Abstract
In contrast to the upfront setting in which the role of high-dose therapy with autologous hematopoietic cell transplantation (HCT) as consolidation of a first remission in patients with multiple myeloma (MM) is well established, the role of high-dose therapy with autologous or allogeneic HCT has not been extensively studied in MM patients relapsing after primary therapy. The International Myeloma Working Group together with the Blood and Marrow Transplant Clinical Trials Network, the American Society of Blood and Marrow Transplantation, and the European Society of Blood and Marrow Transplantation convened a meeting of MM experts to: (1) summarize current knowledge regarding the role of autologous or allogeneic HCT in MM patients progressing after primary therapy, (2) propose guidelines for the use of salvage HCT in MM, (3) identify knowledge gaps, (4) propose a research agenda, and (5) develop a collaborative initiative to move the research agenda forward. After reviewing the available data, the expert committee came to the following consensus statement for salvage autologous HCT: (1) In transplantation-eligible patients relapsing after primary therapy that did NOT include an autologous HCT, high-dose therapy with HCT as part of salvage therapy should be considered standard; (2) High-dose therapy and autologous HCT should be considered appropriate therapy for any patients relapsing after primary therapy that includes an autologous HCT with initial remission duration of more than 18 months; (3) High-dose therapy and autologous HCT can be used as a bridging strategy to allogeneic HCT; (4) The role of postsalvage HCT maintenance needs to be explored in the context of well-designed prospective trials that should include new agents, such as monoclonal antibodies, immune-modulating agents, and oral proteasome inhibitors; (5) Autologous HCT consolidation should be explored as a strategy to develop novel conditioning regimens or post-HCT strategies in patients with short (less than 18 months remissions) after primary therapy; and (6) Prospective randomized trials need to be performed to define the role of salvage autologous HCT in patients with MM relapsing after primary therapy comparing it to “best non-HCT” therapy. The expert committee also underscored the importance of collecting enough hematopoietic stem cells to perform 2 transplantations early in the course of the disease. Regarding allogeneic HCT, the expert committee agreed on the following consensus statements: (1) Allogeneic HCT should be considered appropriate therapy for any eligible patient with early relapse (less than 24 months) after primary therapy that included an autologous HCT and/or high-risk features (ie, cytogenetics, extramedullary disease, plasma cell leukemia, or high lactate dehydrogenase); (2) Allogeneic HCT should be performed in the context of a clinical trial if possible; (3) The role of postallogeneic HCT maintenance therapy needs to be explored in the context of well-designed prospective trials; and (4) Prospective randomized trials need to be performed to define the role salvage allogeneic HCT in patients with MM relapsing after primary therapy.
Keywords: Myeloma, Stem cell transplantation, Salvage therapy
INTRODUCTION
Multiple myeloma (MM) is a disease characterized by malignant plasma cell proliferation, bone destruction, and immunodeficiency. The median age at diagnosis is approximately 70 years. MM is responsible for about 1% of all cancer-related deaths in western countries [1].
Modern therapy now includes induction therapy with combinations of immune-modulatory drugs (IMiDs), such as thalidomide and lenalidomide, and proteasome inhibitors (bortezomib and carfilzomib) in combination with steroids, alkylators, or anthracyclines. When possible, patients who are deemed “transplantation eligible” undergo consolidation therapy with high-dose melphalan and autologous hematopoietic cell transplantation (HCT). This therapeutic strategy has improved the median overall survival (OS) for patients with MM from 36 months to more than 5 years in patients with standard risk MM [2–4].
Despite modern therapy, most patients with MM will live to see their disease recur. Several prognostic factors for disease progression have been identified, such as beta-2 microglobulin, albumin (International Staging System [ISS] score), plasma cell labeling index, elevated serum lactate dehydrogenase, and certain chromosomal abnormalities, such as t(4;14) and t(14;16), either partial or complete deletion of chromosome 17, deletion of 8q21, and 1p loss or 1q gains [5–9].
The availability of both proteasome inhibitors and IMiDs has led to the development of multiple combination chemotherapy regimens as salvage therapies that include different permutations of these drugs. Although response rates of the combinations are higher, it is uncertain whether any specific combination is associated with better survival or whether sequential therapy would be equally effective [10].
These new combinations have resulted in dramatic OS improvements for MM patients relapsing after primary therapy over the last decade. The magnitude of improvement was examined by Kumar et al. The median OS after relapse increased from 11.8 months for patients relapsing before 2000 to 24 months for patients relapsing beyond that date. The OS benefit was seen primarily in patients receiving 1 of the IMiDs or proteasome inhibitors. The prognosis for patients who become resistant to both agents is extremely poor. Among 286 patients identified, 74% received subsequent therapy (range, 0 to 8), with 44% of patients achieving at least a minor response. The median OS and event-free survival from the time of registration were 9 and 5 months, respectively. These patients have been successfully treated with novel therapeutic approaches, including carfilzomib and/or pomalidomide, but will invariably succumb to their disease [10–12].
In contrast to the upfront setting, in which the role of high-dose therapy as consolidation of a first remission is well established, the role of a second course of high-dose therapy with autologous or allogeneic HCT has not been extensively studied. To that effect, the International Myeloma Foundation through its International Myeloma Working Group, together with the Blood and Marrow Transplant Clinical Trials Network, the National Marrow Donor Program, the European Society of Blood and Marrow Transplantation (EBMT), and the American Society of Blood and Marrow Transplantation, convened a meeting of MM experts regarding the role of autologous or allogeneic HCT in the treatment of patients who had progressed after primary therapy with or without autologous HCT consolidation. The goals of the meeting were as follows:
Summarize current knowledge regarding the role of autologous or allogeneic HCT in MM patients progressing after primary therapy
Propose guidelines for the use of salvage HCT in MM
Identify knowledge gaps
Propose a research agenda
Develop a collaborative initiative to move the research agenda forward
Herein are the results of those deliberations held in Minnesota on October 27, 2014. The meeting was supported in part by an unrestricted grant from Sanofi Pharmaceuticals to the International Myeloma Foundation and from the BMT CTN U01.
CURRENT STANDARDS FOR TREATMENT OF RELAPSED AND/OR REFRACTORY MYELOMA
Before the advent of IMiDs and proteasome inhibitors, patients with relapsed MM had limited therapeutic options. Salvage therapy with alkylators, anthracycline, steroids, vinca alkaloids, or combinations had response rates of 50% or less with short remission durations and poor prognoses [13,14]. Salvage therapy for MM has been greatly improved by the availability of new agents and combinations. Table 1 summarizes the most relevant phase II trials exploring new combinations of IMiDs and proteasome inhibitors in patients with relapsed and or refractory MM.
Table 1.
Phase II Trials of New Combination Therapies for Relapsed and Refractory Myeloma
| Ref | ORR (CR) | PFS/OS, mo | Comments |
|---|---|---|---|
| Immunomodulatory drug doublets | |||
| Thal/Dex [15] | 47% (13%) | 14/38 | RRMM |
| Len/Dex [16–18] | 60.6% (15%) | 11/38 | RRMM |
| Pom/Dex [19] | 31% (1%) | 4/12.7 | RRMM |
| Proteasome inhibitor doublets | |||
| Bor/Dex [20] | 43% (9%) | 6.2/29.8 | RRMM |
| Bor/PegAnthr [21] | 44% (4%) | 9/NS | RRMM |
| Car/Dex [22] | 55% (5%) | 7/NS | RRMM |
| Triple drug combinations | |||
| VTD [23] | 63% (6%) | 6/22 | RRMM |
| RVD [24] | 61% (8%) | 7.7/37 | RRMM |
| CyBor Steroids [25] | 95% (NS) | NS/NS | RRMM |
| KRD [26] | 76.9% (5.7%) | 15.4/NS | RRMM |
| Elo Len Dex [27] | 82% (4%) | NR/NR | RRMM |
Ref indicates reference; ORR, overall response rate; Thal, thalidomide; Dex, dexamethasone; RRMM, relapse/refractory multiple myeloma; Len, lenalidomide; Pom, pomalidomide; Bor, bortezomib; PegAnthr, pegylated anthracycline; NS, not stated; Car, carfilzomib; VTD, bortezomib, thalidomide, dexamethasone; RVD, lenalidomide, bortezomib, dexamethasone; CyBor, cyclophosphamide, bortezomib; KRD, carfilzomib, lenalidomide, dexamethasone; Elo, elotuzumab; NR, not reached.
In the last 2 years, 4 randomized trials have been performed and reported assessing optimal treatment of patients relapsing after 1 to 3 prior lines of treatment. The first trial was reported by Garderet et al. and compared the efficacy and safety of bortezomib-thalidomide-dexamethasone versus thalidomide-dexamethasone in patients relapsing after an autologous HCT. A total of 269 patients were randomized. Complete remission (CR) and near CR rates were significantly better for bortezomib-thalidomide-dexamethasone than for thalidomide-dexamethasone (45% versus 25%), which translated into an superior median time to progression (19.5 versus 13.8 months) and a trend towards improved 24-month survival (71% versus 65%; P = .093). Grade 3 neuropathy and grades 3 and 4 infection and thrombocytopenia were significantly higher in the bortezomib-thalidomide-dexamethasone arm [28].
Stewart et al. reported the results of a randomized trial of carfilzomib, lenalidomide, and dexamethasone versus lenalidomide and dexamethasone in patients with MM failing 1 to 3 prior therapies (ASPIRE Trial). ASPIRE enrolled 792 patients with relapsed or refractory MM. The objective response rate was 87% for carfilzomib, lenalidomide, and dexamethasone versus 67% for lenalidomide and dexamethasone, with a significantly higher rate of CRs in the carfilzomib, lenalidomide, and dexamethasone arm (32% versus 9%; P < .0001). Median progression-free survival (PFS) in the carfilzomib, lenalidomide, and dexamethasone arm was 26.3 months versus 17.6 months for the lenalidomide and dexamethasone arm. Median OS has not been reached in either group, but there was a trend toward longer survival in the carfilzomib arm [29].
San Miguel et al. reported the results of a phase III trial comparing panobinostat, bortezomib, and dexamethasone to bortezomib and dexamethasone in patients with MM failing 1 to 3 prior therapies. Of 768 randomized patients, 387 received panobinostat, bortezomib, and dexamethasone and 382 received placebo, bortezomib, and dexamethasone. Panobinostat, bortezomib, and dexamethasone showed superior PFS when compared with placebo, bortezomib, and dexamethasone (12.0 versus 8.1 months; hazard ratio, .63; P < .0001) with no OS difference reported. Complete plus near complete response rates were 28% and 16%, with median response duration of 13.1 and 10.9 months, respectively [30].
Lonial et al. reported the results of a phase III trial comparing the combination of elotuzumab plus lenalidomide plus dexamethasone to placebo plus lenalidomide plus dexamethasone (Eloquent 2) [31]. Overall, 321 patients were assigned to the elotuzumab group and 325 to the control group. After a median follow-up of 24.5 months, the rate of PFS at 1 year in the elotuzumab group was 68% compared with 57% in the control group; at 2 years, the rates were 41% and 27%, respectively. Median PFS in the elotuzumab group was 19.4 months versus 14.9 months in the control group (hazard ratio for progression or death in the elotuzumab group, .70; 95% confidence interval, .57 to .85; P < .001).
Therefore, by the end of 2015, the MM community has completed 4 randomized trials in patients with relapsed MM (failing 1 to 3 prior therapies) in which all of those reported to date have demonstrated that more intense therapy (triplets versus doublets) is associated with improvements in depth of response, and that depth of response is associated with improvements in PFS although a definitive survival benefit has not been shown. These results are summarized in Table 2.
Table 2.
Results of Phase III Trials Comparing Two to Three Drug Combinations in Patients with Myeloma Failing One to Three Prior Therapies
| Ref | ORR (CR) | PFS/OS | Comments |
|---|---|---|---|
| [28] | VTD 88% (28%) TD 72% (13%) |
19.8/NS 13.8/NS |
First relapse after autologous |
| [29] | KRD 87% (31.8%) RD 66.6% (9.3%) |
26.3/NR 17.6/NR |
1 to 3 prior therapies |
| [30] | Pano Bor Dex 60.7% (NS) Bor Dex 54.6% (NS) |
11.9/NS 8.08/NS |
1 to 3 prior therapies |
| [31] | Rev Dex Rev Dex Elo |
14.9/NS 19.4/NS |
1 to 3 prior therapies |
TD indicates thalidomide, dexamethasone; RD, lenalidomide, dexamethasone; Pano Bor Dex, panobinostat, bortezomib, dexamethasone; Bor Dex, bortezomib, dexamethasone; Rev Dex, lenalidomide, dexamethasone.
With the increasing efficacy of salvage therapy for MM, the role of high-dose therapy with HCT support (so-called salvage HCT) to further enhance the depth of response is being increasingly explored and formed the basis of discussion for the expert committee.
WHAT IS THE DEFINITION OF SALVAGE HCT?
For the purpose of discussion, the expert committee defined salvage HCT as either an autologous or allogeneic HCT performed on MM patients who had failed a prior line of therapy. The committee recognized that this definition would encompass multiple scenarios, ranging from transplantation-naïve patients failing frontline treatment to patients who had failed multiple therapies without ever having an HCT.
ROLE OF AUTOLOGOUS HCT AS TREATMENT FOR PATIENTS RELAPSING AFTER PRIMARY THERAPY IN TRANSPLANTATION-NAÏVE PATIENTS
The optimal treatment for transplantation-eligible patients who relapsed after an initial therapy that did not include high-dose therapy consolidation remains uncertain, although it is the comparator arm for all randomized trials of early versus delayed HCT. Reinduction treatment with combination chemotherapy is the standard and most experts agreed that high-dose therapy consolidation should be considered the standard of care at this time for this patient population. The expert committee agreed that this patient population, although heterogeneous, is worthy of prospective trials designed to address the issue of optimal reinduction therapy and consolidation to determine whether their natural history is different than that for patients relapsing after a prior autograft.
ROLE OF AUTOLOGOUS HCT FOR PATIENTS RELAPSING AFTER PRIOR AUTOGRAFT
Retrospective Trials
To date, multiple reports of salvage autologous HCT have been published and are summarized in Table 3. In all reports, chemosensitivity and remission duration after first autograft were the most important prognostic factors for subsequent long-term disease control. However, it is still uncertain whether all patients would benefit from salvage autograft regardless of remission duration. Most reports identified that the number of lines of prior therapy had a significant impact on outcomes and suggested that salvage autologous HCT should not be relegated to a “last-ditch effort” in patients who have failed all prior therapies, but it should be considered an integral component of initial salvage strategies. Of particular importance is the retrospective analysis performed by Grövdal et al. in which treatment outcomes of 1061 consecutive patients from 24 hospitals in Denmark, Finland, Norway, and Sweden who had received an autologous HCT as consolidation of a first remission were reported. During the study period, 564 patients progressed and were treated with either conventional chemotherapy (n = 91); IMiDs and/or proteasome inhibitors (n = 362), or a second autologous HCT after either approach (n = 111). Patients who received salvage therapy that included a second HCT had a statistically significantly longer median survival; 4 years compared with 3.3 years for those who received salvage therapy with IMiDs and/or proteasome inhibitors but without a second autologous HCT and 2.5 years for those who received conventional chemotherapy. Of note, in this analysis, even patients undergoing a second autograft with initial remission durations of less than 12 months still benefited from the procedure when compared with the other groups [41].
Table 3.
Retrospective Analysis of Salvage Autologous HCT Outcomes (Series with More Than 40 Patients)
| Ref | n | NRM (%) | Median PFS in Months | Prognostic Factor |
|---|---|---|---|---|
| [32] | 44 | 2 | 12 | First RD |
| [33] | 81 | 3 | 16 | First RD, ≥VGPR |
| [34] | 41 | 7 | 8.5 | Age > 65, lines of therapy |
| [35] | 55 | 5 | 14 | First RD |
| [36] | 83 | NS | 15.6 | NS |
| [37] | 60 | NS | 11 | NS |
| [38] | 98 | 4 | 10.3 | First RD |
| [39] | 187 | 2 | 11 | First RD |
| [40] | 200 | 3 | 15 | First RD, reinduction with novel drugs and response, ISS at salvage autologous HCT |
| [41] | 111 | NS | 18 | Improved outcomes for second HCT when compared with IMiDs and proteasome inhibitors, stage, hemoglobin |
RD indicates remission duration; VGPR, very good partial response.
Current Activity in North America
Data from the Center for International Blood and Marrow Transplant Research (CIBMTR) data base show that since 1995, 22,069 MM patients were reported to have relapsed after autologous HCT. Data regarding salvage HCT were available on 80% of relapsed patients: most of them (15,529 [87%]) did not receive a subsequent HCT, 1606 (9%) received another autologous HCT (mostly single, 1524 [85%] or rarely a tandem, 82 [<1%]). Another 624 patients (3.6%) underwent an allogeneic HCT at some point during their treatment course as salvage therapy. However, since 2001, there is a constant increase in the salvage HCT activity, with more than 300 patients per year after 2010 (Figure 1A,B).
Figure 1.
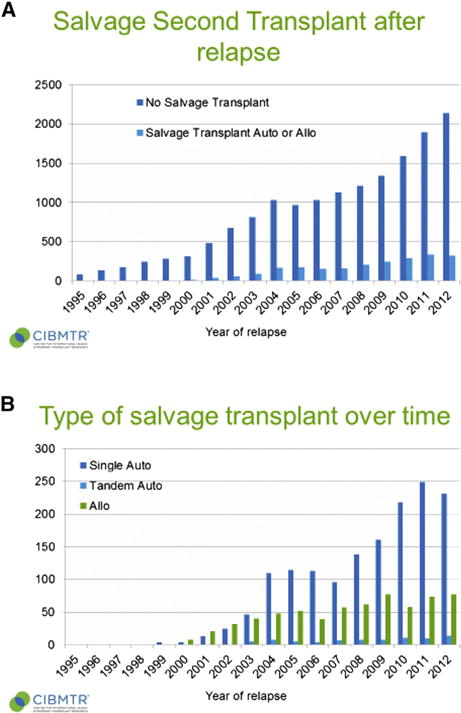
(A) Number or salvage HCT performed for MM in North America over time (CIBMTR registry). (B) Type of salvage HCT performed for MM over time (CIBMTR registry).
Current Activity in Europe
In the EBMT database, since 1995, 33,415 MM patients were reported to be in first relapse after autologous HCT. In that setting, various salvage treatments were performed: most of the patients, 26,622 (80%), did not receive a subsequent HCT, 5275 (15.8%) received a subsequent autologous HCT (either 1, 4443 [13.3%], 2, 260 [.8%], or a tandem auto followed by allogeneic HCT, 572 [1.7%]). Another 1527 patients (5%) underwent an allogeneic transplantation at 1 point of their treatment course as a salvage therapy. Since 1995, there is a constant increase in the salvage HCT numbers, reaching more than 500 patients per year after 2012 (Figure 2A,B).
Figure 2.
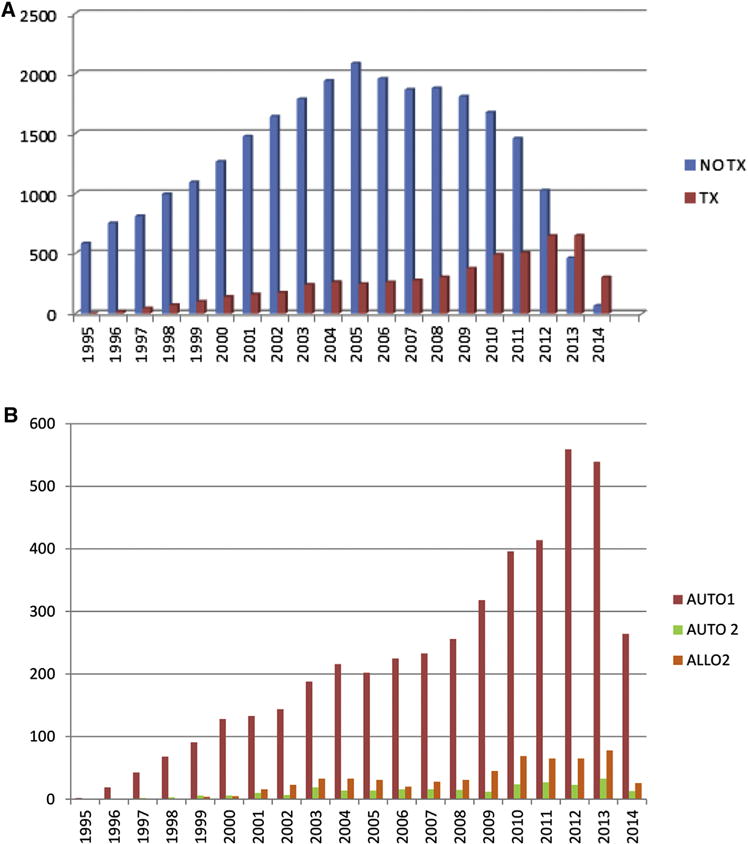
(A) Since 1995, evolution of salvage treatment after autologous transplantation in Europe: no transplantation versus autologous transplantation. 2014 data is incomplete (EBMT registry). (B) Since 1995, evolution of salvage autologous transplantation in Europe: single (AUTO1) versus double (AUTO2) versus tandem autoallo (ALLO2). 2014 data is incomplete (EBMT registry).
Prospective Trials of Salvage Autologous HCT
The first prospective randomized trial studying autologous HCT versus less-intensive alkylating agent consolidation (weekly cyclophosphamide) after first relapse and reinduction with a bortezomib-containing regimen has been reported [42]. This multicenter, randomized, open-label, phase III study recruited patients at least 18 years old with MM who needed treatment for first progressive or relapsed disease at least 18 months after a previous autologous HCT from 51 centers across the United Kingdom. Before randomization, eligible patients received bortezomib, doxorubicin, and dexamethasone induction therapy and then underwent peripheral blood stem cell mobilization and harvesting, if applicable. Eligible patients (with adequate stem cell harvest) were randomly assigned (1:1) to either high-dose melphalan 200 mg/m2 plus salvage HCT or to oral cyclophosphamide (400 mg/m2 per week for 12 weeks). This trial showed a clear advantage in terms of time to progression, 19 versus 11 months (P < .0001) for the transplantation arm compared with the chemotherapy arm and, in fact, the trial was stopped earlier because it met its predefined endpoint. However, the control arm is considered nowadays suboptimal. With limited follow up, an OS difference has not been shown.
The Nordic group published recently a phase II study analyzing salvage autologous HCT in 53 bortezomib- and lenalidomide-naïve patients who previously received 1 autologous HCT [43]. The reinduction was bortezomib based and the conditioning regimen included bortezomib as well. The second PFS was similar to the initial PFS, 19 versus 20 months (P = .80). Median OS was almost 5.5 years with acceptable toxicity [43].
RESULTS OF CONSENSUS SURVEY REGARDING ROLE OF AUTOLOGOUS HCT IN RELAPSED MYELOMA
Before the consensus conference, participants were asked to rate their agreement with a variety of statements regarding salvage HCT, giving a score of 0 if they strongly agreed with the statement and a score of 10 if they strongly disagreed.
When asked if salvage autologous HCT should be considered for all patients relapsing after primary therapy, if eligible, and with an initial remission duration of more than 24 months, there was a clear consensus agreement (Figure 3A). When asked if salvage autologous HCT should be considered for all patients relapsing after primary therapy, if eligible, and with an initial remission duration of less than 6 months, there was a clear consensus with most experts strongly disagreeing with that approach, as seen in Figure 3B. The degree of agreement was significantly less for patients with remissions lasting only 12 to 24 months, as is seen in Figure 3C.
Figure 3.
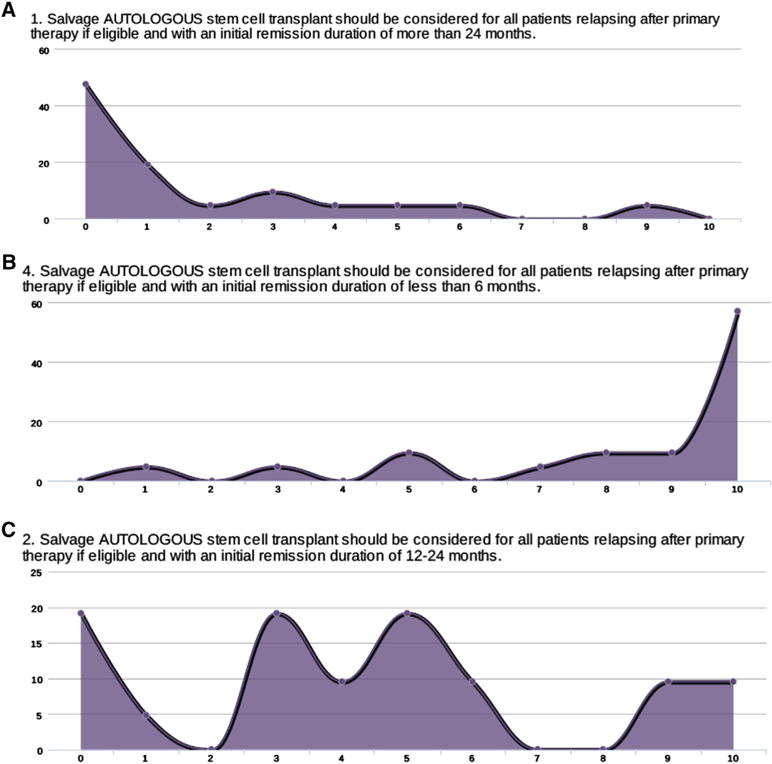
(A) Expert consensus on role of autologous HCT as consolidation therapy of an initial remission after first autograft of greater than 24 months (0 strongly agree and 10 strongly disagree, ordinate axis is the number of people who voted). (B) Expert consensus on role of autologous HCT as consolidation therapy of an initial remission after first autograft of less than 6 months (0 strongly agree and 10 strongly disagree, ordinate axis is the number of people who voted). (C) Expert consensus on role of autologous HCT as consolidation therapy of an initial remission after first autograft of between 12 and 24 months (0 strongly agree and 10 strongly disagree, ordinate axis is the number of people who voted).
CONSENSUS GUIDELINES FOR SALVAGE AUTOLOGOUS HCT
The consensus committee agreed on the following guideline statements:
In transplantation-eligible patients relapsing after primary therapy that did NOT include an autologous HCT, high-dose therapy with autologous HCT as part of salvage therapy should be considered standard.
High-dose therapy and autologous HCT should be considered appropriate therapy for any patients relapsing after primary therapy that includes an autologous HCT with initial remission duration of more than 18 months.
High-dose therapy and autologous HCT can be used as a bridging strategy to allogeneic HCT.
The role of postsalvage HCT maintenance needs to be explored in the context of well-designed prospective trials that should include new agents, such as monoclonal antibodies, IMiDs, and oral proteasome inhibitors.
Autologous HCT consolidation should be explored as a strategy to develop novel conditioning regimens or post-HCT strategies in patients with short remission (less than 18 months).
Prospective randomized trials need to be performed to define the role of salvage autologous HCT in patients with MM relapsing after primary therapy comparing to “best non-HCT” therapy.
The expert committee also underscored the importance of collecting enough hematopoietic stem cells to perform 2 transplantations early in the course of the disease. This is particularly important, as 30% of patients in the National Cancer Research Institute (NCRI) Myeloma X Relapse trial randomized to second autograft were unable to proceed to HCT because of poor collection. Recently, the International Myeloma Working Group wrote guidelines for mobilization, suggesting that plerixafor could be used in patients who did not have enough cells collected for a salvage HCT as a mobilization strategy [44].
ROLE OF ALLOGENEIC HCT AS TREATMENT FOR PATIENTS RELAPSING AFTER PRIMARY THERAPY RETROSPECTIVE STUDIES
Most studies focusing on transplantation procedures as salvage treatment strategies are separate evaluations of either a second salvage autograft or a salvage allograft. Data from comparative studies are very few and limited by their retrospective nature and/or their small study populations. The largest registry analysis was performed by Freytes et al. using data from the CIBMTR. The outcomes of a second autotransplant (n = 137) were compared to those of a salvage allograft (n = 152) after nonmyeloablative or reduced-intensity conditioning (NST/RIC) from 1995 to 2008. Non-relapse mortality (NRM) at 1 year after transplantation was higher in the NST/RIC cohort, 13% versus 2%. Three-year PFS and OS for the NST/RIC cohort were 6% and 20%, respectively, and inferior to the outcomes for the autologous transplantation cohort (12% and 46%, respectively) [45]. These results contrast with 2 recent donor versus no donor analyses performed in patients relapsing after an initial autograft. Patriarca et al. retrospectively analyzed 169 consecutive patients who had relapsed after an autograft and had undergone HLA typing immediately after relapse. The 2-year NRM was 22% among the 75 patients who had an identified HLA-compatible donor (donor group) versus 1% for those without a donor (no donor group). The 2-year PFS was 42% in the donor group and 18% in the no-donor group (P < .0001) with similar 2-year OS of 54% and 53% for the donor and no donor groups, respectively. Better response did not translate into better OS given a higher treatment-related mortality (TRM) in the donor group. An update of this study is eagerly awaited to observe possible differences in OS with longer follow up [46]. In another small donor versus no-donor comparison, de Lavallade et al. showed that patients with relapsed MM and an HLA-identical sibling who underwent reduced-intensity allograft had a significantly better event-free survival than patients without an HLA-identical sibling (46% versus 8% at 3 years) [47]. A small number of single-institution comparison have also been performed and are summarized in Table 4 [45–50].
Table 4.
Retrospective Comparisons of Autologous versus Allogeneic HCT for Patients Relapsing after an Initial Autograft
| Ref | n | NRM/RR | PFS/OS | Comments |
|---|---|---|---|---|
| [45] | Auto: 137 Allo: 152 |
4%/91% 15%/83% |
4%/29% at 5 yr 2%/9% at 5 yr |
CIBMTR analysis only RIC conditioning multiple lines of therapy |
| [46] | Auto: 94 Allo: 75% |
1%/81% 22%/48% |
18%/54% at 2 yr 42%/53% at 2 yr |
Donor versus no donor analysis on consecutive patients at first relapse after auto HCT who had HLA typing. |
| [47] | Auto: 13 Allo: 19 |
0%/86% 33%/22% |
8%/92% at 3 yr 46%/93% at 3 yr |
Donor versus no donor analysis; RIC conditioning; multiple lines of therapy |
| [48] | Auto: 42 Allo: 42 |
10%/72% 43%/33% |
NS/54% at 3 yrs NS/29% at 3 yrs |
Ablative conditioning; case-matched series |
| [49] | Auto: 26 Allo: 14 |
7%/NS 11%/NS |
6.8 mo/29 mo 7.3 mo/13 mo |
Retrospective not case controlled; RIC conditioning |
| [50] | Auto: 27 Allo: 19 |
3.7%/NS 5.3%/NS |
19 mo/23 mo 6 mo/19 mo |
Retrospective only first relapse; multiple conditioning regimens |
RR indicates relapse rate; auto, autologous; allo, allogeneic.
Most reports of allografting as salvage therapy either after first-line autologous transplantation or after more lines of therapies have been single-institution or registry analysis and have been reviewed extensively elsewhere [51–53]. All together, these studies showed the feasibility of allogeneic transplantation in relapsed MM. However, given the heterogeneous patient cohorts and differences in conditioning regimens and supportive care, its real role and curative potential have not been clearly established.
At the recent meeting of the American Society of Hematology in San Francisco (December 2014), Michallet et al. presented an EBMT registry study on allografting in MM, which included 7333 patients who underwent transplantation at a median age of 51 years between January 1990 and December 2012. Sixty-four percent of patients underwent transplantation after the year 2004. The upfront use of an allograft was observed to gradually decrease after the year 2000 to the current 12%. Remarkably, an allograft was more frequently used in recent years and, in 2012, most allografts, overall 33%, were performed in patients who relapsed after a first autograft. This may suggest an overall preference in using an allograft at first relapse after a standard autograft. The 1588 patients who received an allograft after a single autograft showed 5-year PFS and OS of 26% and 33%, respectively, whereas the 930 who received it after failing 2 lines of treatment showed 5-year PFS and OS of 24% and 29%, respectively, and the 296 who underwent transplantation with an allograft after at least 3 lines of treatment showed 5-year PFS and OS of 15% and 23%, respectively [54]. This report demonstrates the impact of lines of therapy on allogeneic HCT outcomes, but also shows that a small fraction of heavily pretreated patients can achieve long-term disease control with allogeneic HCT.
Prospective Trials
Published prospective trials on the use of an allograft in the setting of relapsed MM are very few. In a prospective multicenter EBMT trial, Kröger et al. investigated the role of allografting from unrelated donors in 49 patients who relapsed after a previous autograft. Conditioning regimen consisted of melphalan (140 mg/m2), fludarabine (90 mg/m2), and antithymocyte globulin (Fresenius) (60 mg/kg body weight). All patients showed leukocyte and platelet engraftment after a median of 15 days and 19 days, respectively. Grades II to IV acute graft-versus-host disease (GVHD) occurred in 25%, and 35% of the patients experienced chronic GVHD. The overall response rate was 95%, including 46% CR. The cumulative incidence of 1-year TRM was 25% and was significantly lower in transplantations from fully HLA-matched donors compared with from mismatched donors (10% versus 53%, P = .001). After a median follow-up of 43 months, the 5-year PFS and OS were 20% and 26%, respectively, and were significantly better in patients who achieved post-transplantation CR (41% versus 7%, P = .04, and 56% versus 16%, P = .02) [55].
The introduction of “new drugs” has made allografting a less attractive treatment option because of its toxicity, at least in newly diagnosed patients. However, the mechanisms of actions of new drugs and immune-mediated graft-versus-myeloma effects are not mutually exclusive. Kroger et al. demonstrated that the addition of IMiDs or proteasome inhibitors to donor lymphocyte infusions after allogeneic HCT could increase the frequency of CR. Thirty-two patients were treated with donor lymphocyte infusions plus either an IMiDs or bortezomib, if no response. Nineteen patients achieved CR by EBMT criteria, of which 17 had no evidence of disease by flow cytometric criteria and 15 by molecular analysis [56]. Thus, continued exploration of post-transplantation therapies with either IMiDs or proteasome inhibitors may demonstrate a definite superiority of allografting as salvage therapy. Phase II trials exploring these concepts have already been performed and are summarized in Table 5.
Table 5.
Prospective Trials of IMiDS or Proteasome Inhibitors in Allografting
| Ref | Intervention, n | NRM/RR | PFS/OS | Comment |
|---|---|---|---|---|
| [56] | DLI only: 6 DLI + IMiD and/or bortezomib: 24 |
0%/NS | CR pts: 58%/90% at 5 yr No CR pt 35%/62% at 5 yr |
59% CR by EBMT criteria; CR improved survival; 33% grade II–IV GVHD |
| [57] | Lenalidomide | 6%/42% | 52%/79% at 3 yr | 28% grades II–IV aGVHD |
| [58] | Lenalidomide 30 | 10%/27% | 63%/78% at 18 mo | 30% aGVHD grades II–IV; 47% of all planned cycles completed; most common dose upon completing study 5 mg QOD |
| [59] | Lenalidomide | NS/NS | 61%/94% at 2 yr | 37% aGVHD II–IV; 11% chronic extensive GVHD; only 10% of patients completed planned therapy |
| [60] | Bortezomib | 25%/54% | 30%/41% @ 3 yr | 25% grade III aGVHD |
| [61] | Bortezomib as part of conditioning | 16.9%/NS | 74%/77% at 2 yr | No post-transplantation maintenance in most patients |
DLI indicates donor lymphocyte infusion; aGVHD, acute graft-versus-host disease; QOD, every other day.
Kröger et al. investigated a none–total body irradiation myeloablative toxicity-reduced allograft consisting of intravenous busulfan (12.2 mg/kg) and cyclophosphamide (120 mg/kg) followed by maintenance therapy with lenalidomide in 33 MM patients relapsing after an autograft. Median remission duration after the autograft was 12 months. After the allograft, 1-year cumulative incidence of TRM was only 6%. Twenty-four patients started maintenance therapy with lenalidomide at a median dose of 5 mg for a median of 6 cycles. Cumulative incidence of relapse at 3 years was 42% and the 3-year estimated probabilities of PFS and OS were 52% and 79%, respectively [57].
Two other reports have been published regarding the role of post-RIC allogeneic HCT lenalidomide maintenance with conflicting results. Kneppers et al. began 10 mg of lenalidomide daily starting from 1 month after HCT in 35 patients with MM, of whom 30 were able to take the medication. Almost one half of the patients (47%) had to stop the drug after 2 cycles because of the development of acute GVHD. Notwithstanding, 37% of patients had a further improvement in their MM response and the 1-year PFS from start of maintenance was 69% [58]. Alsina et al. performed a multicenter trial to determine the safety and toxicity of maintenance lenalidomide after allogeneic HCT. Thirty patients with high-risk MM were enrolled at 8 centers between 2009 and 2012. The median time from HCT to initiation of lenalidomide maintenance was 96 days. Eleven patients (37%) completed maintenance and 10 mg daily was the most commonly delivered dose (44%). Most common reasons for discontinuation were acute GVHD (37%) and disease progression (37%). As seen in the German and Dutch trials, acute GVHD was seen in 38% of patients, NRM was 11%, with a PFS of 63% at 3 years and an OS of 78% at 3 years [59]. These results demonstrate that postallogeneic HCT lenalidomide maintenance is feasible and results in further reduction of MM tumor burden; however, whether this results in improved long-term outcomes is uncertain.
Caballero-Velázquez et al. evaluated bortezomib within a RIC and as maintenance after allografting in patients relapsing after a prior autograft. The conditioning consisted of fludarabine (30 mg/m2 i.v. on days −9 to −5), melphalan (70 mg/m2 i.v. on days −4, −3), and bortezomib (1.3 mg/m2 i.v. on day −2); maintenance treatment consisted of cycles of i.v. bortezomib (on days 1, 8, and 15). Sixteen patients were prospectively enrolled. Nine of 16 (56%) and 5 of 16 (31%) achieved CR and partial remission, respectively. Acute grade III GVHD was observed in 25%. Nonhematological toxicities consisted of peripheral neuropathy in 2 patients, liver toxicity in 2, and pulmonary toxicity in 1. Three-year cumulative incidences of TRM, relapse, and OS were 25%, 54%, and 41% respectively [60].
Nishihori et al. explored a similar approach but as consolidation of a first remission. Twenty-two MM patients with a very good partial response or better received fludarabine (30 mg/m2 i.v. if with bortezomib and 40 mg/m2 i.v. when without bortezomib, × 4 days) plus melphalan (70 mg/m2 i.v. ×2 days) with (n = 13) or without (n = 9) bortezomib (1.3 mg/m2). The risk of moderate to severe chronic GVHD at 2 years was 46% but the 2-year PFS was 74.8%, comparing favorably with the 52% 2-year PFS seen in similar patients who underwent an autologous HCT [61].
In the light of these recent findings, 2 large cooperative groups are planning phase II trials integrating bortezomib into the conditioning regimens followed by some form of post-transplantation therapy, including either an IMiD or a proteasome inhibitor. These trials will be offered as frontline consolidation therapy of patients with high-risk disease or as consolidation for first relapse after an initial autograft.
There have been no prospective randomized trials of allogeneic versus autologous HCT in the relapsed setting. Recently, Gahrton et al. compared outcomes of patients who relapsed on their upfront tandem autologous/reduced-intensity allogeneic HCT (auto/RICallo) versus autologous HCT. OS after progression was significantly better in the auto/RICallo group than in the autologous group (50% versus 27% at 60 months from time of progression). Salvage allograft was performed at progression in 11 patients (17%) in the auto/RICallo group and in 17 (8%) in the auto group. At last followup, a preliminary analysis showed that 3 patients obtained CR and 5 patients were alive; 2 in persistent CR in the among patients who received a salvage allograft relapsing after an auto/RICallo. Among the 17 patients who received an allograft as salvage of an autograft relapse, 8 patients obtained CR and 4 patients were alive, all in CR more than 4 years after salvage allogeneic HCT. These findings suggest that an allograft is a valid option at progression after either upfront auto/RICallo or an autograft [62].
CONSENSUS SURVEY REGARDING ROLE OF ALLOGENEIC HCT IN RELAPSED MYELOMA
Before the consensus conference, the attendants were asked to rate their agreement with a variety of statements regarding salvage HCT, giving a score of 0 if they strongly agreed with the statement and a score of 10 if they strongly disagreed.
In contrast to the autologous setting, there was much less consensus regarding the role of allografting for patients relapsing after autologous HCT, whatever the duration of remission. The results of the survey, when asked if salvage allogeneic HCT should be considered for all patients relapsing after primary therapy, if eligible, and with an initial remission duration of more than 24 months, between 12 and 24 months, and less than 6 months, can be seen in Figure 4A–C.
Figure 4.
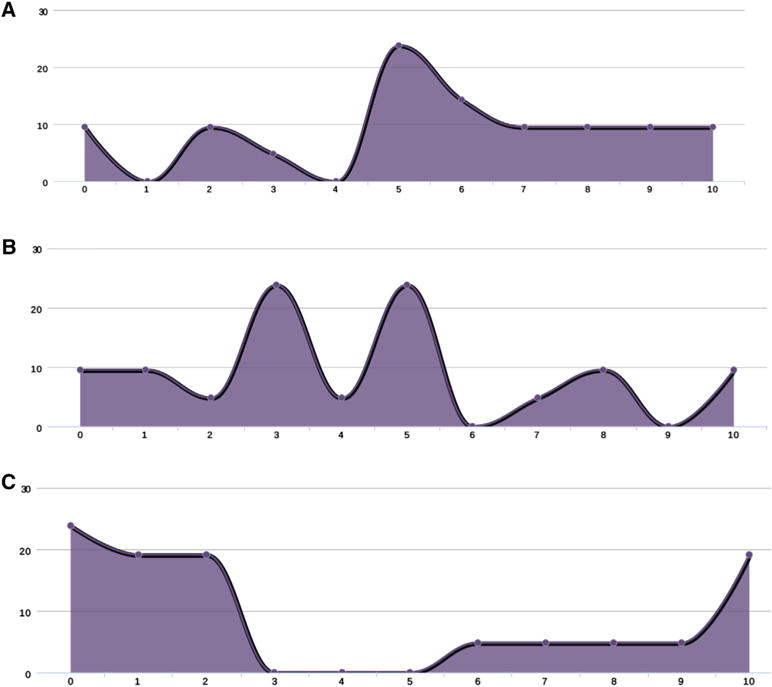
(A) Expert consensus on role of allogeneic HCT as consolidation therapy of an initial remission after first autograft of greater than 24 months (0 strongly agree and 10 strongly disagree, ordinate axis is the number of people who voted). (B). Expert consensus on role of allogeneic HCT as consolidation therapy of an initial remission after first autograft between 12 and 24 months (0 strongly agree and 10 strongly disagree, ordinate axis is the number of people who voted). (C) Expert consensus on role of allogeneic HCT as consolidation therapy of an initial remission after first autograft of less than 6 months (0 strongly agree and 10 strongly disagree, ordinate axis is the number of people who voted).
CONSENSUS GUIDELINES REGARDING ROLE OF ALLOGENEIC HCT IN RELAPSED MYELOMA
The expert committee agreed on the following guideline statements:
Allogeneic HCT should be considered appropriate therapy for any eligible patient with early relapse (less than 24 months) after primary therapy that included an autologous HCT or with high-risk features (ie, cytogenetics, extramedullary disease, plasma cell leukemia, or high lactate dehydrogenase) provided that they responded favorably to salvage therapy before allogeneic HCT.
Whenever possible, allogeneic HCT should be performed in the context of a clinical trial.
The role of postallogeneic HCT maintenance therapy needs to be further explored.
Prospective randomized trials need to be performed to define the role of salvage allogeneic HCT in patients with MM relapsing after primary therapy.
HCT FOR PATIENTS WITH REFRACTORY DISEASE
The Spanish myeloma group looked at the outcome of outcome of primary refractory MM, which they defined as never having achieved a minimal response or better [63]. Patients underwent either tandem autologous or autologous HCT followed by RIC allogeneic transplantation. Patients progressing under induction therapy had shorter OS from first transplantation than the stable disease group. However, induction therapy for all patients did not include any novel agents but was with multiagent conventional chemotherapy. In the Mayo series, 50 patients with primary refractory MM, defined as failure to achieve a partial response or greater, were compared to 101 patients with chemosensitive disease. Twenty percent of the chemorefractory group achieved a CR with the transplantation and had a 1-year PFS of 70% [64]. None of these patients received modern day therapy with IMiDs and proteasome inhibitors.
A more contemporaneous analysis from the CIBMTR may be more informative [65]. Five hundred ninety-three patients who underwent autologous HCT after failing to achieve less than partial remission to first-line induction therapy were identified. The patients were divided into 2 groups: those who received additional salvage therapy and those who did not before HCT. On multivariate analysis, there was no impact of pretransplantation salvage on TRM, PFS, or OS, thereby suggesting that those with suboptimal response to induction could still derive a benefit from high-dose chemotherapy.
KNOWLEDGE GAPS AND MOST COMPELLING QUESTIONS
The expert committee recognized that further randomized trials of the optimal type and timing of salvage HCT are essential. Particularly, can patients relapsing after primary therapy be risk stratified into high-risks groups, with patients with high-risk disease proceeding to more aggressive salvage therapies, including allogeneic HCT, and patients with very low-risk disease continued on maintenance therapy without high-dose consolidation? The International Myeloma Working Group is currently performing such an analysis and these results will be essential to guide therapy in the relapse setting.
Although recent data suggest that attaining a deep response with primary therapy predicts prolonged response before relapse, the impact of depth of response in the salvage setting is less certain. The addition of new technologies, such as flow cytometry and deep gene sequencing, now allows for monitoring and detection of minimal residual disease. How this information will impact the treatment of patients relapsing after primary therapy is still uncertain, but it will likely guide us in deciding the intensity of salvage therapies [66].
Likewise, the possibility that some cytogenetic or molecular subgroups may not benefit from high-dose therapy needs to be carefully explored. Pharmacogenomic predictors of response to melphalan are currently under investigation [67].
As the role of post-transplantation therapy has been well established in the setting of frontline autologous HCT, the expert committee recommended that prospective trials exploring optimal postsalvage HCT maintenance therapy be considered a priority, particularly those exploring novel immunotherapy strategies postsalvage HCT (ie, vaccines, cellular therapies, monoclonal antibodies, novel proteasome inhibitors, histone deacetylase [HDAC] inhibitors, and others). With the use of prolonged therapy after primary therapy, such as maintenance, it will be critically important to investigate novel agents and combinations to overcome resistance to the agent used during maintenance (more commonly lenalidomide, bortezomib, or even the combination). Novel conditioning regimens should also be explored in this setting.
CURRENT PROTOCOL PORTFOLIO
Numerous trials are currently registered on the ClinicalTrials.gov website involving HCT in the salvage setting. NCT01745588 aims to compare salvage HCT with pomalidomide/dexamethasone and clarithromycin as a maintenance schedule against a non-HCT strategy of pomalidomide, clarithromycin, and dexamethasone. NCT01242267 aims to address the augmentation of high-dose melphalan with increasing doses of thalidomide in a phase I/II setting. NCT00938626 is aiming to utilize Bite antibodies to augment T cell activation before salvage HCT in a phase I/II. The Intergroupe Francophone du Myelome is conducting a phase 2 study (NCT02244125) for patients in first relapse following the IFM 2009/DFCI trial (VRD [bortezomib (velcade) lenalidomide and dexamethasone] induction followed by upfront or delayed HCT followed by a VRD consolidation and 1-year lenalidomide maintenance). All patients are treated with the combination of pomalidomide plus cyclophosphamide and dexamethasone. The primary endpoint is response after 4 cycles. If patients are not progressive, they undergo an autologous HCT if they did not get it upfront followed by pomalidomide plus cyclophosphamide and dexamethasone consolidation and pomalidomide plus dexamethasone maintenance. The German-speaking multiple myeloma study group currently enrolls patients in the “RELAPSE” trial, comparing an early versus a late auto HCT in patients in patients with 1 to 3 prior therapies (EudraCT 2009-013856-61, ISRCTN 16345835).
The European Myeloma Network (EMN-alloRIC2010, Eudra-CT number: 2010-018594-37, ClinicalTrials.gov Identifier: NCT01460420) has a trial that aims at optimizing clinical outcomes by including bortezomib in a melphalanbased conditioning regimen and using a combination of bortezomib and lenalidomide as post-transplantation maintenance. Candidates are high-risk myeloma patients, younger than 70 years, with early relapse after first primary therapy with new drugs and autologous HCT.
The next NCRI myeloma trial is a randomized trial exploring the role of an augmented melphalan conditioning regimen by adding bortezomib followed by a second randomization to either ixazomib or placebo for consolidation and maintenance in patients relapsing after an initial autograft.
The Blood and Marrow Transplant Clinical Trials Network will perform a phase II randomized trial of ixazomib maintenance after allogeneic HCT. The conditioning regimen will be melphalan-bortezomib. Both patients with high-risk myeloma and in first relapse after an autologous HCT will be eligible.
There is an Medical Research Council study that is being developed for relapsed disease after primary therapy.
CONCLUSIONS
Patients with relapsed MM have considerable options for salvage treatment. Randomized trials are demonstrating that, as with frontline therapy, depth of response to therapy predicts outcomes. Thus, optimal use of high-dose therapy in the salvage setting needs to be extensively explored through well-designed prospective trials that include and explore the newly developed agents for MM at different phases of the HCT process (induction, conditioning, and maintenance). In the meantime, both autologous and allogeneic HCT should be considered valid clinical options based on this consensus statement.
Acknowledgments
Financial disclosure: Blood and Marrow Transplant Clinical Trials Network U10HL069294 from the National Heart, Lung, and Blood Institute and the National Cancer Institute U10HL069294) and by the Be The Match Foundation (Grant # U10HL069294, and an Unrestricted educational grant to the International Myeloma Foundation from SANOFI Corporation.
Footnotes
Authorship statement: S.G. and L.G. contributed equally to this manuscript and work and should be considered cofirst authors. N.K. and E.S. contributed equally to the organization of the Consensus Conference and the development of the manuscript and should be considered as cosenior authors.
References
- 1.Kyle RA, Rajkumar SV. Multiple myeloma. N Engl J Med. 2004;351:1–10. doi: 10.1056/NEJMra041875. [DOI] [PubMed] [Google Scholar]
- 2.Hoering A, Crowley J, Shaugnessy JD, et al. Complete remission in multiple myeloma examined as time-dependent variable in terms of both onset and duration in Total Therapy protocols. Blood. 2009;114:1299–1305. doi: 10.1182/blood-2009-03-211953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.San Miguel JF, Mateos MV, Ocio E, Garcia-Sanz R. Multiple myeloma: treatment evolution. Hematology. 2012;(Suppl 1):S3–S6. doi: 10.1179/102453312X13336169154971. [DOI] [PubMed] [Google Scholar]
- 4.Cavo M, Rajkumar SV, Palumbo A, et al. International Myeloma Working Group consensus approach to the treatment of multiple myeloma patients who are candidates for autologous stem cell transplantation. Blood. 2011;117:6063–6073. doi: 10.1182/blood-2011-02-297325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Avet-Loiseau H, Koreth J, Cutler CS, et al. High-dose therapy with single autologous transplantation versus chemotherapy for newly diagnosed multiple myeloma: A systematic review and meta-analysis of randomized controlled trials. Biol Blood Marrow Transplant. 2007;13:183–190. doi: 10.1016/j.bbmt.2006.09.010. [DOI] [PubMed] [Google Scholar]
- 6.Hahn T, Wingard J, Anderson K, et al. The role of cytotoxic therapy with hematopoietic stem cell transplantation in the therapy of multiple myeloma: an evidence-based review. Biol Blood Marrow Transplant. 2003;9:4–37. doi: 10.1053/bbmt.2003.50002. [DOI] [PubMed] [Google Scholar]
- 7.Avet Loiseau H, Attal M, Campion L, et al. Long-term analysis of the IFM 99 trials for myeloma: cytogenetic abnormalities [t(4;14), del(17p), 1q gains] play a major role in defining long-term survival. J Clin Oncol. 2012;30:1949–1952. doi: 10.1200/JCO.2011.36.5726. [DOI] [PubMed] [Google Scholar]
- 8.Sutlu T, Alici E, Jansson M, et al. The prognostic significance of 8p21 deletion in multiple myeloma. Br J Haematol. 2009;144:266–268. doi: 10.1111/j.1365-2141.2008.07454.x. [DOI] [PubMed] [Google Scholar]
- 9.Gmidène A, Saad A, Avet-Loiseau H. 8p21.3 deletion suggesting a probable role of TRAIL-R1 and TRAIL-R2 as candidate tumor suppressor genes in the pathogenesis of multiple myeloma. Med Oncol. 2013;30:489. doi: 10.1007/s12032-013-0489-8. [DOI] [PubMed] [Google Scholar]
- 10.Jakubowiak A. Management strategies for relapsed/refractory multiple myeloma: current clinical perspectives. Semin Hematol. 2012;49:S16–S32. doi: 10.1053/j.seminhematol.2012.05.003. [DOI] [PubMed] [Google Scholar]
- 11.Kumar SK, Therneau TM, Gertz MA, et al. Clinical course of patients with relapsed multiple myeloma. Mayo Clin Proc. 2004;79:867–874. doi: 10.4065/79.7.867. [DOI] [PubMed] [Google Scholar]
- 12.Kumar SK, Lee JH, Lahuerta JJ, et al. Risk of progression and survival in multiple myeloma relapsing after therapy with IMiDs and bortezomib: a multicenter international myeloma working group study. Leukemia. 2012;26:149–154. doi: 10.1038/leu.2011.196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alegre A, Granda A, Martinez C, et al. Different patterns of relapse after autologous peripheral blood stem cell transplantation in multiple myeloma: clinical results of 280 cases from the Spanish Registry. Haematologica. 2002;87:609–614. [PubMed] [Google Scholar]
- 14.Lenhoff S, Hjorth M, Turnisson I, et al. Intensive therapy for multiple myeloma in patients younger than 60 years. Long term results focusing on the effect of the degree of response and relapse pattern after transplantation. Haematologica. 1996;91:1228–1233. [PubMed] [Google Scholar]
- 15.Anagnostopoulos A, Weber D, Rankin K, et al. Thalidomide and dexamethasone for resistant multiple myeloma. Br J Haematol. 2003;121:768–771. doi: 10.1046/j.1365-2141.2003.04345.x. [DOI] [PubMed] [Google Scholar]
- 16.Weber DM, Chen C, Niesvizky R, et al. Lenalidomide plus dexamethasone for relapsed multiple myeloma in North America. N Engl J Med. 2007;357:2133–2142. doi: 10.1056/NEJMoa070596. [DOI] [PubMed] [Google Scholar]
- 17.Dimopoulos M, Spencer A, Attal M, et al. Lenalidomide plus dexamethasone for relapsed or refractory multiple myeloma. N Engl J Med. 2007;357:2123–2132. doi: 10.1056/NEJMoa070594. [DOI] [PubMed] [Google Scholar]
- 18.Dimopoulos MA, Chen C, Spencer A, et al. Long-term follow-up on overall survival from the MM-009 and MM 010 phase III trials of lenalidomide plus dexamethasone in patients with relapsed or refractory multiple myeloma. Leukemia. 2009;23:2147–2152. doi: 10.1038/leu.2009.147. [DOI] [PubMed] [Google Scholar]
- 19.San Miguel J, Weisel K, Moreau P, et al. Pomalidomide plus low-dose dexamethasone versus high-dose dexamethasone alone for patients with relapsed and refractory multiple myeloma (MM-003): a randomised, open-label, phase 3 trial. Lancet Oncol. 2013;14:1055–1066. doi: 10.1016/S1470-2045(13)70380-2. [DOI] [PubMed] [Google Scholar]
- 20.Richardson PG, Sonneveld P, Schuster M, et al. Extended follow-up of a phase 3 trial in relapsed multiple myeloma: final time-to-event results of the APEX trial. Blood. 2007;110:3557–3560. doi: 10.1182/blood-2006-08-036947. [DOI] [PubMed] [Google Scholar]
- 21.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 22.Papadopoulos KP, Siegel DS, Vesole D, et al. Phase I study of 30 minute infusion of carfilzomib as single agent or in combination with low dose dexamethasone in patients with relapsed and/or refractory multiple myeloma. J Clin Oncol. 2014;32:3522–3533. doi: 10.1200/JCO.2013.52.3522. [DOI] [PubMed] [Google Scholar]
- 23.Pineda-Roman M, Zangari M, van Rhee F, et al. VTD combination therapy with bortezomib-thalidomide-dexamethasone is highly effective in advanced and refractory multiple myeloma. Leukemia. 2008;22:1419–1427. doi: 10.1038/leu.2008.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Richardson PG, Jagannath S, Jakubowiak AJ, et al. Phase II trial of lenalidomide, bortezomib, and dexamethasone in patients (pts) with relapsed and relapsed/refractory multiple myeloma (mm): updated efficacy and safety data after >2 years of follow-up [ASH Annual Meeting Abstracts] Blood. 2010;116:3049. [Google Scholar]
- 25.Reece DE, Rodriguez GP, Chen C, et al. Phase I–II trial of bortezomib plus oral cyclophosphamide and prednisone in relapsed and refractory multiple myeloma. J Clin Oncol. 2008;26:4777–4783. doi: 10.1200/JCO.2007.14.2372. [DOI] [PubMed] [Google Scholar]
- 26.Wang M, Martin T, Bensinger W, et al. Phase 2 dose expansion study of PX-171-006 of carfilzomib, lenalidomide, and low dose dexamethasone in relapsed or progressive multiple myeloma. Blood. 2013;122:3122–3128. doi: 10.1182/blood-2013-07-511170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lonial S, Vij R, Harousseau JL, et al. Elotuzumab in combination with lenalidomide and low dose dexamethasone in relapsed or refractory myeloma. J Clin Oncol. 2012;30:1953–1959. doi: 10.1200/JCO.2011.37.2649. [DOI] [PubMed] [Google Scholar]
- 28.Garderet L, Iacobelli S, Moreau P, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 randomized phase III trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2475–2482. doi: 10.1200/JCO.2011.37.4918. [DOI] [PubMed] [Google Scholar]
- 29.Stewart AK, Rajkumar V, Dimopoulos MA, et al. Carfilzomib, lenalidomide and dexamethasone for relapsed multiple myeloma. N Engl J Med. 2015;372:142–152. doi: 10.1056/NEJMoa1411321. [DOI] [PubMed] [Google Scholar]
- 30.San Miguel JF, Hungria VT, Yoon SS, et al. Panobinostat plus bortezomib and dexamethasone versus placebo plus bortezomib and dexamethasone in patients with relapsed or relapsed and refractory multiple myeloma: a multicentre, randomized, double blind phase 3 trial. Lancet Oncol. 2014;11:1195–1206. doi: 10.1016/S1470-2045(14)70440-1. [DOI] [PubMed] [Google Scholar]
- 31.Lonial S, Dimopoulos M, Palumbo A, et al. Elotuzumab therapy for relapsed or refractory multiple myeloma. N Engl J Med. 2015;373:621–631. doi: 10.1056/NEJMoa1505654. [DOI] [PubMed] [Google Scholar]
- 32.Shah N, Ahmed F, Bashir Q, et al. Durable remission with salvage second auto-transplants in patients with multiple myeloma. Cancer. 2012;118:3549–3555. doi: 10.1002/cncr.26662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Jimenez-Zepeda VH, Mikhael J, Winter A, et al. Second autologous stem cell transplantation as salvage therapy for multiple myeloma: impact on progression-free and overall survival. Biol Blood Marrow Transplant. 2012;18:773–779. doi: 10.1016/j.bbmt.2011.10.044. [DOI] [PubMed] [Google Scholar]
- 34.Olin RL, Vogl DT, Porter DL, et al. Second auto-SC is safe and effective salvage therapy for relapsed multiple myeloma. Bone Marrow Transplant. 2009;43:417–422. doi: 10.1038/bmt.2008.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Fenk R, Liese V, Neubauer F, et al. Predictive factors for successful salvage high-dose therapy in patients with multiple myeloma relapsing after autologous blood stem cell transplantation. Leuk Lymphoma. 2011;52:1455–1462. doi: 10.3109/10428194.2011.575967. [DOI] [PubMed] [Google Scholar]
- 36.Alvares CL, Davies FE, Horton C, et al. The role of second autografts in the management of myeloma at first relapse. Haematologica. 2006;91:141–142. [PubMed] [Google Scholar]
- 37.Silva Rondon C, Hassoun H, Chimento D, et al. Outcomes following salvage autologous stem cell transplant for multiple myeloma. Biol Blood Marrow Transplant. 2012;18:S254. [Google Scholar]
- 38.Gonzalvez WI, Gertz MA, Lacy MQ, et al. Second auto stem cell transplant for treatment of relapsed multiple myeloma. Bone Marrow Transplant. 2012;10:1–6. doi: 10.1038/bmt.2012.183. [DOI] [PubMed] [Google Scholar]
- 39.Michaelis LC, Saad A, Zhong X, et al. Savage second hematopoietic cell transplantation in myeloma. Biol Blood Marrow Transplant. 2013;19:760–766. doi: 10.1016/j.bbmt.2013.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sellner L, Heiss C, Benner A, et al. Autologous retransplantation for patients with recurrent multiple myeloma. A single-center experience with 200 patients. Cancer. 2013;119:2438–2446. doi: 10.1002/cncr.28104. [DOI] [PubMed] [Google Scholar]
- 41.Grövdal M, Nahi H, Gahrton G, et al. Autologous stem cell transplantation versus novel drugs or conventional chemotherapy for patients with relapsed multiple myeloma after previous ASCT. Bone Marrow Transplant. 2015;50:808–812. doi: 10.1038/bmt.2015.39. [DOI] [PubMed] [Google Scholar]
- 42.Cook G, Williams C, Brown JM, et al. High-dose chemotherapy plus autologous stem-cell transplantation as consolidation therapy in patients with relapsed multiple myeloma after previous autologous stem-cell transplantation (NCRI Myeloma X Relapse [intensive trial]): a randomised, open-label, phase 3 trial. Lancet Oncol. 2014;15:874–885. doi: 10.1016/S1470-2045(14)70245-1. [DOI] [PubMed] [Google Scholar]
- 43.Gimsing P, Hjertner O, Abildgaard N, et al. Salvage bortezomib-dexamethasone and high dose melphalan (HDM) and autologous stem cell support (ASCT) in myeloma patients at first relapse after HDM and ASCT. A phase-2 trial. Bone Marrow Transplant. 2015;50:1306–1311. doi: 10.1038/bmt.2015.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Giralt S, Stadtmauer EA, Harousseau JL, et al. International myeloma working group (IMWG) consensus statement and guidelines regarding the current status of stem cell collection and high-dose therapy for multiple myeloma and the role of plerixafor (AMD 3100) Leukemia. 2009;10:1904–1912. doi: 10.1038/leu.2009.127. [DOI] [PubMed] [Google Scholar]
- 45.Freytes CO, Vesole DH, LeRademacher L, et al. Second transplants for multiple myeloma relapsing after a previous autotransplantd–reduced-intensity allogeneic versus autologous transplantation. Bone Marrow Transplant. 2014;49:416–421. doi: 10.1038/bmt.2013.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Patriarca F, Einsele H, Spina F, et al. Allogeneic stem cell transplantation in multiple myeloma relapsed after autograft: a multicenter retrospective study based on donor availability. Biol Blood Marrow Transplant. 2012;18:617–626. doi: 10.1016/j.bbmt.2011.07.026. [DOI] [PubMed] [Google Scholar]
- 47.De Lavallade H, El-Cheikh J, Faucher C, et al. Reduced-intensity conditioning allogeneic SCT as a salvage treatment for relapsed multiple myeloma. Bone Marrow Transplant. 2008;41:953–960. doi: 10.1038/bmt.2008.22. [DOI] [PubMed] [Google Scholar]
- 48.Mehta J, Tricot G, Jagannath S, et al. Salvage autologous or allogeneic transplantation for multiple myeloma refractory to or relapsing after a first-line autograft. Bone Marrow Transplant. 1998;21:887–892. doi: 10.1038/sj.bmt.1701208. [DOI] [PubMed] [Google Scholar]
- 49.Qazilbash MH, Saliba R, De Lima M, et al. Second autologous or allogeneic transplantation after the failure of first autograft in patients with multiple myeloma. Cancer. 2006;106:1084–1089. doi: 10.1002/cncr.21700. [DOI] [PubMed] [Google Scholar]
- 50.Wirk B, Byrne M, Dai Y, Moreb JS. Outcomes of salvage autologous versus allogeneic hematopoietic cell transplantation for relapsed multiple myeloma after initial autologous hematopoietic cell transplantation. J Clin Med Res. 2013;5:174–184. doi: 10.4021/jocmr1274w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Giralt S, Koehne G. Allogeneic hematopoietic stem cell transplantation for multiple myeloma: What place, if any? Curr Hematol Malig Rep. 2013;8:284–290. doi: 10.1007/s11899-013-0185-y. [DOI] [PubMed] [Google Scholar]
- 52.Lokhorst H, Einsele H, Vesole D, et al. International Myeloma Working Group consensus statement regarding the current status of allogeneic stem-cell transplantation for multiple myeloma. J Clin Oncol. 2010;28:4521–4530. doi: 10.1200/JCO.2010.29.7929. [DOI] [PubMed] [Google Scholar]
- 53.Bensinger W. Allogeneic stem cell transplantation for multiple myeloma. Hematol Oncol Clin North Am. 2014;28:891–902. doi: 10.1016/j.hoc.2014.06.001. [DOI] [PubMed] [Google Scholar]
- 54.Michallet M, Sobh M, Iacobelli S, et al. Allogeneic hematopoietic stem cell transplantation for multiple myeloma: evolution and outcomes over more than two decades within EBMT centers [ASH Annual Meeting Abstracts] Blood. 2014;124 Abstract 2554. [Google Scholar]
- 55.Kröger N, Shimoni A, Schilling G, et al. Unrelated stem cell transplantation after reduced intensity conditioning for patients with multiple myeloma relapsing after autologous transplantation. Br J Haematol. 2010;148:323–331. doi: 10.1111/j.1365-2141.2009.07984.x. [DOI] [PubMed] [Google Scholar]
- 56.Kröger N, Zabelina T, Badbaran A, et al. Post-transplant immunotherapy with donor-lymphocyte infusion and novel agents to upgrade partial into complete and molecular remission in allografted patients with multiple myeloma. Exp Hematol. 2009;37:791–798. doi: 10.1016/j.exphem.2009.03.008. [DOI] [PubMed] [Google Scholar]
- 57.Kröger N, Zabelina T, Klyuchnikov E, et al. Toxicity-reduced, myeloablative allograft followed by lenalidomide maintenance as salvage therapy for refractory/relapsed myeloma patients. Bone Marrow Transplant. 2013;48:403–407. doi: 10.1038/bmt.2012.142. [DOI] [PubMed] [Google Scholar]
- 58.Kneppers E, Van der Holt B, Kersten MJ, et al. Lenalidomide maintenance after nonmyeloablative allogeneic stem cell transplantation in multiple myeloma is not feasible: results of the HOVON 76 Trial. Blood. 2011;118:2413–2419. doi: 10.1182/blood-2011-04-348292. [DOI] [PubMed] [Google Scholar]
- 59.Alsina M, Becker P, Zhong X, et al. Lenalidomide maintenance for high-risk multiple myeloma after allogeneic hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2014;20:1183–1189. doi: 10.1016/j.bbmt.2014.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Caballero-Velázquez T, López-Corral L, Encinas C, et al. Phase II clinical trial for the evaluation of bortezomib within the reduced intensity conditioning regimen (RIC) and post-allogeneic transplantation for high-risk myeloma patients. Br J Haematol. 2013;162:474–482. doi: 10.1111/bjh.12410. [DOI] [PubMed] [Google Scholar]
- 61.Nishihori T, Ochoa Bayona JL, Kim J, et al. Allogeneic hematopoietic cell transplantation for consolidation of VGPR or CR for newly diagnosed multiple myeloma. Bone Marrow Transplant. 2013;48:1179–1184. doi: 10.1038/bmt.2013.37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gahrton G, Iacobelli S, Bjorkstrand B, et al. Autologous/reduced-intensity allogeneic stem cell transplantation vs autologous transplantation in multiple myeloma: long-term results of the EBMT-NMAM2000 study. Blood. 2013;121:5055–5063. doi: 10.1182/blood-2012-11-469452. [DOI] [PubMed] [Google Scholar]
- 63.Rosinol L, Garcia-Sanz R, Lahuerta JJ, et al. Benefit from autologous stem cell transplantation in primary refractory myeloma? Different outcomes in progressive versus stable disease. Haematologica. 2012;97:616–621. doi: 10.3324/haematol.2011.051441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Kumar S, Lacy MQ, Dispenzieri A, et al. High dose therapy and autologous stem cell transplantation for multiple myeloma poorly responsive to initial therapy. Bone Marrow Transplant. 2004;34:161–167. doi: 10.1038/sj.bmt.1704545. [DOI] [PubMed] [Google Scholar]
- 65.Vij R, Kumar S, Zhang MJ, et al. Impact of pretransplant therapy and depth of disease response before autologous transplantation for multiple myeloma. Biol Blood Marrow Transplant. 2015;21:335–341. doi: 10.1016/j.bbmt.2014.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Mailankody S, Korde N, Lesokhin A, et al. Minimal residual disease in multiple myeloma: bringing the bench to the bedside. Nat Rev Clin Oncol. 2015;12:286–295. doi: 10.1038/nrclinonc.2014.239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Falgreen S, Dybkaer K, Young KH, et al. Predicting response to multidrug regimens in cancer patients using cell line experiments and regularized regression models. BMC Cancer. 2015;15:235. doi: 10.1186/s12885-015-1237-6. [DOI] [PMC free article] [PubMed] [Google Scholar]


