Abstract
Background and Aims
Treatments with morphine or opioid agonists cause constipation. Lubiprostone is approved for treatment of adult idiopathic constipation and constipation-predominant IBS in adult women. We tested whether lubiprostone can reverse morphine-suppression of mucosal secretion in human intestine and explored the mechanism of action.
Methods
Fresh segments of jejunum discarded during Roux-En-Y gastric bypass surgeries were used. Changes in short-circuit current (ΔIsc) were recorded in Ussing flux chambers as a marker for electrogenic chloride secretion during pharmacological interactions between morphine, prostaglandin receptor antagonists, chloride channel blockers and lubiprostone.
Results
Morphine suppressed basal Isc. Lubiprostone reversed morphine suppression of basal Isc. Lubiprostone, applied to the mucosa in concentrations ranging from 3 nM to 30 μM, evoked increases in Isc in concentration-dependent manner when applied to the mucosal side of muscle-stripped preparations. Blockade of enteric nerves did not change stimulation of Isc by lubiprostone. Removal of chloride or application of bumetanide or NPPB suppressed or abolished responses to lubiprostone. Antagonists acting at CFTR channels and prostaglandin EP4 receptors, but not at E1, EP1-3 receptors, partially suppressed stimulation of Isc by lubiprostone.
Conclusions
Antisecretory action of morphine results from suppression of excitability of secretomotor neurons in the enteric nervous system. Lubiprostone, which does not affect enteric neurons directly, bypasses the action of morphine by directly opening mucosal chloride channels.
Keywords: Constipation, Opioid analgesics, Mucosal secretion, Enteric nervous system, Prostaglandins, Irritable bowel syndrome, CFTR, ClC-2 channels
Introduction
Treatment of pain with morphine and allied opioid agonists has constipating side effects [1, 2]. Opiate-induced non-propulsive motility and suppressed mucosal secretion, each of which reflects suppression of motor neuronal excitability in the enteric nervous system (ENS), are underlying factors in opiate-related constipation [3–5]. Schiller et al. [6, 7] concluded, from in vivo studies in humans, that the antidiarrheal actions of loperamide or codeine to reduce stool volume result from delayed intestinal transit and prolonged mucosal contact with the luminal contents, not stimulation of mucosal absorption rate. Opiates, in animals and humans, slow intestinal transit by elevating contractile tone in the intestinal circular muscle coat. Delayed transit is linked with rhythmic contractions at electrical slow wave frequency and with infrequent high-amplitude, non-propulsive phasic contractions [8–10]. This kind of contractile behavior reflects depressed excitability of inhibitory musculomotor neurons in the ENS and release of the autogenic circular muscle coat from inhibitory neural input [11, 12]. What’s more, presynaptic inhibitory action of morphine suppresses release of acetylcholine at nicotinic synapses in the ENS microcircuits [13, 14]. Suppression of nicotinic neurotransmission by morphine disrupts propulsive motility due to interruption of synaptic integration within the neural networks of the ENS. Coordination of contractile behavior of the muscle coats into organized motor patterns requires functional synaptic connectivity inside the networks.
Suppressed intestinal transit and prolonged time for absorption of electrolytes and water to proceed was supported in the early 1980s as an explanation for the constipating symptoms associated with opiate treatment [6, 7]. Subsequent progress in understanding ENS control of mucosal secretion and the function of secretomotor neurons in the submucosal division of the ENS introduces an additional factor into mechanisms of action of opioids [15]. Firing of action potentials by secretomotor neurons evokes secretion of HCO3, NaCl, H2O and mucus. Opiates silence the secretomotor neurons by hyperpolarizing their membrane potential [11, 12]. Suppression of secretomotor firing reduces secretion, which in concert with delayed intestinal transit, results in lowered liquidity of the small and large intestinal contents. Drier-harder stools are the result in the large intestine.
Morphine’s inhibitory action on secretomotor neurons and cholinergic neurotransmission in ENS motility circuitry can account for much of its constipating action. Nevertheless, stimulation of sympathetic noradrenergic input to the ENS, from the central nervous system, might underlie some of the constipating action [16–18]. Sympathetic postganglionic fibers, after entering the bowel, release norepinephrine at their synaptic contacts with ENS neurons. Norepinephrine, when released inside the ENS, suppresses fast and slow excitatory synaptic transmission in the neural microcircuits. At the same time, it hyperpolarizes the membrane potential and suppresses firing in secretomotor neurons in the submucosal plexus [12, 19]. Noradrenergic and opioid receptors act synergistically, in secretomotor control, to suppress neurogenic mucosal secretion.
Lubiprostone
Lubiprostone is a member of a group of compounds called prostones. They are naturally occurring bicyclic fatty acids formed by enzymatic oxidation of the 15-hydroxyl group of prostaglandins to a keto group by 15-hydroxyprostaglandin dehydrogenase [20]. Lubiprostone is FDA-approved in the United States for the treatment of adult chronic idiopathic constipation and constipation-predominant irritable bowel syndrome in adult women. Exposure of flat-sheet preparations of guinea pig small or large intestine to lubiprostone in Ussing flux chambers, evokes increases in short-circuit current (Isc) that reflect stimulation of mucosal chloride (Cl−) secretion [21].
A proposed mechanism of lubiprostone action on intestinal secretion is opening of Cl− channels in small and large intestinal epithelium [21]. However, unequivocal identification of the Cl− channels by which lubiprostone stimulates functional intestinal Cl− secretion is unresolved and contradictory. The cystic fibrosis transmembrane conductance regulator (CFTR) and ClC-2 channels are possibilities for activation by lubiprostone [22, 23].
Structural similarity of lubiprostone to prostaglandins factors into a proposal that stimulation of mucosal Cl− secretion by lubiprostone in the intestine reflects stimulation of prostaglandin receptors linked to a G protein-coupled adenyl cyclase-cAMP-protein kinase A enterocyte signal transduction pathway [24, 25]. On the other hand, results from flat-sheet preparations of guinea pig small and large intestine in Ussing chambers and intracellular electrophysiological recording of secretomotor neuronal activity do not support prostaglandin receptor stimulation as a mechanism of lubiprostone action in the guinea pig [21].
The present study was designed to evaluate the extent to which results from our earlier work with lubiprostone on guinea pig intestine might translate to human small bowel [21, 23]. Changes in short-circuit current (Isc) across the mucosa of human small intestinal preparations in Ussing flux chambers were used as a marker for morphine- or lubiprostone-evoked changes in Cl− secretion. The preparations were segments of jejunum removed during Roux-En-Y gastric bypass surgeries and transported immediately to the laboratory. Chloride is the predominate anion involved in morphine-evoked changes in Isc [26, 27]. We tested if stimulation of electrogenic Cl− secretion by lubiprostone will offset the inhibitory action of morphine on secretion and might therefore reverse constipating effects of morphine. Some of the results have been published in abstract form [28].
Materials and Methods
Fresh preparations for Ussing chamber studies were procured from human small intestine. They were obtained from segments of jejunum discarded during Roux-En-Y gastric bypass surgeries and studied immediately in the laboratory. Fifty-three percent of the patients were female, their ages ranged from 37 to 50 years and body mass index ranged from 44 to 67.4 kg/m2. The human protocols were reviewed and approved by the Institutional Review Board of the Ohio State University Office of Research Risks Protection (Protocol 02H0208). The intestinal segments were flushed with ice-cold Krebs solution and opened along the mesenteric border. Composition of the Krebs solution was in mM: 120 NaCl, 6 KCl, 2.5 CaCl2, 1.2 MgCl2, 1.35 NaH2PO4, 14.4 NaHCO3, and 11.5 glucose. The muscularis externa and myenteric plexus were removed by microdissection and referred to as “muscle-stripped preparations.” The submucosal plexus remained intact with the mucosa.
The methods for study of human intestinal mucosal secretion in Ussing chambers were the same as described earlier [21, 23, 29]. Krebs solution in the Ussing chambers was gassed with 95% O2/5% CO2 and buffered at pH 7.4.
Mucosal preparations were studied simultaneously in four to eight standard Ussing chambers. Each chamber was equipped with a pair of Ag/AgCl electrodes connected via Krebs-agar bridges to calomel half-cells for measurement of transmural potential difference (PD). A second pair of electrodes connected to WPI DVC-1000 voltage/current clamp amplifiers (World Precision Instruments, Sarasota, FL) compensated for the solution resistance between the PD-sensing bridges. Flat-sheet preparations were mounted between halves of the Ussing chambers, which had chamber openings with a total cross-sectional area of 1.13 cm2. The tissues were bathed on both sides from circulation reservoirs, which contained 10 mL of Krebs solution maintained at 37°C by circulation from a temperature-controlled water bath. The current necessary to change the transmucosal PD by 2.5 mV served to monitor tissue conductance, calculated according to Ohm’s law, as a viability measure for the tissue. Isc was read-out from the WPI DVC-1000 in voltage clamp mode. Concentration–response relations were obtained by adding the agents to either the chamber compartment bathing the mucosa (mucosal side) or the compartment that bathed the muscle-stripped side (serosal side). Drug-evoked changes in Isc were measured and expressed as ΔIsc. The data were normalized to the cross-sectional area of the preparations (i.e., 1.13 cm2). Morphine was dissolved in Krebs solution and lubiprostone in DMSO. Volumes not more than 10 lL were added to the 10-mL circulation reservoirs.
Chemicals
Lubiprostone, free of prostaglandin contamination, was synthesized by R-Tech Ueno, Ltd (Japan) (>99.8% purity) and shipped and stored as frozen aliquots of 2 mM solutions in 100% DMSO. Dimethyl sulfoxide (DMSO), obtained as frozen aliquots from the same source, was used as a vehicle for lubiprostone and for testing effects of the vehicle itself. Tetrodotoxin, bumetanide, 4,4′-Diisothiocyanatostilbene-2, 2′-disulfonic acid disodium salt hydrate (DIDS), CFTR(inh)-172, carbachol, prostaglandin E2 and morphine sulphate pentahydrate were purchased from Sigma–Aldrich (St. Louis, MO). The source for 5-nitro-2-2(-penylpropylamino) benzoic acid (NPPB) and the selective prostaglandin PGE4 receptor antagonist, L-161,982 were purchased from Tocris Bioscience (Ellisville, MO). The prostaglandin receptor antagonists, GW627368X, AH6809 and SC-19220 were purchased from Cayman Chemical Co. (Ann Arbor, MI). Stock solutions were prepared in Krebs solution, deionized H2O or DMSO. Volumes added to 10-mL bath solutions never exceeded 10 μL.
Statistical Analysis
Ussing chamber data are presented as means ± SE with n values referring to numbers of patients and preparations. Continuous curves for concentration–response relationships were constructed with the following least-squares fitting routine using Sigmaplot® software (SPSS Inc., Chicago, IL): V = Vmax/[1 + EC50/C)nH], where V is the observed increased Isc, Vmax is the maximal response, C is the corresponding drug concentration, EC50 is the concentration that induces the half-maximal response, and nH is the apparent Hill coefficient. Student’s t test or paired t test was used to determine significance with P < 0.05 considered to be significant.
Results
Lubiprostone
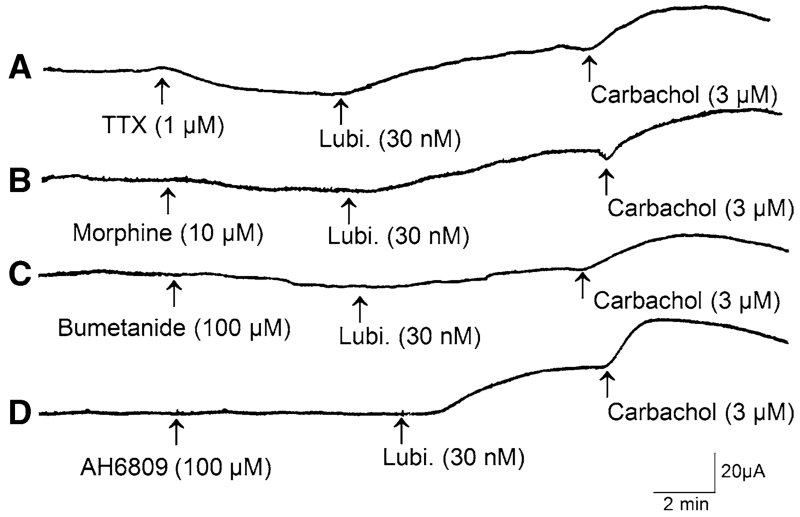
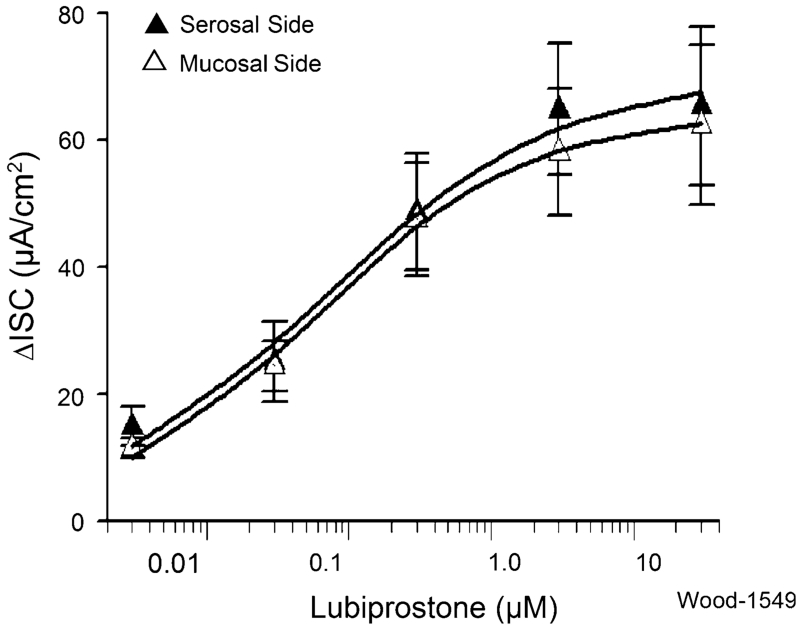
Figure 1 illustrates how individual protocols were configured to study changes in Isc in response to lubiprostone in the presence of strategic pharmacological agents, with TTX, morphine, bumetanide and a prostaglandin EP receptor antagonist shown as examples of strategic agents. Lubiprostone alone elevated Isc in each of 16 preparations obtained from eight gastric bypass patients (Fig. 2). Applications to the serosal or mucosal side of preparations in four concentrations, ranging from 3 nM to 12 μM, evoked increases in Isc in a concentration-dependent manner. Concentration–response curves did not reach a plateau at the maximal concentration of 12 μM used in the study (Fig. 2). When calculated for the data range between 3 nM and 12 μM, the EC50 for serosal side application was 65 nM and the EC50 for mucosal application was 57 nM, when computed for the same range of concentrations. The differences for EC50s, computed in this manner, between serosal- and mucosal-side applications were not significant (P > 0.05). There was no significant time lag for the lubiprostone-evoked responses to reach their peak irrespective of mucosal or serosal side application.
Fig. 1.
Protocols involved placing lubiprostone in either the serosal- or mucosal-side compartment of Ussing chambers, applying pharmacological agents and recording changes in short-circuit current (ΔIsc) on a chart recorder or digital data acquisition system. a Record of ΔIsc to illustrate effect of TTX on response to lubiprostone. Application of TTX evoked characteristic reduction in baseline Isc. Lubiprostone in the presence of TTX evoked an increase in Isc. b Record of ΔIsc to illustrate effect of lubiprostone on response to morphine. Application of morphine reduced baseline Isc. Lubiprostone in the presence of morphine evoked an increase in Isc. c Record of ΔIsc to illustrate effect of bumetanide on response to lubiprostone. Application of bumetanide reduced baseline Isc. Stimulatory response to lubiprostone was suppressed in the presence of bumetanide. d Record of ΔIsc to illustrate effect of AH6809, an EP1,2,3 receptor antagonist, on response to lubiprostone. Application of AH6809 did not change baseline Isc or stimulatory response to lubiprostone. At the end of each experiment, the muscarinic agonist, carbachol, was added as a test for viability of the preparations
Fig. 2.
Cumulative concentration–response relations for lubiprostone-evoked increases in ΔIsc in human jejunum. Lubiprostone, applied to the serosal side or mucosal side of 16 preparations from eight patients in five concentrations ranging from 3 nM to 12 μM, evoked increases in Isc in a concentration-dependent manner. Concentration–response curves did not reach a maximum plateau at 12 μM. When calculated for the data range between 0.3 nM and 12 μM, the EC50 for serosal side application (black triangle) was 65 nM and the EC50 for mucosal application was 57 nM. Differences for EC50s between serosal- and mucosal-side applications, as calculated, were not significant (P > 0.05). Values are expressed as means ± SE
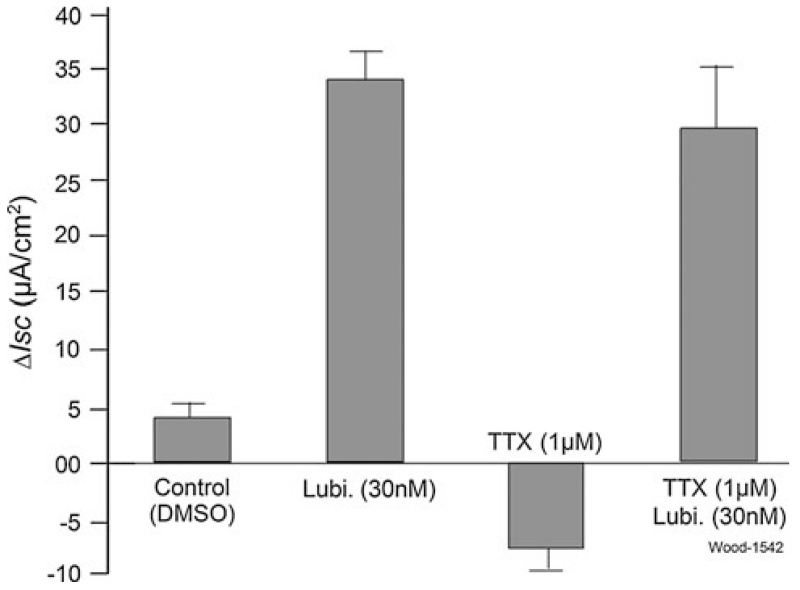
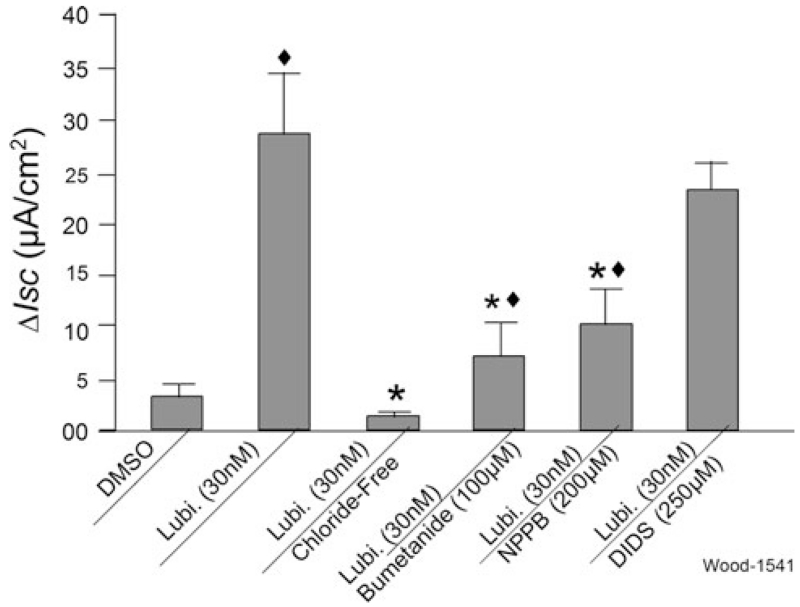
Blockade of nerves in the ENS by 1 μM TTX, applied to the serosal side, had no effect on stimulation of Isc by serosally applied 30 nM lubiprostone (Fig. 3). Application of TTX alone evoked canonical suppression of baseline Isc (Figs. 1a, 3) [30]. Substitution of gluconate for Cl− in the bathing medium reduced to near zero all responses to lubiprostone, when applied in the serosal compartment (Fig. 4). Inhibition of the basolateral Na/K/2Cl co-transporter by bumetanide partially suppressed increases in Isc evoked by lubiprostone (Figs. 1c, 4). NPPB, a nonspecific Cl− channel blocker, partly suppressed lubiprostone-evoked increases in Isc (Fig. 4). DIDS, which is a canonical blocker of anion channels and chloride-bicarbonate exchangers [31], did not significantly suppress lubiprostone-evoked increases in Isc when applied to the mucosal or serosal sides of the preparations (Fig. 4, data for serosal application not shown).
Fig. 3.
Effect of tetrodotoxin (TTX) on lubiprostone-evoked ΔIsc in human jejunum. Application of TTX blocked ENS secretomotor neurons and thereby reduced baseline Isc. Pretreatment with TTX did not change stimulation of Isc by 30 nM lubiprostone applied in the serosal side of the Ussing chambers (P > 0.05). Values are expressed as means ± SE. N = 7 patients, 12 preparations
Fig. 4.
Effects of Cl− free media, bumetanide, NPPB or DIDS on lubiprostone-evoked ΔIsc in human jejunum. Substitution of gluconate for Cl− in the bathing solution (chloride-free) suppressed or abolished all responses to 30 nM lubiprostone. Bumetanide, an inhibitor of the basolateral Na/K/2Cl co-transporter, and NPPB, a potent nonspecific Cl− channel blocker, suppressed increases in Isc evoked by 30 nM lubiprostone. Mucosal application of DIDS, which blocks chloride–bicarbonate exchangers and is a canonical low affinity and nonspecific Cl− channel blocker, did not alter increases in Isc evoked by 30 nM lubiprostone. Values are expressed as means ± SE. N = 6 patients, 10 preparations. Compared with lubiprostone, *P < 0.01; compared with DMSO, ◆P < 0.01
CdCl2
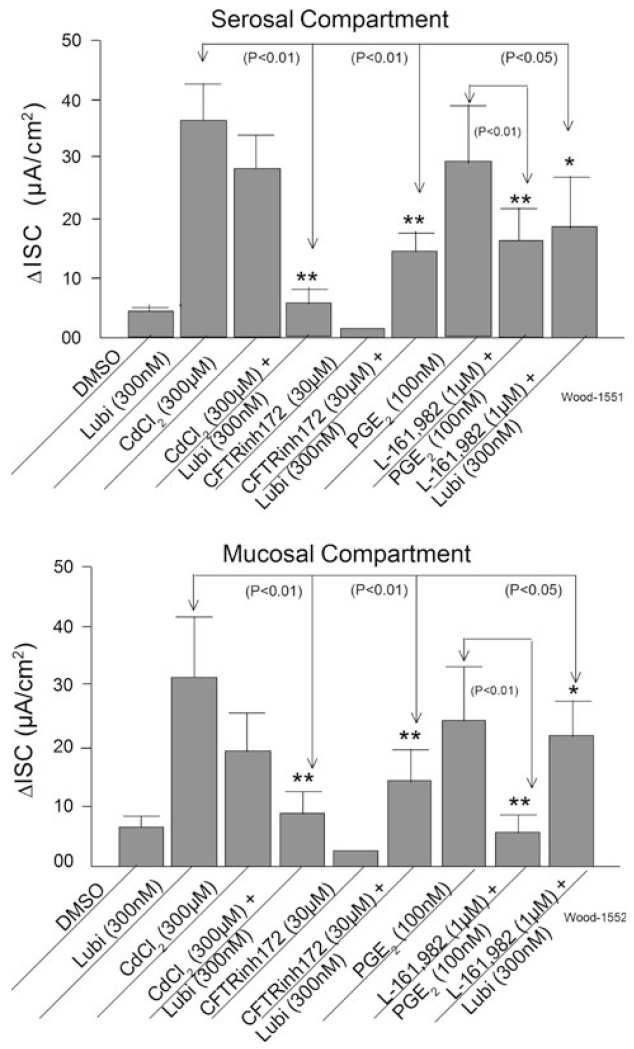
Serosal or mucosal application of 300 μM CdCl2, which suppressed K+ conductance and thereby depolarized the membrane potential of the enterocytes, increased Isc (Fig. 5). The mean responses to lubiprostone when placed on either serosal or mucosal sides of the preparations, in the presence of CdCl2, were suppressed by 85.4% for the serosal and by 72.4% for the mucosal sides (Fig. 5).
Fig. 5.
Effects of cadmium (CdCl2), CFTR(inh)-172, and L-161-982 on lubiprostone-evoked ΔIsc in human small intestine. CdCl2, which suppressed K+ conductance, depolarized the enterocytes and thereby reduced the driving force on intracellular Cl−, suppressed lubiprostone-evoked ΔIsc. Suppression of CFTR channel conductance by CFTR(inh)-172 reduced responses to lubiprostone. Prostaglandin PGE2 mimicked the action of lubiprostone. The EP4 receptor antagonist, L-161,982, suppressed the responses to PGE2 and the lubiprostone-evoked responses. The effects were generally the same for applications in the serosal and mucosal compartments of the Ussing chambers. Values are expressed as means ± SE (*P < 0.05; **P < 0.01). N = 4 patients, 6 preparations
CFTR Antagonist
Presence of the selective CFTR antagonist, CFTR(inh)-172, in the bathing medium on the serosal side of the preparation suppressed increases in Isc evoked by serosal application of lubiprostone by 61% (Fig. 5). CFTR(inh)-172 suppressed lubiprostone-evoked Isc by 55% when the protocol was repeated for the mucosal sides of the preparations (Fig. 5).
Prostaglandins
Lubiprostone was reported by others to contract the longitudinal muscle coat of the stomach and to inhibit neuronally mediated contractions of the colonic circular muscle coat in preparations from rats and humans. These actions were suppressed by an EP1 receptor antagonist and an EP4 receptor antagonist respectively, suggesting to the authors that lubiprostone might act like a prostaglandin to stimulate EP receptors [24, 25]. We tested this by applying lubiprostone following pre-incubation with one or the other of the following prostaglandin EP receptor antagonists: (1) SC-19220, a selective EP1 receptor antagonist; (2) GW627368X and L-161,982 selective PGE4 receptor antagonists; (3) AH6809, an EP-1,-2,-3, antagonist [32–35]. Stimulation of Isc by lubiprostone was not suppressed by AH6890, SC-19220, nor GW627368X (data not shown).
Based on an ad hoc finding with L-161,982, we examined PGE2 and involvement of its action at the PGE4 receptor in relation to lubiprostone. Action of PGE2 to stimulate intestinal transmucosal Isc has been described for several species [36]. Like 300 nM lubiprostone, application of 100 nM PGE2 in either the mucosal or serosal compartment of the Ussing chamber evoked increases in Isc (Fig. 5). The presence of the selective PGE4 receptor antagonist, L-161.982, in the bathing medium on the serosal side of the preparations suppressed the mean PGE2-evoked responses by 45% and suppressed the PGE2-evoked responses by 78% when present in the mucosal side compartment of the chambers (Fig. 5). Presence of L-161,982 in the bathing medium on the serosal side of the preparations suppressed the mean lubiprostone-evoked responses by 49% and suppressed the lubiprostone-evoked responses by 32% when present in the mucosal side compartments of the chambers. L-161.982 (1 μM) alone did not change basal Isc (data not shown).
Morphine
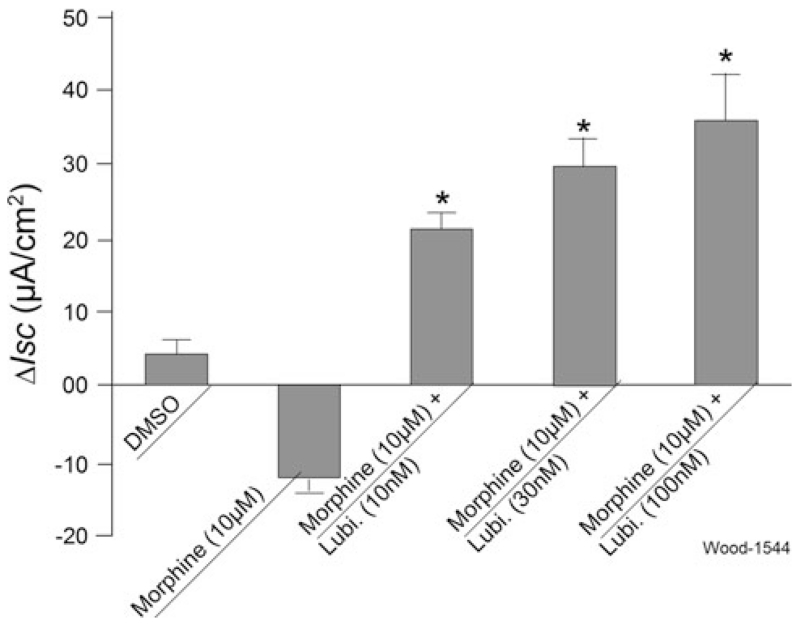
Exposure to morphine on the serosal side of the preparations reduced basal Isc in a manner reminiscent of findings by others [26, 27]. Morphine (10 μM) was added to the serosal side of 12 preparations from seven gastric-bypass patients 5 min before application of a concentration range of 10, 30 and 100 nM lubiprostone (Fig. 6). Lubiprostone reversed the morphine-induced suppression of basal Isc, in a concentration-dependent manner (Figs. 1b, 6). Pretreatment with 1 μM TTX suppressed basal Isc, as described earlier [23, 30], and suppressed or abolished the inhibitory action of morphine on basal Isc (data not shown).
Fig. 6.
Lubiprostone reversed morphine-induced suppression of Isc in human jejunum. Morphine alone suppressed basal Isc. Lubiprostone (10, 30 or 100 nM), applied on the same side 5 min later, reversed morphine-induced suppression in a concentration-dependent manner. Values are expressed as means ± SE (*P < 0.01). N = 7 patients, 12 preparations
Discussion
Concentration dependence and EC50 values in the nanomolar range for stimulation of Isc by lubiprostone in human jejunum are reminiscent of our earlier results for guinea pig small intestine [21] and those of Bijvelds et al. [25] for human ileum. Our EC50 values for the jejunum are estimates because the concentration–response curves never reached full saturation plateaus at the highest concentration (i.e., 12 μM, Fig. 2). Like the results for intestinal preparations, nanomolar concentrations of lubiprostone also stimulate Isc and Cl− secretion across confluent epithelial cell monolayers in culture [25, 37, 38].
Stimulation of Isc, by lubiprostone when applied on the mucosal or serosal sides of the preparations, was paradoxical because expression of ClC-2 and CFTR channels, where lubiprostone might act, is generally thought to be at the apical membrane of the enterocytes. The channels should therefore be opened optimally by mucosal, not serosal side, application. Nevertheless, this has not been found for either the human intestine in this study or for guinea pig intestine or confluent T84 cell monolayers in earlier reports [21, 37]. Paths, over which lubiprostone in the serosal side of the chambers might move to reach the apical membranes on the mucosal side in the present study, are unclear. A leak pathway through “edge damage” is a possibility. Artifacts due to edge damage have often been a concern for tissues set up in standard Ussing chambers. On the other hand, DMSO, which was the solvent, readily penetrates epithelia (e.g., epidermis) and might have ferried lubiprostone from serosal to mucosal sides of the tissue. Aside from edge damage and solvent drag, expression of ClC-2 channels in apical as well as basolateral sites might underlie the lubiprostone-evoked Isc found when it was applied separately to mucosal or serosal compartments of the chamber. Immunohistochemical localization in intact mucosa shows expression of ClC-2 in the basolateral membrane [39] and in the vicinity of tight junctions [40].
Our results for lubiprostone at the tissue level in human jejunum are consistent with the interpretation of results obtained by others at the cellular level with ClC-2 transfected HEK-293 cells and confluent T84 and A6 cell monolayers [25, 37, 38]. Lubiprostone opens epithelial Cl− channels and this was reflected as stimulation of Isc for human jejunal preparations in the present study. Reduction of Cl− in the bathing medium, suppression of the basolateral Na/K/2Cl co-transporter and application of the potent nonspecific Cl− channel blocker, NPPB, each suppressed lubiprostone-evoked increases in Isc, which is consistent with opening of Cl− channels by lubiprostone in human jejunum. DIDS, the canonical Cl− channel and Cl-HCO3 exchange blocker, had negligible effects on lubiprostone-evoked Isc in human or guinea pig small intestine [21]. Negligible affinity of DIDS for ClC-2 channels is a likely explanation for its lack of suppression of lubiprostone-evoked secretion. DIDS blocks outwardly rectifying Cl− channels and has minimal effect on inwardly rectifying Cl− channels [39]. Lubiprostone, at low concentrations, selectively activates ClC-2 channels [37].
We did not identify the specific Cl− channel/s opened by lubiprostone. Whether stimulation of Isc by lubiprostone, in our study, reflects exclusive opening of ClC-2 channels, exclusive opening of CFTR channels or a mixture of the two is unresolved. Application of lubiprostone to confluent cultures of T84, HEK-293 or A6 cells increases chloride conductance in both ClC-2 and CFTR [37, 38]. However, according to Bao et al. [37], lubiprostone activates ClC-2 at “much” lower concentrations than required to activate CFTR. On the other hand, Bijvelds et al. [25] reported that lubiprostone-evoked Isc across confluent monolayers of T84 cells was suppressed only by the CFTR channel antagonist CFTR(inh)-172, and interpreted this as evidence for exclusive involvement of CFTR. Partial suppression of the action of 300 nM lubiprostone by CFTR(inh)-172 in our study is consistent with a mixed action to open ClC-2 and CFTR channels. This differs from the guinea pig small intestine where CFTR(inh)-172 does not suppress lubiprostone-evoked increases in Isc [21].
Tetrodotoxin
The stimulatory action of lubiprostone on Isc reflects a non-neural action directly on the epithelium because it was unaffected by the nerve-blocking agent, TTX. Had lubiprostone action been an indirect effect resulting from excitation of submucosal secretomotor neurons and excitatory neurotransmitter release at the neuroepithelial junctions, then TTX, which blocks action potential generation in axons of enteric neurons, would have inhibited the response. Moreover, lubiprostone has no action on electrical and synaptic behavior of neurons in the submucosal plexus as recorded with intracellular microelectrodes and this is likewise consistent with a non-neuronal mechanism of action [21].
Paracrine Mediators
Results of electrophysiological studies in ENS neurons make unlikely a possibility that stimulation of Isc by lubiprostone reflected stimulation of paracrine release of mediators from nonneural sources, such as enteric mast cells, enteroendocrine cells and/or enterochromaffin cells. Microelectrode studies in ENS neurons document that release of paracrine mediators (e.g., histamine, serotonin and eicosanoids) in food allergies and inflammatory states is reflected by excitation of neurons in the ENS [41].
Absence of any association between stimulation of Isc by lubiprostone and excitation of neurons in the ENS suggests that its action was not mediated by release and action of paracrine mediators.
Morphine
Guinea pig intestinal preparations in Ussing flux chambers exhibit a persistent basal Isc, which reflects neurogenic secretion evoked by on-going discharge of secretomotor neurons in the submucosal plexus [30]. Blockade of basal neurogenic activity by the neuronal blocking agent, TTX, suppresses basal Isc in guinea pig mucosal preparations [30]. Morphine behaved in a similar manner to TTX in suppressing basal Isc in the human jejunum. This reflected the action of morphine to suppress ongoing discharge of secretomotor neurons and thereby suppress basal secretion. This kind of morphine action is mediated by mu-type opioid receptors, which are expressed by ENS neurons in the guinea pig and human intestine [42]. Binding of morphine and other opioids to these receptors increases potassium conductance, hyperpolarizes the membrane potential and suppresses excitability [4]. Suppression of secretomotor neuronal excitability is believed to be a mechanism by which opiates suppress neurogenic mucosal secretion and thereby promote a constipated state in humans and animal models.
Lubiprostone
Reversal of morphine-evoked suppression of basal Isc by lubiprostone does not result from any direct action on secretomotor neurons because blockade of neuronal conduction in the ENS by TTX has no significant effect on stimulation of Isc by lubiprostone either in human small bowel in the present work or in guinea pig intestine [21]. As mentioned earlier, application of lubiprostone to secretomotor neurons in guinea pig preparations does not change the neurons’ electrophysiological behavior [21].
Reversal by lubiprostone of morphine suppression of basal Isc was likely a reflection of opening of electrogenic enterocyte Cl− channels at the time ENS neuronal excitability was suppressed by morphine. The basal Isc, recorded in the absence of morphine, reflected ongoing stimulation of Cl− secretion by the neurotransmitters, vasoactive intestinal peptide and acetylcholine, released from tonically-active secretomotor neurons [15]. Morphine suppression of secretomotor neuronal excitability eliminated this component of basal Isc (see Figs. 1, 6), which then was replaced by lubiprostone-evoked chloride secretion.
Prostaglandins
Neither the EP1-2-3 receptor antagonist, AH6809, the EP1 antagonist, Sc-19220, nor the EP4 antagonist, GW-627368X, suppressed Isc responses to lubiprostone in guinea pig intestinal preparations in Ussing chambers [21] or in human jejunum in the present study. On the other hand, the EP4 antagonist, L-161,982, did partially suppress responses to 100 nM lubiprostone in the present study. This opens the possibility that lubiprostone, in addition to a direct action to open chloride channels, might interact with the EP4 prostaglandin receptor in human small intestine. This possibility was suggested by Bijvelds et al. [25] for T84 cells, which are of human origin, and for mouse intestine.
Acknowledgments
The authors appreciate helpful consultation with Prof. Helen J. Cooke during performance of the Ussing chamber studies and to Yu-zhong Wang for technical assistance with the Ussing chamber setups. National Institutes of Health grants RO1 DK 37238, RO1 DK 57075 to J. D. Wood, RO1 DA18860 and KO1 DA14600 to L. M. Bohn, KO8 DK60468 to Y. Xia, a Pharmaceutical Manufacturers of America Foundation Research Starter Award to Sumei Liu and a grant-in-aid from Sucampo Pharmaceuticals, Inc. supported the work.
Footnotes
Conflicts of interest The authors disclose no conflicts of interest.
Contributor Information
Xiaohong Sun, Department of Physiology and Cell Biology, The Ohio State University College of Medicine, 304 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210, USA.
Xiyu Wang, Department of Anesthesiology, The Ohio State University College of Medicine, Columbus, OH, USA, wang.773@osu.edu.
Guo-Du Wang, Department of Physiology and Cell Biology, The Ohio State University College of Medicine, 304 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210, USA, wang.772@osu.edu.
Yun Xia, Department of Anesthesiology, The Ohio State University College of Medicine, Columbus, OH, USA, Yun.Xia@osumc.edu.
Sumei Liu, Department of Internal Medicine, The Ohio State University College of Medicine, Columbus, OH, USA; Department of Physiology and Cell Biology, The Ohio State University College of Medicine, 304 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210, USA.
Meihua Qu, Department of Physiology and Cell Biology, The Ohio State University College of Medicine, 304 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210, USA, qumeihua@hotmail.com.
Bradley J. Needleman, Department of Surgery, The Ohio State University College of Medicine, Columbus, OH, USA, Bradley.needleman@osumc.edu
Dean J. Mikami, Department of Surgery, The Ohio State University College of Medicine, Columbus, OH, USA, dean.mikami@osumc.edu
W. Scott Melvin, Department of Surgery, The Ohio State University College of Medicine, Columbus, OH, USA, scott.melvin@osumc.edu.
Laura M. Bohn, Department of Pharmacology, The Ohio State University College of Medicine, Columbus, OH, USA
Ryuji Ueno, Sucampo Pharmaceuticals, Inc., Bethesda, MD, USA, rueno@sucampo.com.
Jackie D. Wood, Department of Physiology and Cell Biology, The Ohio State University College of Medicine, 304 Hamilton Hall, 1645 Neil Avenue, Columbus, OH 43210, USA
References
- 1.Droney J, Ross J, Gretton S, Welsh K, Sato H, Riley J. Constipation in cancer patients on morphine. Support Care Cancer. 2008;16:453–459. doi: 10.1007/s00520-007-0373-1. [DOI] [PubMed] [Google Scholar]
- 2.Glare P, Walsh D, Sheehan D. The adverse effects of morphine: a prospective survey of common symptoms during repeated dosing for chronic cancer pain. Am J Hosp Palliat Care. 2006;23:229–235. doi: 10.1177/1049909106289068. [DOI] [PubMed] [Google Scholar]
- 3.Liu S, Hu HZ, Ren J, Gao C. Pre- and postsynaptic inhibition by nociceptin in guinea pig small intestinal myenteric plexus in vitro. Am J Physiol Gastrointest Liver Physiol. 2001;281:G237–G246. doi: 10.1152/ajpgi.2001.281.1.G237. [DOI] [PubMed] [Google Scholar]
- 4.Morita K, North RA. Opiate activation of potassium conductance in myenteric neurons: inhibition by calcium ion. Brain Res. 1982;242:145–150. doi: 10.1016/0006-8993(82)90504-2. [DOI] [PubMed] [Google Scholar]
- 5.Morita K, North RA. Opiates and enkephalin reduce the excitability of neuronal processes. Neuroscience. 1981;6:1943–1951. doi: 10.1016/0306-4522(81)90034-8. [DOI] [PubMed] [Google Scholar]
- 6.Schiller LR, Davis GR, Santa Ana CA, Morawski SG, Fordtran JS. Studies of the mechanism of the antidiarrheal effect of codeine. J Clin Invest. 1982;70:999–1008. doi: 10.1172/JCI110711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schiller LR, Santa Ana CA, Morawski SG, Fordtran JS. Mechanism of the antidiarrheal effect of loperamide. Gastroenterology. 1984;86:1475–1480. [PubMed] [Google Scholar]
- 8.Frantzides CT, Cowles V, Salaymeh B, Tekin E, Condon RE. Morphine effects on human colonic myoelectric activity in the postoperative period. Am J Surg. 1992;163:144–148. doi: 10.1016/0002-9610(92)90267-u. [DOI] [PubMed] [Google Scholar]
- 9.Sarna SK, Otterson MF. Small intestinal amyogenesia and dysmyogenesia induced by morphine and loperamide. Am J Physiol. 1990;259:G282–G289. doi: 10.1152/ajpgi.1990.258.2.G282. [DOI] [PubMed] [Google Scholar]
- 10.Telford GL, Condon RE, Szurszewski JH. Opioid receptors and the initiation of migrating myoelectric complexes in dogs. Am J Physiol. 1989;256:G72–G77. doi: 10.1152/ajpgi.1989.256.1.G72. [DOI] [PubMed] [Google Scholar]
- 11.Wood JD, Galligan JJ. Function of opioids in the enteric nervous system. Neurogastroenterol Motil. 2004;16:17–28. doi: 10.1111/j.1743-3150.2004.00554.x. [DOI] [PubMed] [Google Scholar]
- 12.Wood JD. Opioids, the enteric nervous system and postoperative ileus. Semin Colon Rectal Surg. 2005;16:188–196. [Google Scholar]
- 13.Cherubini E, Morita K, North RA. Opioid inhibition of synaptic transmission in the guinea-pig myenteric plexus. Br J Pharmacol. 1985;85:805–817. doi: 10.1111/j.1476-5381.1985.tb11079.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.North RA. Twelfth Gaddum memorial lecture. Drug receptors and the inhibition of nerve cells. Br J Pharmacol. 1989;98:13–28. doi: 10.1111/j.1476-5381.1989.tb16855.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cooke HJ. Neural and humoral regulation of small intestinal electrolyte transport. In: Johnson LR, Christensen J, Jackson MJ, et al., editors. Physiology of the Gastrointestinal Tract. Raven Press; New York: 1987. pp. 1307–1350. [Google Scholar]
- 16.Brown DR, Miller RJ. Adrenergic mediation of the intestinal antisecretory action of opiates administered into the central nervous system. J Pharmacol Exp Ther. 1984;231:114–119. [PubMed] [Google Scholar]
- 17.Coupar IM, Taylor DA. Evidence for tryptaminergic and noradrenergic involvement in the antisecretory action of morphine in the rat jejunum. J Pharm Pharmacol. 1987;39:363–369. doi: 10.1111/j.2042-7158.1987.tb03399.x. [DOI] [PubMed] [Google Scholar]
- 18.Jiang Q, Sheldon RJ, Porreca F. Opioid modulation of basal intestinal fluid transport in the mouse: actions at central, but not intestinal, sites. J Pharmacol Exp Ther. 1990;253:784–790. [PubMed] [Google Scholar]
- 19.Hu HZ, Gao N, Zhu MX, et al. Slow excitatory synaptic transmission mediated by P2Y1 receptors in the guinea-pig enteric nervous system. J Physiol (Lond) 2003;550:493–504. doi: 10.1113/jphysiol.2003.041731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Anggard E. The biological activities of three metabolites of prostaglandin E 1. Acta Physiol Scand. 1966;66:509–510. doi: 10.1111/j.1748-1716.1966.tb03231.x. [DOI] [PubMed] [Google Scholar]
- 21.Fei G, Wang YZ, Liu S, et al. Stimulation of mucosal secretion by lubiprostone (SPI-0211) in guinea pig small intestine and colon. Am J Physiol Gastrointest Liver Physiol. 2009;296:G823–G832. doi: 10.1152/ajpgi.90447.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wood JD. Enteric nervous system: sensory physiology, diarrhea and constipation. Curr Opin Gastroenterol. 2010;26:102–108. doi: 10.1097/MOG.0b013e328334df4f. [DOI] [PubMed] [Google Scholar]
- 23.Fei G, Raehal K, Liu S, et al. Lubiprostone (SPI-0211) reverses the inhibitory action of morphine on intestinal secretion in guinea pig and mouse. J Pharmacol Exp Ther. 2010;334:333–340. doi: 10.1124/jpet.110.166116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bassil AK, Borman RA, Jarvie EM, et al. Activation of prostaglandin EP receptors by lubiprostone in rat and human stomach and colon. Br J Pharmacol. 2008;154:126–135. doi: 10.1038/bjp.2008.84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bijvelds MJ, Bot AG, Escher JC, et al. Activation of intestinal Cl− secretion by lubiprostone requires the cystic fibrosis transmembrane conductance regulator. Gastroenterology. 2009;137:976–985. doi: 10.1053/j.gastro.2009.05.037. [DOI] [PubMed] [Google Scholar]
- 26.Fondacaro JD, Kolpak DC, Burnham DB, et al. Cecectomized rat. A model of experimental secretory diarrhea in conscious animals. J Pharmacol Methods. 1990;24:59–71. doi: 10.1016/0160-5402(90)90050-u. [DOI] [PubMed] [Google Scholar]
- 27.Sheldon RJ, Riviere PJ, Malarchik ME, et al. Opioid regulation of mucosal ion transport in the mouse isolated jejunum. J Pharmacol Exp Ther. 1990;253:144–151. [PubMed] [Google Scholar]
- 28.Sun X, Xia Y, Liu S, et al. Lubiprostone reverses the inhibitory action of morphine on mucosal secretion in the human jejunum. Gastroenterology. 2008;134:A122. doi: 10.1007/s10620-010-1515-8. Abstract 844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Fang X, Liu S, Wang XY, et al. Neurogastroenterology of tegaserod (HTF 919) in the submucosal division of the guinea-pig and human enteric nervous system. Neurogastroenterol Motil. 2008;20:80–93. doi: 10.1111/j.1365-2982.2007.00983.x. [DOI] [PubMed] [Google Scholar]
- 30.Carey HV, Cooke HJ. Tonic activity of submucosal neurons influences basal ion transport. Life Sci. 1989;44:1083–1088. doi: 10.1016/0024-3205(89)90335-4. [DOI] [PubMed] [Google Scholar]
- 31.Alrefai WA, Ramaswamy K, Dudeja PK. Mechanism(s) of chloride transport in human distal colonic apical membrane vesicles. Dig Dis Sci. 2001;46:2209–2218. doi: 10.1023/a:1011971117097. [DOI] [PubMed] [Google Scholar]
- 32.Chan CL, Jones RL, Lau HY. Characterization of prostanoid receptors mediating inhibition of histamine release from anti-IgE-activated rat peritoneal mast cells. Br J Pharmacol. 2000;29:589–597. doi: 10.1038/sj.bjp.0703072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Botella A, Delvaux M, Fioramonti J. Receptor subtypes involved in dual effects induced by prostaglandin E2 in circular smooth muscle from dog colon. J Pharmacol Exp Ther. 1995;273:1008–1014. [PubMed] [Google Scholar]
- 34.Wilson RJ, Giblin GMP, Roomans S, et al. GW627368X ((N-{2-[4-(4, 9-diethoxy-1-oxo-1, 3-dihydro-2H-benzo[f]isoindol-2-yl) phenyl]acetyl} benzene sulphonamide): a novel, potent and selective prostanoid EP4 receptor antagonist. Br J Pharmacol. 2006;148:326–339. doi: 10.1038/sj.bjp.0706726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Balzary RW, Cocks TM. Lipopolysaccharide induces epithelium- and prostaglandin E2-dependent relaxation of mouse isolated trachea through activation of cyclooxygenase (COX)-1 and COX-2. J Pharmacol Exp Ther. 2006;317:806–812. doi: 10.1124/jpet.105.097634. [DOI] [PubMed] [Google Scholar]
- 36.Cooke HJ, Reddix RA. Neural regulation of intestinal electrolyte transport. In: Johnson LR, Alpers DH, Christensen J, et al., editors. Physiology of the Gastrointestinal Tract. Raven Press; New York: 1994. pp. 2083–2132. [Google Scholar]
- 37.Bao HF, Liu L, Self J, et al. A synthetic prostone activates apical chloride channels in A6 epithelial cells. Am J Physiol Gastrointest Liver Physiol. 2008;295:G234–G251. doi: 10.1152/ajpgi.00366.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cuppoletti J, Malinowska DH, Tewari KP, et al. SPI-0211 activates T84 cell chloride transport and recombinant human ClC-2 chloride currents. Am J Physiol Cell Physiol. 2004;287:C1173–C1183. doi: 10.1152/ajpcell.00528.2003. [DOI] [PubMed] [Google Scholar]
- 39.Gyömörey K, Yeger H, Ackerley C, et al. Expression of the chloride channel ClC-2 in the murine small intestine epithelium. Am J Physiol Cell Physiol. 2000;279:C1787–C1794. doi: 10.1152/ajpcell.2000.279.6.C1787. [DOI] [PubMed] [Google Scholar]
- 40.Pena-Munzenmayer G, Catalan M, Cornejo I, et al. Basolateral localization of native ClC-2 chloride channels in absorptive intestinal epithelial cells and basolateral sorting encoded by a CBS-2 domain di-leucine motif. J Cell Sci. 2005;118:4243–4252. doi: 10.1242/jcs.02525. [DOI] [PubMed] [Google Scholar]
- 41.Wood JD. Enteric neuroimmuno physiology and pathophysiology. Gastroenterology. 2004;127:635–657. doi: 10.1053/j.gastro.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 42.Sternini C, Patierno S, Selmer IS. Kirchgessner A. The opioid system in the gastrointestinal tract. Neurogastroenterol Motil. 2004;16:3–16. doi: 10.1111/j.1743-3150.2004.00553.x. [DOI] [PubMed] [Google Scholar]