal blow technique” or nasal swab at scheduled visits at randomization and during each treated RTI at home by a trained parent or guardian. Samples were frozen for later analysis for respiratory virus detection by polymerase chain reaction-based diagnostic assays.16 This assay detects the following viruses: rhinovirus or enteroviruses (not distinguishable by this assay); coronaviruses; adenoviruses B, C, and E; influenza A and B; parainfluenza viruses I-IV; respiratory syncytial virus A and B; metapneumovirus; and bocavirus.
Participants were genotyped for the IL-8 rs4073 single-nucleotide polymorphism by polymerase chain reaction amplification of the region containing the polymorphism and selective restriction endonuclease digestion (restriction fragment-length polymorphism). Further details are provided in Supplement 2.
Outcome Measures
The primary outcome measure was the number of treated RTIs not progressing to severe LRTI among participants experiencing at least 1 treated RTI, which was based on a predefined level of parent/guardian-reported symptom severity that triggered the prescription of an additional rescue intervention. Parents or guardians were instructed to call the clinical center if any of the following levels of symptoms that represented a severe LRTI occurred: (1) having symptoms that were more than mild after 3 albuterol administrations over 1 hour, (2) requiring albuterol administrations more often than once every 4 hours, (3) requiring more than 6 albuterol treatments over a 24-hour period, or (4) having moderate to severe cough or wheeze for 5 or more days since study medication was initiated. If the study physician concurred that the patient was experiencing this degree of symptomatology, the primary end point was reached. Early termination status was assigned if the child developed any of the following prior to, or on the same day as, initiating study medication according to the individualized care plan: (1) presence of predefined severe respiratory symptoms requiring emergent care, (2) need for open-label systemic corticosteroids, (3) symptoms consistent with uncontrolled persistent asthma, or (4) withdrawal from the study by physician discretion for respiratory-related problems (see Supplement 2 for further details). Episodes culminating in early termination were not considered to have met the primary outcome. Study participation was completed after the first severe LRTI or after assignment of early termination status, in which case the primary end point was not reached. An RTI was deemed not to have progressed to severe LRTI if a severe RTI did not occur within 14 days of initiating study medication. Dropout status was assigned if the participant withdrew consent, was withdrawn from the study by physician discretion for nonrespiratory-related reasons or was lost to follow-up. Secondary prespecified outcome measures included numbers of urgent care visits, emergency department visits, and hospitalizations. Measures of disease impairment reflected by symptom severity and albuterol use during treated RTI were assessed through parental completion of the validated Preschool Asthma Diary17 daily during treated RTIs, beginning on the first day of each illness and continuing until all symptoms had resolved for 2 consecutive days. We also prespecified comparisons of parent-reported, drug-related adverse effects and development of azithromycin-resistant pathogens.
Statistical Analysis
The primary outcome, number of treated RTIs not progressing to severe LRTI, is similar to familiar “time to event” outcomes except that time is measured discretely by the occurrence of RTIs rather than by the passage of time. The primary analysis tested the null hypothesis that azithromycin and placebo do not differ using a discrete-time proportional hazards regression model including the following covariates: study site, age at randomization (12-42 months vs 43-71 months), modified API18 status, season during which the RTI occurred (a time-dependent covariate), and whether the child enrolled before or after the study was extended to 78 weeks. The primary outcome was considered to be right-censored if the participant dropped out or reached the end of follow-up prior to experiencing a fourth treated RTI. The proportional hazards assumption was assessed by comparing the goodness-of-fit of the proportional hazards model with the goodness-of-fit of the nonproportional hazards model using the Akaike information criterion.19
Secondary outcomes of health care utilization and respiratory-related symptoms during RTIs were explored using repeated measures analysis of variance with compound symmetry to account for multiple treated RTIs in the same individual. RTIs during which symptom data were missing (9% of RTIs) were not included in the analysis. Treatment-effect modification was explored in prespecified subgroups of participants, defined by age at randomization, sex, modified API status, presence of viral infection during RTI, season during which the RTI occurred, and IL-8 rs4073 genotype.11 The statistical significance of possible differences between subgroups in the treatment effect was tested with interaction terms. No adjustments for multiple tests were made. To assess if azithromycin decreased the frequency of subsequent RTIs, time to second RTI was compared between study groups using Kaplan-Meier curves and the log-rank test. All tests were 2-sided and based on a significance criterion of a P value less than .05. All analyses were performed with the use of SAS (SAS Institute), version 9.3.
The target sample size of 600 children (300 per treatment group) was chosen so that the study would have power of at least 0.9 with 2-sided α of .05 if the hazard ratio (HR) for progressing to severe LRTI with azithromycin compared with placebo was 0.65 or smaller. These calculations assumed an average of 2.75 RTI per child-year with overall dropout rate of 33%. A prespecified interim feasibility analysis conducted when 50% of the children had completed 6 months of follow-up revealed that the average RTI rate per child-year was close to 2.0 and that the power for a hazard rate of 0.65 would be approximately 0.80. The data and safety monitoring board and AsthmaNet Steering Committee approved continuation of the trial.
Results
Participants
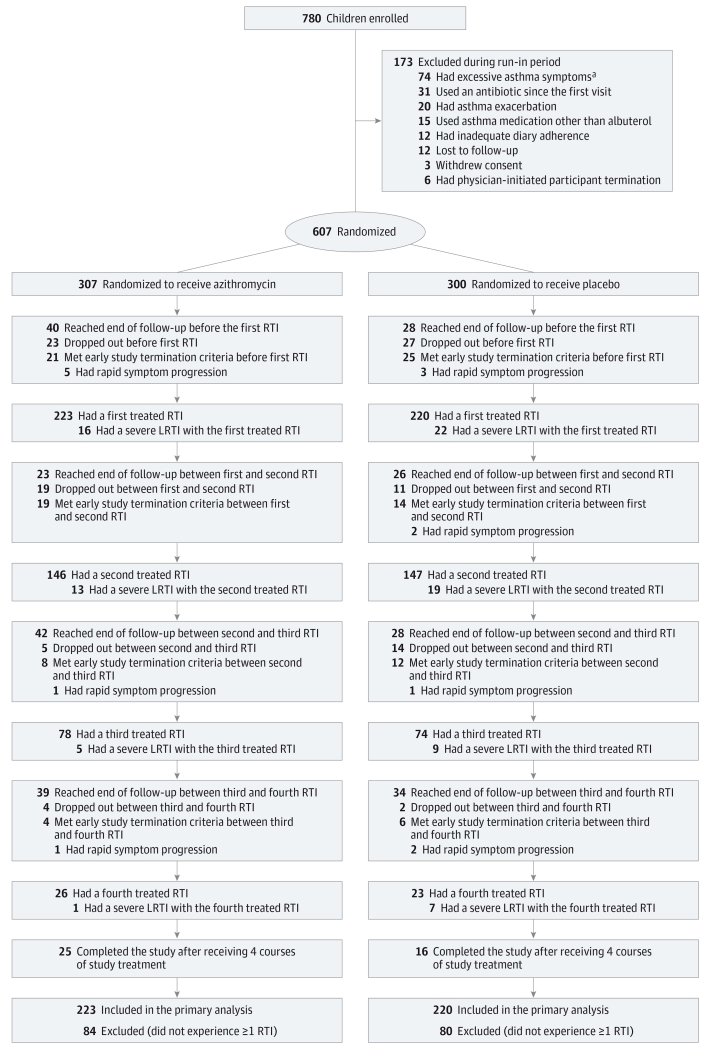
Of 780 participants enrolled, 607 underwent randomization: 307 participants to the azithromycin group and 300 participants to the placebo group (Figure 1). Baseline demographic and clinical characteristics for all 607 randomized participants and for the subgroup that experienced at least 1 treated RTI are shown in Table 1 and Table 2. Among participants who experienced at least 1 treated RTI and were included in the primary analysis, treatment groups were comparable except for a greater proportion with day-care attendance in the azithromycin group. Atopy was common, with 39.1% demonstrating allergic sensitization to at least 1 allergen and 46.8% meeting criteria for being at high risk for asthma by virtue of a positive modified API.18 At baseline, any nasal virus was detected among 261 of 588 randomized participants (44.4%), 188 of 432 participants with at least 1 treated RTI (43.5%), 73 of 156 participants with no RTI (46.8%), 99 of 220 participants receiving azithromycin with at least 1 treated RTI (45.0%), and 89 of 212 participants receiving placebo with at least 1 treated RTI (42.0%).
Figure 1. Flow Diagram of the Study Enrollment and Outcomes for Preschool Children With a History of Respiratory Tract Illness.
LRTI indicates lower respiratory tract illness. No information was recorded on potential participants screened but not enrolled.
a Presence of excessive asthma symptoms was defined as, on average, more than 4 days per week or more than 1 nighttime awakening requiring albuterol during the 2-week run-in period (for controller naïve children) or during the latter 2 weeks of a 4 week run-in period (for children receiving low-dose inhaled corticosteroids or montelukast monotherapy at enrollment).
Table 1. Demographic Characteristics of Study Participants.
| All Randomized Participants (N = 607) |
Participants With at Least 1 Treated RTI (n = 443) |
Participants With No RTI (n = 164) |
Participants With at Least 1 Treated RTI | ||
|---|---|---|---|---|---|
| Azithromycin (n = 223) |
Placebo (n = 220) |
||||
| Demographics, No. (%) | |||||
| Age at enrollment, mean (SD), mo | 41.5 (16.5) | 41.4 (16.5) | 41.81 (16.27) | 42.5 (16.4) | 40.2 (16.6) |
| 12-42 | 327 (53.9) | 241 (54.4) | 86 (52.4) | 115 (51.6) | 126 (57.3) |
| 43-71 | 280 (46.1) | 202 (45.6) | 78 (47.6) | 108 (48.4) | 94 (42.7) |
| Boys | 365 (60.1) | 274 (61.9) | 91 (55.)5 | 139 (62.3) | 135 (61.4) |
| Entered study on controller medication | 48 (7.9) | 41 (9.3) | 7 (4.3) | 25 (11.2) | 16 (7.3) |
| Race | |||||
| American Indian or Alaskan Native | 8 (1.3) | 7 (1.6) | 1 (0.6) | 3 (1.3) | 4 (1.8) |
| Asian | 10 (1.6) | 7 (1.6) | 3 (1.8) | 4 (1.8) | 3 (1.4) |
| Black or African American | 157 (25.9) | 89 (20.1) | 68 (41.5) | 47 (21.1) | 42 (19.1) |
| White | 362 (59.6) | 290 (65.5) | 72 (43.9) | 141 (62.3) | 149 (67.7) |
| More than 1 race specified | 70 (11.5) | 50 (11.3) | 20 (12.2) | 28 (12.6) | 22 (10.0) |
| Ethnicity | |||||
| Hispanic or Latino | 183 (30.1) | 130 (29.3) | 53 (32.3) | 63 (28.3) | 67 (30.5) |
| Not Hispanic or Latino | 424 (69.9) | 313 (70.7) | 111 (67.7) | 160 (71.7) | 153 (69.5) |
| Height, mean (SD), cm | 98.5 (11.7) | 98.3 (11.7) | 98.87 (11.67) | 99.2 (11.4) | 97.4 (12.1) |
| Weight, mean (SD), kg | 16.8 (4.6) | 16.8 (4.6) | 17.0 (4.6) | 17.1 (4.5) | 16.4 (4.6) |
Abbreviation: RTI, respiratory tract illness.
Table 2. Characteristics of Study Participants.
| All Randomized Participants (N = 607) |
Participants With at Least 1 Treated RTI (n = 443) |
Participants With No RTI (n = 164) |
Participants With at Least 1 Treated RTI | ||
|---|---|---|---|---|---|
| Azithromycin (n = 223) |
Placebo (n = 220) |
||||
| Exposures, No. (%) | |||||
| Day-care attendance | 307 (50.6) | 220 (49.7) | 87 (53.1) | 123 (55.2) | 97 (44.1) |
| Tobacco smoke exposure, No./total (%) | 240/601 (39.9) | 164/439 (37.4) | 76/162 (46.9) | 89/221 (40.3) | 75/218 (34.4) |
| Pet in home | 280 (46.1) | 228 (51.5) | 52 (31.7) | 117 (52.5) | 111 (50.5) |
| Feature of Previous Wheezing | |||||
| No. of wheezing episodes in the past year, mean (SD) |
4.45 (3.15) | 4.45 (3.14) | 4.45 (3.19) | 4.49 (3.41) | 4.41 (2.86) |
| No. of urgent and/or ED visits in the past year, mean (SD) |
2.48 (1.64) | 2.53 (1.71) | 2.34 (1.41) | 2.52 (1.72) | 2.54 (1.71) |
| Hospitalized in the past year, No. (%) | 87 (14.3) | 58 (13.1) | 29 (17.7) | 34 (15.2) | 24 (10.9) |
| No. of hospitalizations in the past year, median (IQR) |
0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) | 0 (0-1) |
| At least 1 course of OCS in past year, No. (%) | 361 (59.5) | 276 (62.3) | 85 (51.8) | 143 (64.1) | 133 (60.5) |
| No. of OCS courses in the past year, median (range) |
1 (0-4) | 1 (0-4) | 1 (0-4) | 1 (0-4) | 1 (0-4) |
| ICS use in past year, No./total (%) | 150/605 (24.8) | 126/441 (28.6) | 24/164 (14.6) | 70/223 (31.3) | 56/218 (25.7) |
| Montelukast use in the past year, No. (%) | 54 (8.9) | 48 (10.8) | 6 (3.7) | 25 (11.2) | 23 (10.5) |
| Symptom Burden During 14-d Run-in Period | |||||
| No. of days in run-in period, median (IQR) |
15 (14-19) | 15 (14-19) | 15 (14-19) | 15 (14-19) | 14 (14-19) |
| Percentage of asthma control days, mean (SD)a |
77.4 (24.1) | 76.6 (23.6) | 79.6 (25.3) | 76.3 (24.5) | 77.0 (22.6) |
| No. of asthma control days per week, mean (SD)a |
5.4 (1.7) | 5.4 (1.7) | 5.6 (1.8) | 5.3 (1.7) | 5.4 (1.6) |
| Percentage of nights with albuterol use, median (range) |
0 (0-43) | 0 (0-43) | 0 (0-36) | 0 (0-24) | 0 (0-43) |
| Percentage of days with albuterol use, median (range) |
0 (0-57) | 0 (0-57) | 0 (0-59) | 0 (0-57) | 0 (0-54) |
| Atopic Features | |||||
| Eczema, No./total (%) | 328/592 (55.4) | 242/430 (56.3) | 86/162 (53.1) | 112/215 (52.1) | 130/215 (60.5) |
| Allergic rhinitis, No./total (%) | 128/581 (22.0) | 100/420 (23.8) | 28/161 (17.4) | 52/211 (24.6) | 48/209 (23.0) |
| Parentalasthma, No./total(%) | 314/587 (53.5) | 222/428 (51.9) | 92/159 (57.9) | 114/213 (53.5) | 108/215 (50.2) |
| Physician-diagnosed asthma, No. (%) | 345 (56.8) | 248 (56.0) | 97 (59.2) | 127 (57.0) | 121 (55.0) |
| Positive modified API, No. (%)b | 284 (46.8) | 208 (47.0) | 76 (46.3) | 104 (46.6) | 104 (47.3) |
| Allergy | |||||
| Sensitivity to any allergen, No./total (%) |
314/596 (52.7) | 230/436 (52.8) | 84/160 (52.2) | 112/219 (51.1) | 118/217 (54.4) |
| No. of allergens (of 16), median (IQR) |
1 (0-3) | 1 (0-3) | 1 (0-3) | 1 (0-3) | 1 (0-3) |
| Sensitivity to ≥1 aeroallergen, No./total (%) |
233/596 (39.1) | 169/436 (38.8) | 64/160 (40.0) | 85/219 (38.8) | 84/217 (38.7) |
| No. of aeroallergens (of 13), median (IQR) |
0 (0-2) | 0 (0-2) | 0 (0-2) | 0 (0-2) | 0 (0-2) |
| Sensitivity to ≥1 food allergen, No./total (%) |
241/596 (40.4) | 179/436 (41.1) | 62/160 (38.8) | 82/219 (37.4) | 97/217 (44.7) |
| Immunoglobulin E, μg/L, median (IQR) | 122.6 (35-408.7) n = 550 |
117.1 (34.8-421.1) n = 400 |
130 (43.7-382.8) n = 150 |
132 (34.8-430.1) n = 201 |
102.5 (31-403) n = 199 |
| Peripheral blood eosinophils, median (IQR), % |
3 (2-5.6) n = 568 |
3 (2-5.3) n = 411 |
3 (2-5.8) n = 157 |
3 (2- 6) n = 205 |
3 (2-5) n = 206 |
| Eosinophils ≥4%, No./total (%) | 234/568 (41.2) | 166/411 (40.4) | 68/157 (43.3) | 85/205 (41.5) | 81/206 (39.3) |
| Study Duration When Enrolled, No. (%) | |||||
| 52 wk | 315 (51.9) | 238 (53.7) | 77 (46.9) | 117 (52.5) | 121 (55.0) |
| 78 wk | 292 (48.1) | 205 (46.3) | 87 (53.1) | 105 (47.5) | 99 (45.0) |
Abbreviations: API, asthma predictive index; ED, emergency department; ICS, inhaled corticosteroids; IQR, interquartile range; OCS, oral corticosteroids; RTI, respiratory tract illness.
An asthma control day was a day without asthma-related symptoms, medication use, or health care utilization.
A participant was classified as having a positive modified API if the individual had experienced at least 4 wheezing episodes in the past year and had1 major criterion (physician-diagnosed atopic dermatitis, parental history of asthma, or allergic sensitization to ≥1 aeroallergen) or 2 minor criteria (wheezing unrelated to colds, blood eosinophils ≥4%, or allergic sensitization to milk, eggs, or peanuts).12
Participant enrollment and disposition are shown in Figure 1. During the study, 164 participants did not experience a treated RTI (azithromycin group, 84; placebo group, 80). Compared with participants who experienced no treated RTI, participants who experienced at least 1 treated RTI were significantly more likely to be white, have lower rates of exposure to smoke or pet exposure, and higher rates of asthma controller (inhaled corticosteroids or montelukast) or oral corticosteroid use in the year prior to enrollment (Table 1). A total of 937 trial-defined RTIs occurred (azithromycin group, 473; placebo group, 464). During a first RTI, 443 participants were treated; during a second RTI, 293; during a 3rd RTI, 152; during a fourth RTI, 49, with comparable distributions of numbers of treated RTIs between treatment groups. Of the 937 RTIs included in the primary analysis, therapy was initiated 2 or more days after the start of the RTI in 21% of cases; 3 or more days after, 12% of cases; 4 or more days after, 7% of cases. Early termination criteria was met by 109 participants (azithromycin group, 52; placebo group, 57). There were 105 participants (azithromycin group, 51; placebo group, 54) who withdrew for other reasons or were lost to follow-up. The overall percentage of early termination and dropout was 35%.
Primary Outcome
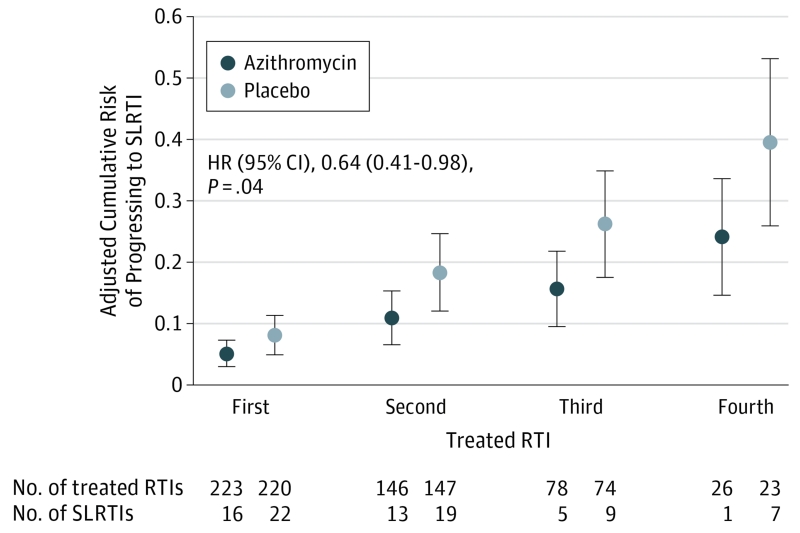
The azithromycin group experienced significantly lower risk of progressing to severe LRTI than the placebo group (HR, 0.64 [95% CI, 0.41-0.98], P = .04; absolute risk for first RTI: 0.05 for azithromycin, 0.08 for placebo; risk difference, 0.03 [95% CI, 0.00-0.06]), after adjustment for study site, age, modified API status,18 season during which the RTI occurred, and whether the child enrolled before or after the study was extended to 78 weeks (Figure 2). The cumulative risk for a severe LRTI over a maximum of 4 RTIs was 0.40 in the placebo group and 0.24 in the azithromycin group. The number needed to treat (NNT) to prevent 1 severe LRTI varied by the number of treated RTIs each child experienced, with the NNT decreasing with each subsequent treated RTI (NNT: for 1 RTI, 33; for 2 RTIs, 14; for 3 RTIs, 10; for 4 RTIs, 7). We examined if a delay in initiation of therapy was a potential treatment effect modifier in our model and found no significant results.
Figure 2. Cumulative Risk of Experiencing an Episode of Severe LRTI Across Treated RTIs for Preschool Children With a History of Severe LRTI.
RTI indicates respiratory tract illness; SLRTI, severe lower RTI. Shown are risks and 95% CIs based on the discrete-time proportional hazards model of treatment effect adjusted for clinical site, age, modified Asthma Predictive Index status, season during which the treated RTI occurred, and whether the child enrolled before or after the study was extended to 78 weeks.
Viral Detection
Viral pathogens were detected at randomization in 47% of children in the azithromycin group and 43% in the placebo group from whom nasal samples were collected (eTable 1A in Supplement 2). Nasal wash samples were obtained during 94% of all treated RTIs during the trial. Viral pathogens were detected during 83% of RTIs in the azithromycin group and 80% in the placebo group. Rhinovirus was the most commonly detected virus at randomization, during RTIs, and during severe LRTIs (eTable 1B in Supplement 2).
Subgroup Analyses
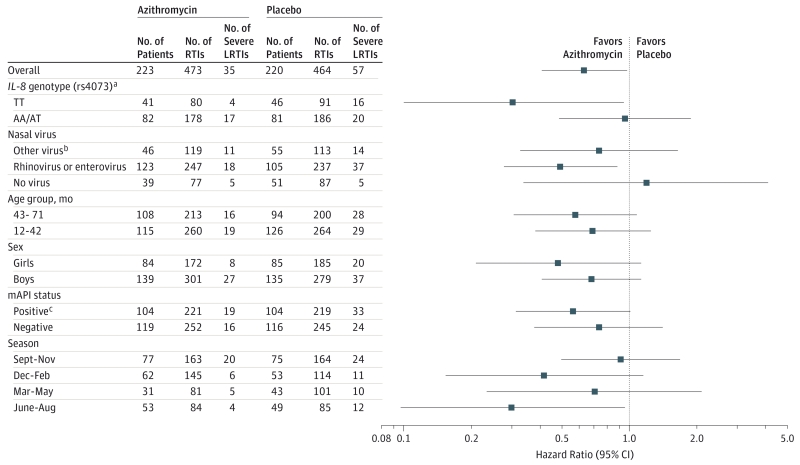
In prespecified subgroup analyses, we tested for the presence of an interaction between treatment group and age group (12-42 months vs 43-71 months at randomization), sex (boys vs girls), modified API status (positive vs negative), presence of virus detected during treated RTI (no virus detected vs rhinovirus or rhinovirus/enterovirus vs other virus), season during which the RTI occurred (September-November, June-August, March-May, and December-February), and IL-8 rs4073 genotype (TT vs AA/AT). No statistically significant interactions were demonstrated for any of these factors, indicating no significant differences in the HRs between subgroups (Figure 3).
Figure 3. Potential Treatment-Effect Differences in Prespecified Subgroups for Risk of an Episode of Severe LRTI Among Preschool Children With a History of Severe LRTI.
IL-8 indicates interleukin-8 gene; LRTI, lower respiratory tract illness; mAPI, modified Asthma Predictive Index. Estimates and CIs were obtained separate discrete-time proportional hazards models, each incorporating an interaction between treatment and the factor indicated on the y-axis. All models included adjustments for clinical site, age, modified API status, season during which the treated respiratory tract illness occurred, and whether the child enrolled before or after the study was extended to 78 weeks. No significant differences between treatment effects (blue circles) are noted between subgroups.
a IL-8 rs4073 genotype results shown include only Hispanic and non-Hispanic white participants with an adjustment for ethnicity.
b Other virus indicates coronaviruses; adenoviruses B, C, and E; influenza A and B; parainfluenza viruses I-IV; respiratory syncytial virus A and B; metapneumovirus; and bocavirus.
c A participant was classified as having a positive mAPI if the individual had experienced at least 4 wheezing episodes in the past year and had 1 major criterion (physician-diagnosed atopic dermatitis, parental history of asthma, or allergic sensitization to ≥1 aeroallergen) or 2 minor criteria (wheezing unrelated to colds, blood eosinophils ≥4%, or allergic sensitization to milk, eggs, or peanuts).12
Secondary Outcomes
Symptom Scores and Albuterol Use During Treated RTIs
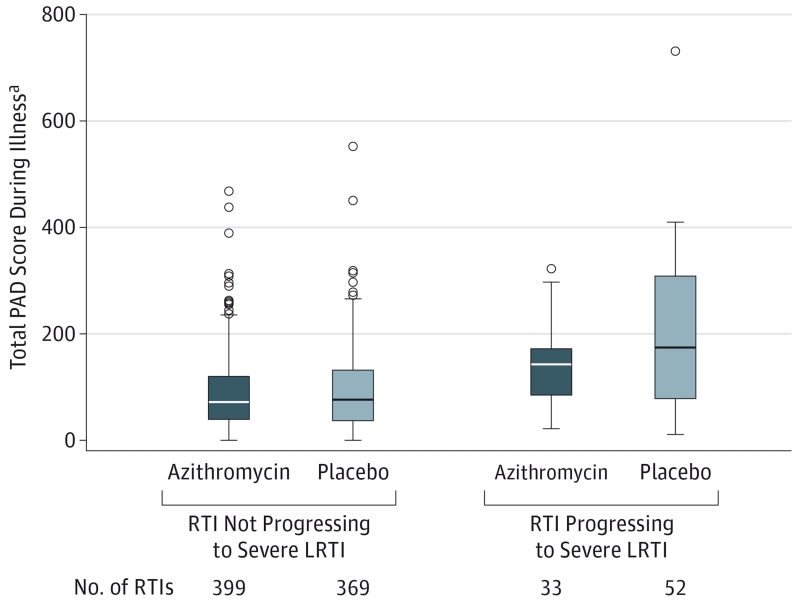
Azithromycin therapy decreased the overall severity of symptoms during severe LRTIs compared with placebo, as reflected by lower mean total symptom scores over the duration of RTI, but not during episodes not progressing to severe LRTI (Figure 4). Total albuterol use during treated RTIs did not differ to a statistically significant extent between treatment groups (eFigure 2 in Supplement 2).
Figure 4. Symptom Scores Over the Duration of Treated RTIs Among Preschool Children With a History of Severe LRTI.
LRTI indicates lower respiratory tract illness; PAD, Preschool Asthma Diary. P values are based on repeated measures analysis of variance with compound symmetry (RTIs not progressing to severe LRTI) or 2-sample t test (RTIs progressing to severe LRTI). The bottom and top edges of the box indicate the first and third quartiles, respectively. The line inside the box indicates the median. The error bars that extend from each end of the box indicate the range of values that are outside of the interquartile range, but not more than 1.5 times the interquartile range above the third quartile. Circles indicate values that are more than 1.5 times the interquartile range above the third quartile.
a The PAD was completed daily starting on the first day an illness kit was used and continued until the participant was symptom-free for 2 days. It contains questions of frequency of respiratory symptoms, each scored on a scale of 1 through 7, with higher scores representing increasingly frequent symptoms, with daily scores ranging from 0 (asymptomatic) to a maximum of 102. The total PAD score is the sum of the daily individual symptom scores over the duration of the illness, with higher scores representing more frequent symptoms.
Health Care Utilization
Urgent care and emergency department visits were infrequent, occurring in 3.6% of participants receiving azithromycin and 5.4% of participants receiving placebo. There were 28 participants hospitalized for respiratory illnesses (azithromycin group, 13; placebo group, 15) over the duration of the trial, 11 of whom (azithromycin group, 5; placebo group, 6) were hospitalized within 14 days of study medication use.
Time to Second Treated RTI
Comparable numbers of RTIs occurred in the azithromycin and placebo groups (azithromycin group, 473; placebo group, 464). Furthermore, there was no statistically significant difference in the time from the first treated RTI to the start of a second treated RTI between treatment groups (eFigure 3 in Supplement 2), suggesting azithromycin treatment did not prevent subsequent RTIs.
Development of Azithromycin-Resistant Organisms
An azithromycin-resistant organism was isolated from 5 of 41 participants treated with azithromycin (12.2%) and 4 of 45 participants not treated with azithromycin (8.9%) at randomization, and from 8 of 40 participants treated with azithromycin (20.0%) and 7 of 41 participants not treated with azithromycin (17.0%) at study completion. Over the duration of the study, 6 of 36 participants treated with azithromycin (16.7%) and 4 of 37 participants not treated with azithromycin (10.8%) acquired azithromycin-resistant organisms. S aureus was the most common azithromycin-resistant organism isolated.
Adverse Events
Gastrointestinal symptoms during treated RTIs, reported by 4 participants (azithromycin group, 3; placebo group, 1), were mild and did not lead to study discontinuation.
Discussion
This randomized clinical trial enrolled preschool children with severe intermittent wheezing in the context of RTIs, representing an early life wheezing phenotype typically cared for in primary care settings. Azithromycin started at the earliest signs of an RTI was effective in significantly reducing the risk of experiencing progression to severe LRTI along with reducing symptom severity during episodes of severe LRTI. Positive effects were detectable irrespective of modified API status, identifying a treatment option for children at both high and low risk for subsequent persistent asthma. Furthermore, azithromycin therapy was well tolerated, with low rates of treatment-related adverse effects. Prevention of severe LRTIs is a highly desirable outcome given recent evidence that oral corticosteroids, the typical rescue strategy for such episodes, may not be effective in reducing symptom burden in the preschool age group,20,21 in contrast to their efficacy in older children with established asthma.
The mechanisms by which azithromycin reduced the risk of progressing to severe LRTI remain uncertain. Azithromycin may have reduced the risk of severe LRTI via its established antibacterial effects, a concept supported by recent evidence of the importance of bacterial pathogens in acute wheezing illnesses. Children with rhinovirus infections along with detectable Streptococcus pneumoniae or Moraxella catarrhalis were at heightened risk of an asthma exacerbation.7 Although in vitro studies have demonstrated that azithromycin reduces rhinovirus replication and increases interferon gene expression,22 we did not detect an interaction between rhinovirus presence and treatment response. Viral infections are associated with neutrophilic airway inflammation and increased expression of the dominant neutrophil chemoattractant IL-8,23,24 and azithromycin has recently been demonstrated to reduce IL-8 levels in nasal secretions during respiratory syncytial virus bronchiolitis.25 However, although polymorphisms in the IL-8 gene also have been shown to modulate IL-8 production,11 we did not observe a significant interaction between reduced risk of progressing to severe LRTI and the IL-8 rs4073 polymorphism.
The use of antibiotics in young children with wheezing illnesses has been a matter of controversy.26 Although not recommended by national asthma guidelines,27 antibiotics (frequently macrolides) are widely used in clinical practice during RTIs and asthma episodes.26,28-30 A major, justified concern in general pediatrics is the overuse of antibiotics for viral illnesses leading to the potential development of antibiotic-resistant pathogens.31,32 Such concern needs to be balanced, however, with the potential for macrolides to improve the quality of life of children with recurrent, severe LRTI. The rate of exposure to study medicine in this study was 1.87 courses per participant-year. Although limited to 86 participants at a single study site, we found numerically higher rates of acquisition of azithromycin-resistant organisms in oropharyngeal samples in participants receiving azithromycin and those who did not, along with evidence of acquisition of azithromycin-resistant organisms even in participants not treated with azithromycin. Real-world rates of development of azithromycin-resistant organisms may be greater, potentially due to failure to complete the full duration of therapy often seen in clinical practice. Given the small sample size, further studies are needed to assess the potential increased risk of antibiotic resistance vs the comparative effectiveness of azithromycin with respect to other asthma medications to prevent severe LRTI.
This study has limitations that need to be considered when interpreting our results. The randomized portion of the trial was extended from 52 to 78 weeks and up to 4 (instead of 3) courses of study medication were allowed. These modifications were decided by the AsthmaNet Steering Committee while blinded to outcome and were approved by the data and safety monitoring board. The extension was justified by the mild nature of the initial viral respiratory seasons during the trial, which threatened to decrease the power of the study. Although results were independent of study duration, it is possible that the protective effect of azithromycin could have been stronger had all participants been followed for 78 weeks. Many participants had received study therapy during a prior RTI without progressing to severe LRTI, but subsequently experienced a severe LRTI or early termination. Once participants experienced a severe LRTI, their study participation was complete. Therefore, the potential efficacy of azithromycin during future RTIs among patients who experience a severe LTRI while receiving azithromycin is uncertain. The decreasing NNT for multiple episodes may represent selection of responders who continued in the trial. This study examined the proactive administration of azithromycin at the early signs of RTIs, and these findings should not be extrapolated to azithromycin’s potential role as a rescue therapy for patients already experiencing severe LRTI symptoms. We enrolled children with intermittent, yet severe, disease, who did not require daily controller therapy—thus, our findings are applicable to such populations, whereas the role of azithromycin in children receiving daily controller therapy requires future investigation. Due to logistical and ethical concerns, lower airway sampling for bacterial infection was not performed. Parental report may have underestimated or overestimated the severity of their child’s respiratory symptoms; however, this strategy has been used in several previous studies.13,14,33 Comparable proportions of participants in both treatment groups experienced rapid symptom progression early during RTIs that did not allow for initiation of study intervention; these rapidly progressing episodes do not appear to be appropriate for early intervention with azithromycin. Acute care emergency department visits and hospitalizations for respiratory illnesses were infrequent, both in the year preceding the study and during the study, potentially reflecting enrollment of a population at low risk for hospitalization. Alternatively, the extensive disease-related education and round-the-clock availability of study personnel for telephone consultation and as-needed study visits may have further reduced hospitalization risks and improved overall outcomes relative to routine care.
In children with the phenotype of wheezing studied herein, clinicians may consider a therapeutic trial of azithromycin early in the course of RTIs based on a parent-initiated individualized plan. Children who demonstrate an azithromycin response, as reflected by less-severe episodes of RTI, may benefit from repeating such therapy with subsequent illnesses. Attention should be paid to the frequency of RTIs prompting azithromycin use and response to the intervention given the concern of antimicrobial resistance. Studies replicating the findings reported herein would provide further support to this conclusion, and future studies comparing the relative benefits of early azithromycin therapy with either daily or intermittent high-dose inhaled corticosteroids may help determine the relative efficacies of these treatment strategies.
Conclusions
Among young children with histories of recurrent severe LRTIs, the use of azithromycin early during an apparent RTI compared with placebo reduced the likelihood of severe LRTI. More information is needed on the development of antibiotic-resistant pathogens with this strategy.
Supplementary Material
Acknowledgments
Funding/Support: The study was funded by grants HL098102, HL098096, HL098075, HL098090, HL098177, HL098098, HL098107, HL098112, HL098103, HL098115, TR001082, TR000439, TR000448, and TR000454 from the National Heart, Lung, and Blood Institute (NHLBI) as part of AsthmaNet, a clinical trials network supported by a cooperative agreement with NHLBI. GlaxoSmithKline, Merck, Teva, Boehringer Ingelheim, and Sunovion provided products for NHLBI AsthmaNet studies.
Role of the Funder/Sponsor: NHLBI Program Officers participated in study design, conduct, and interpretation of the data. The study was monitored by the AsthmaNet data and safety monitoring board, which also reviewed and approved the final manuscript.
Additional Contributions: We thank the study participants, the AsthmaNet clinical research centers (see Supplement 2 for complete listing), the Data Coordinating Center, and Francine M. Ducharme, MD, MSc (University of Montreal), for permission to use the Preschool Asthma Diary. Dr Ducharme received no compensation for her contribution.
Footnotes
TRIAL REGISTRATION clinicaltrials.gov Identifier: NCT01272635
Group Information: Members of the National Heart, Lung, and Blood Institute’s AsthmaNet are listed in Supplement 2.
Author Contributions: Drs Bacharier and Guilbert had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Bacharier, Guilbert, Mauger, Beigelman, Fitzpatrick, Jackson, Burnham, Chmiel, Covar, Holguin, Israel, Kelly, Lazarus, Lemanske, Meade, Peters, Phipatanakul, Pongracic, Raissy, Teague, Thyne, Szefler, Martinez.
Acquisition, analysis, or interpretation of data: Bacharier, Guilbert, Mauger, Boehmer, Beigelman, Fitzpatrick, Jackson, Baxi, Benson, Burnham, Cabana, Castro, Chmiel, Covar, Daines, Gaffin, Gentile, Israel, Lazarus, Lemanske, Ly, Meade, Morgan, Moy, Olin, Peters, Phipatanakul, Raissy, Ross, Sheehan, Sorkness, Teague, Thyne, Martinez.
Drafting of the manuscript: Bacharier, Guilbert, Mauger, Chmiel, Holguin, Raissy, Szefler, Martinez.
Critical revision of the manuscript for important intellectual content: All authors.
Obtained funding: Bacharier, Guilbert, Mauger, Jackson, Cabana, Chmiel, Holguin, Israel, Lemanske, Moy, Peters, Phipatanakul, Pongracic, Thyne, Szefler, Martinez.
Administrative, technical, or material support: Bacharier, Guilbert, Mauger, Beigelman, Fitzpatrick, Benson, Burnham, Cabana, Chmiel, Covar, Daines, Gaffin, Kelly, Lemanske, Meade, Morgan, Moy, Peters, Raissy, Thyne, Szefler, Martinez.
Study supervision: Bacharier, Guilbert, Mauger, Beigelman, Fitzpatrick, Jackson, Baxi, Castro, Chmiel, Covar, Daines, Gentile, Lazarus, Moy, Olin, Phipatanakul, Pongracic, Raissy, Ross, Sorkness, Szefler.
Conflict of Interest Disclosures: All authors have completed and submitted the ICMJE Form for Disclosure of Potential Conflicts of Interest. Dr Bacharier reports receiving personal fees from Aerocrine, GlaxoSmithKline, Genentech/Novartis, Merck, Schering, Cephalon, DBV Technologies, Teva, Boehringer Ingelheim, AstraZeneca, WebMD, and Sanofi. Dr Guilbert reports receiving grant funding from Teva, GlaxoSmithKline, the Centers for Disease Control and Prevention, the Department for Health and Human Services, the National Institutes of Health (NIH), the University of Wisconsin-Madison Medical and Education Research Committee, Abbott, Array BioPharma, Mylan, Forest Research Institute, Roche, MedImmune, KaloBios, Vertex, Roxane Laboratories, CompleWare, Cystic Fibrosis Foundation Therapeutics, and Roche/Genentech; personal fees from the American Board of Pediatrics, Teva, GlaxoSmithKline, Regeneron, and Merck; and royalties from Up To Date. Dr Fitzpatrick reports receiving personal fees from Merck, GlaxoSmithKline, Genentech, and Boehringer Ingelheim. Dr Jackson reports receiving grant funding from National Institute of Allergy and Infectious Diseases and personal fees from GlaxoSmithKline. Dr Baxi reports grant funding from Sunovion. Dr Castro reports receiving grant funding from Genentech, Amgen, Teva, Novartis, GlaxoSmithKline, sanofi-aventis, Vectura, MedImmune, Johnson & Johnson, Invion, Pfizer, and KaloBios; and personal fees from GlaxoSmithKline, Genentech, Boston Scientific, Boehringer Ingelheim, Elsevier, NeoStem, Teva, and Holaira; and holding stock in Sparo. Dr Chmiel reports receiving personal fees from Genentech, Boehringer Ingelheim, Gilead, the American Board of Pediatrics, the Cystic Fibrosis Foundation, NIH, and Celtaxsys; and grant funding from the Cystic Fibrosis Foundation and NIH. Dr Covar reports receiving grant funding from GlaxoSmithKline and Roche. Dr Daines reports grant funding from NIH. Dr Gentile reports receiving personal fees from Merck and Teva. Dr Israel reports receiving personal fees from AstraZeneca, Merck, Philips Respironics, Regeneron, and UpToDate; receiving grant funding from Genentech; giving expert testimony for Campbell, Campbell, Edwards & Conroy, Ficksman & Conley, Fox Rothschild, and Ryan Ryan Deluca; being a member of the data and safety monitoring board for Novartis; and receiving travel expenses from Research In Real Life and Teva. Dr Kelly reports receiving personal fees from GlaxoSmithKline, AstraZeneca, Merck, and Novartis. Dr Lemanske reports receiving grant funding from Pharmaxis; personal fees from Merck, Sepracor, SABoney and Associates, GlaxoSmithKline, American Institute of Research, Genentech, Double Helix, Boehringer Ingelheim, University of Michigan, Alleghany General Hospital, American Academy of Pediatrics, West Allegheny Health, California Chapter 4, Colorado Allergy and Asthma Society, Pennsylvania Allergy and Asthma Society, Howard Pilgrim Health Care, California Society of Allergy, Asthma, and Immunology, New York Allergy and Asthma Society, World Allergy Organization, Asia Pacific Association of Pediatric Allergy, Respirology, and Immunology, Western Society of Allergy, Asthma, and Immunology, American Academy of Allergy, Asthma and Immunology, Elsevier, UpToDate, Kuwait Society of Allergy and Clinical Immunology, Lurie Children’s Hospital, Boston Children’s Hospital, HealthSTAR Communications, Children’s Hospital Los Angeles, and Northwestern University. Dr Morgan reports receiving grant funding from the Cystic Fibrosis Foundation and personal fees from the Cystic Fibrosis Foundation, Genentech, and the University of Arizona. Dr Peters reports receiving personal fees from Integrity Continuing Education, Merck, UpToDate, Array, AstraZeneca, Aerocrine, Airsonett AB, Boehringer Ingelheim, Experts in Asthma, Gilead, GlaxoSmithKline, Merck, Ono Pharmaceuticals, Pfizer, Pharmaceutical Product Development, Quintiles, Sunovion, Saatchi & Saatchi, Targacept, Teva, and Theron. Dr Ross reports receiving grant funding from the NIH and the Ohio Department of Jobs and Family Services and personal fees from Cleveland Clinic. Dr Teague reports receiving grant funding from the American Lung Association Asthma Clinical Research Center and personal fees from Merck. Dr Szefler reports receiving personal fees from Merck, Boehringer Ingelheim, GlaxoSmithKline, Genentech, Aerocrine, Novartis, and Roche; and grant funding from GlaxoSmithKline. Dr Martinez reports receiving grant funding from National Institute of Environmental Health Sciences and personal fees from Abbott. No other disclosures were reported.
Correction: This article was corrected on January 12, 2016, to fix incorrect data in Figure 3.
REFERENCES
- 1.Martinez FD, Wright AL, Taussig LM, Holberg CJ, Halonen M, Morgan WJ, The Group Health Medical Associates Asthma and wheezing in the first 6 years of life. N Engl J Med. 1995;332(3):133–138. doi: 10.1056/NEJM199501193320301. [DOI] [PubMed] [Google Scholar]
- 2.Ly NP, Gold DR, Weiss ST, Celedón JC. Recurrent wheeze in early childhood and asthma among children at risk for atopy. Pediatrics. 2006;117(6):e1132–e1138. doi: 10.1542/peds.2005-2271. [DOI] [PubMed] [Google Scholar]
- 3.Moorman JE, Akinbami LJ, Bailey CM, et al. National surveillance of asthma: United States, 2001-2010. Vital Health Stat 3. 2012;(35):1–67. [PubMed] [Google Scholar]
- 4.Lemanske RF, Jr, Jackson DJ, Gangnon RE, et al. Rhinovirus illnesses during infancy predict subsequent childhood wheezing. J Allergy Clin Immunol. 2005;116(3):571–577. doi: 10.1016/j.jaci.2005.06.024. [DOI] [PubMed] [Google Scholar]
- 5.Khetsuriani N, Kazerouni NN, Erdman DD, et al. Prevalence of viral respiratory tract infections in children with asthma. J Allergy Clin Immunol. 2007;119(2):314–321. doi: 10.1016/j.jaci.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bisgaard H, Hermansen MN, Bønnelykke K, et al. Association of bacteria and viruses with wheezy episodes in young children: prospective birth cohort study. BMJ. 2010;341:c4978. doi: 10.1136/bmj.c4978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kloepfer KM, Lee WM, Pappas TE, et al. Detection of pathogenic bacteria during rhinovirus infection is associated with increased respiratory symptoms and asthma exacerbations. J Allergy Clin Immunol. 2014;133(5):1301–1307. 1307.e1–3. doi: 10.1016/j.jaci.2014.02.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Johnston SL, Blasi F, Black PN, Martin RJ, Farrell DJ, Nieman RB, TELICAST Investigators The effect of telithromycin in acute exacerbations of asthma. N Engl J Med. 2006;354(15):1589–1600. doi: 10.1056/NEJMoa044080. [DOI] [PubMed] [Google Scholar]
- 9.Simpson JL, Powell H, Boyle MJ, Scott RJ, Gibson PG. Clarithromycin targets neutrophilic airway inflammation in refractory asthma. Am J Respir Crit Care Med. 2008;177(2):148–155. doi: 10.1164/rccm.200707-1134OC. [DOI] [PubMed] [Google Scholar]
- 10.Marguet C, Jouen-Boedes F, Dean TP, Warner JO. Bronchoalveolar cell profiles in children with asthma, infantile wheeze, chronic cough, or cystic fibrosis. Am J Respir Crit Care Med. 1999;159(5 Pt 1):1533–1540. doi: 10.1164/ajrccm.159.5.9805028. [DOI] [PubMed] [Google Scholar]
- 11.Hull J, Thomson A, Kwiatkowski D. Association of respiratory syncytial virus bronchiolitis with the interleukin 8 gene region in UK families. Thorax. 2000;55(12):1023–1027. doi: 10.1136/thorax.55.12.1023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Guilbert TW, Morgan WJ, Zeiger RS, et al. Atopic characteristics of children with recurrent wheezing at high risk for the development of childhood asthma. J Allergy Clin Immunol. 2004;114(6):1282–1287. doi: 10.1016/j.jaci.2004.09.020. [DOI] [PubMed] [Google Scholar]
- 13.Bacharier LB, Phillips BR, Zeiger RS, et al. Episodic use of an inhaled corticosteroid or leukotriene receptor antagonist in preschool children with moderate-to-severe intermittent wheezing. J Allergy Clin Immunol. 2008;122(6):1127–1135.e8. doi: 10.1016/j.jaci.2008.09.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zeiger RS, Mauger D, Bacharier LB, et al. CARE Network of the National Heart, Lung, and Blood Institute Daily or intermittent budesonide in preschool children with recurrent wheezing. N Engl J Med. 2011;365(21):1990–2001. doi: 10.1056/NEJMoa1104647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rivera-Spoljaric K, Chinchilli VM, Camera LJ, et al. Signs and symptoms that precede wheezing in children with a pattern of moderate-to-severe intermittent wheezing. J Pediatr. 2009;154(6):877–881.e4. doi: 10.1016/j.jpeds.2008.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jackson DJ, Gangnon RE, Evans MD, et al. Wheezing rhinovirus illnesses in early life predict asthma development in high-risk children. Am J Respir Crit Care Med. 2008;178(7):667–672. doi: 10.1164/rccm.200802-309OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ducharme FM, Lemire C, Noya FJ, et al. Preemptive use of high-dose fluticasone for virus-induced wheezing in young children. N Engl J Med. 2009;360(4):339–353. doi: 10.1056/NEJMoa0808907. [DOI] [PubMed] [Google Scholar]
- 18.Guilbert TW, Morgan WJ, Krawiec M, et al. Prevention of Early Asthma in Kids Study. Childhood Asthma Research and Education Network The Prevention of Early Asthma in Kids study: design, rationale and methods for the Childhood Asthma Research and Education network. Control Clin Trials. 2004;25(3):286–310. doi: 10.1016/j.cct.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 19.Klein JP, Moeschberger ML. Survival Analysis: Techniques for Censored and Truncated Data. Springer-Verlag; New York, NY: 2003. [Google Scholar]
- 20.Panickar J, Lakhanpaul M, Lambert PC, et al. Oral prednisolone for preschool children with acute virus-induced wheezing. N Engl J Med. 2009;360(4):329–338. doi: 10.1056/NEJMoa0804897. [DOI] [PubMed] [Google Scholar]
- 21.Beigelman A, King TS, Mauger D, et al. Childhood Asthma Research and Education Network of National Heart, Lung, and Blood Institute. Do oral corticosteroids reduce the severity of acute lower respiratory tract illnesses in preschool children with recurrent wheezing? J Allergy Clin Immunol. 2013;131(6):1518–1525. doi: 10.1016/j.jaci.2013.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gielen V, Johnston SL, Edwards MR. Azithromycin induces antiviral responses in bronchial epithelial cells. Eur Respir J. 2010;36(3):646–654. doi: 10.1183/09031936.00095809. [DOI] [PubMed] [Google Scholar]
- 23.Gern JE, Martin MS, Anklam KA, et al. Relationships among specific viral pathogens, virus-induced interleukin-8, and respiratory symptoms in infancy. Pediatr Allergy Immunol. 2002;13(6):386–393. doi: 10.1034/j.1399-3038.2002.01093.x. [DOI] [PubMed] [Google Scholar]
- 24.Kobayashi Y. The role of chemokines in neutrophil biology. Front Biosci. 2008;13:2400–2407. doi: 10.2741/2853. [DOI] [PubMed] [Google Scholar]
- 25.Beigelman A, Isaacson-Schmid M, Sajol G, et al. Randomized trial to evaluate azithromycin’s effects on serum and upper airway IL-8 levels and recurrent wheezing in infants with respiratory syncytial virus bronchiolitis. J Allergy Clin Immunol. 2015;135(5):1171–1178.e1. doi: 10.1016/j.jaci.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kozyrskyj AL, Dahl ME, Ungar WJ, Becker AB, Law BJ. Antibiotic treatment of wheezing in children with asthma: what is the practice? Pediatrics. 2006;117(6):e1104–e1110. doi: 10.1542/peds.2005-2443. [DOI] [PubMed] [Google Scholar]
- 27.Program NAEaP . Expert Panel Report III: Guidelines for the diagnosis and management of asthma: Vol Publication No. 08-4051. US Dept of Health and Human Services; Bethesda, MD: 2007. [Google Scholar]
- 28.Paul IM, Maselli JH, Hersh AL, Boushey HA, Nielson DW, Cabana MD. Antibiotic prescribing during pediatric ambulatory care visits for asthma. Pediatrics. 2011;127(6):1014–1021. doi: 10.1542/peds.2011-0218. [DOI] [PubMed] [Google Scholar]
- 29.De Boeck K, Vermeulen F, Meyts I, Hutsebaut L, Franckaert D, Proesmans M. Coprescription of antibiotics and asthma drugs in children. Pediatrics. 2011;127(6):1022–1026. doi: 10.1542/peds.2009-3068. [DOI] [PubMed] [Google Scholar]
- 30.Sarpong EM, Miller GE. Narrow- and Broad-Spectrum Antibiotic Use among U.S. Children. Health Serv Res. 2015;50(3):830–846. doi: 10.1111/1475-6773.12260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hersh AL, Jackson MA, Hicks LA, American Academy of Pediatrics Committee on Infectious Diseases Principles of judicious antibiotic prescribing for upper respiratory tract infections in pediatrics. Pediatrics. 2013;132(6):1146–1154. doi: 10.1542/peds.2013-3260. [DOI] [PubMed] [Google Scholar]
- 32.Costelloe C, Metcalfe C, Lovering A, Mant D, Hay AD. Effect of antibiotic prescribing in primary care on antimicrobial resistance in individual patients: systematic review and meta-analysis. BMJ. 2010;340:c2096. doi: 10.1136/bmj.c2096. [DOI] [PubMed] [Google Scholar]
- 33.Guilbert TW, Morgan WJ, Zeiger RS, et al. Long-term inhaled corticosteroids in preschool children at high risk for asthma. N Engl J Med. 2006;354(19):1985–1997. doi: 10.1056/NEJMoa051378. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.