SUMMARY
The esophagogastric junction contractile integral (EGJ-CI), designed similar to distal contractile integral (DCI), has been proposed as a metric to evaluate EGJ barrier function. We determined normative values and evaluated EGJ-CI in predicting esophageal acid exposure time (AET) and symptomatic outcome in this observational cohort study. High-resolution manometry (HRM) studies were reviewed in 188 patients (55.2 ± 0.9 years, 64% female) undergoing ambulatory pH monitoring off therapy. Dominant symptoms and global symptom severity (GSS) were determined on questionnaires initially and upon follow-up. EGJ-CI was measured using the DCI tool placed across the EGJ and compared to normal controls (n = 21, 27.6 ± 0.6 years, 52% female). EGJ-CI was calculated both for a single respiratory cycle (SRC, in mmHg.cm.s) and corrected for respiratory cycle (CRC, mmHg.cm). Univariate and multivariate analyses determined the predictive potential of EGJ-CI in terms of AET and post-therapy GSS at follow-up, controlling for medical versus surgical therapy. Mean EGJ-CI values were significantly lower when AET was abnormal; EGJ-CI/SRC and EGJ-CI/CRC were 86% concordant (r = 0.84). Using receiver operating characteristic analysis, values below 121.8 mmHg.cm.s (EGJ-CI/SRC) and 39.3 mmHg.cm (EGJ-CI/CRC) predicted abnormal AET best (sensitivity 0.61 and 0.65, specificity 0.61 and 0.57, respectively). On univariate and multivariate analysis, the EGJ-CI discriminated normal from abnormal AET better than conventional LES parameters (P ≤ 0.02). After 2.7 ± 0.1 years follow-up, EGJ-CI below identified thresholds predicted better symptom response to antireflux surgery compared to medical therapy (P = 0.009). EGJ-CI is a novel HRM metric that has potential to complement or replace currently used basal LES and EGJ parameters.
Keywords: esophagogastric junction, gastroesophageal reflux disease, high-resolution manometry
INTRODUCTION
The esophagogastric junction (EGJ) serves as a barrier between the esophagus and the stomach. In health, the EGJ has both a sphincter component (lower esophageal sphincter [LES]) and a superimposed diaphragmatic crural component to provide a mechanical and physiologic barrier to spontaneous reflux of gastric content into the esophagus.1 While transient LES relaxation is considered to be the most frequent mechanism of gastroesophageal reflux (GER), structural and physiologic weakness in the EGJ barrier can also promote GER.2,3 Structural weakness can manifest as a separation between the intrinsic LES and the diaphragmatic crura, which defines a hiatus hernia.4 Physiologic weakness can manifest as low LES tone or a hypotensive LES. Since the LES tone is dynamic and augments with inspiration, end-expiratory LES pressure has been utilized to define basal LES tone independent of diaphragmatic contribution. As would be anticipated, patients referred for antireflux surgery (ARS) have a higher likelihood of a disrupted EGJ barrier, and reestablishment of barrier function with ARS leads to symptom improvement in GER.5
With the advent of high-resolution manometry (HRM), vigor of peristaltic events in the esophageal body is evaluated using a computerized space–time algorithm that takes duration, amplitude and length of the contracting segment into consideration, termed the distal contractile integral (DCI).6 Indeed, the DCI has been adapted to define both hypercontractile and hypocontractile esophageal body conditions.7,8 A metric similar to the DCI concept has been proposed for interrogation of the EGJ barrier function. The first report by Hoshino et al. consisted of measuring the DCI of the LES (termed LES pressure integral) over a 10-second period of time.9 The LES pressure integral was further refined by Nicodeme et al. to be independent of respiration, by measuring the DCI value at the LES above a threshold of 2 mmHg above the gastric baseline, and dividing the recorded value by the duration of complete respiratory cycles (three cycles in this study).10 This new metric, termed the EGJ-contractile integral (EGJ-CI) since it takes the EGJ barrier into account, segregated GER patients as defined by abnormal pH-impedance studies from healthy controls. However, the impact of the EGJ-CI on symptom outcome and its ability to predict abnormal esophageal acid exposure remain uncertain. Further, the measurement technique described required multiple steps to set the threshold for the DCI measurement across the EGJ.
In this study, we evaluated the EGJ-CI in a cohort of patients undergoing ambulatory pH monitoring off acid suppressive therapy and normal healthy controls with the aims of: (i) simplification of the calculation process by measuring the DCI above the gastric baseline, both averaged to a single respiratory cycle (SRC) and corrected for respiratory cycle (CRC) to be independent of respiration, (ii) determining and comparing normative values, and (iii) assessing the predictive value of the EGJ-CI in terms of esophageal acid exposure and symptom improvement with antireflux therapy.
METHODS
All adult patients (>18 years) undergoing esophageal HRM using the Given system (Given Imaging/Sierra Scientific, Los Angeles, CA) in conjunction with ambulatory pH monitoring off antisecretory therapy over a 6-year period (2006–2012) were eligible for inclusion. All subjects completed a symptom questionnaire detailing dominant symptoms and global symptom severity (GSS) on a 100-point visual analog scale at the time of their esophageal physiologic studies. For inclusion, subjects were prospectively contacted for repeat completion of the same symptom questionnaire and for determination of therapy and outcome. Exclusion criteria included incomplete esophageal HRM or ambulatory pH monitoring data, incomplete symptom data, prior foregut surgery, primary motor disorders (including achalasia spectrum disorders and esophageal outflow obstruction), and lack of follow-up questionnaire data. Informed consent was obtained from each subject to include review of clinical data and completion of survey questions related to the study. The control group was composed of healthy asymptomatic patients who had no history of gastrointestinal symptoms, no upper gastrointestinal tract surgery, on no regular medications, and no other medical conditions. These control subjects underwent HRM as part of the institutional normative data assessment, after informed consent. The study protocol was approved by the Human Research Protection Office (Institutional Review Board) at Washington University in St Louis.
Calculation of EGJ-CI
HRM studies were performed after an overnight fast using a 36-channel solid-state catheter system with high-fidelity circumferential sensors at 1 cm intervals (Given Imaging/Sierra Scientific, Los Angeles, CA). After calibration, the catheter was passed through an anesthetized nasal canal. A 20-second swallow-free period was first obtained after the subject remained still and resting quietly in the recumbent position (landmark period), from which basal LES pressures were obtained. Ten swallows were recorded using 4–5 mL of ambient temperature water spaced >20 seconds apart. Studies were acquired and analyzed using dedicated computerized HRM acquisition, display and analysis systems (ManoView; Given Imaging/Sierra Scientific, Los Angeles, CA).
In addition to standard analysis of the motor pattern using the Chicago Classification,11 specific attention was focused on EGJ metrics. Mean basal LES pressure, end-expiratory LES pressure, LES length, and integrated relaxation pressure (IRP) were recorded. The presence or absence of a measurable hiatus hernia was determined. For the purposes of this study, a hiatus hernia consisted of consistent and complete spatial separation between the identified LES and diaphragmatic crural contraction, measured in 1-cm increments. Therefore, we designated no separation as type I, separation ≤2 cm as type II, and separation ≥3 cm as type III, as described by Pandolfino et al.12 This definition was chosen so as to segregate settings where the EGJ-CI could be measured across both LES and diaphragmatic crura, or alternatively, across the LES alone in the presence of a hiatus hernia.
The landmark phase recording was first identified and confirmed to be separate from swallows and artifacts, obtained during a period of quiet rest after the patient settled down.13 Three respiratory cycles were identified and duration measured (Fig. 1). The gastric baseline was recorded, and this value was set as the threshold for the DCI calculation algorithm. The DCI software measurement tool was then forced across the EGJ, with the proximal and distal margins approximating the respective contours of the EGJ or LES as identified by a sharp change in pressure values and color change from both the esophageal body and the gastric lumen, respectively (Fig. 1). In the presence of a type III hiatus hernia, the LES barrier function was determined with the DCI measurement box positioned across the LES (Fig. 1B). The EGJ-CI was recorded for the three respiratory cycles. This was divided by 3 to obtain the EGJ-CI for a single respiratory cycle (EGJ-CI/SRC), and by the duration of the respiratory cycles for EGJ-CI independent of respiration (EGJ-CI/CRC).
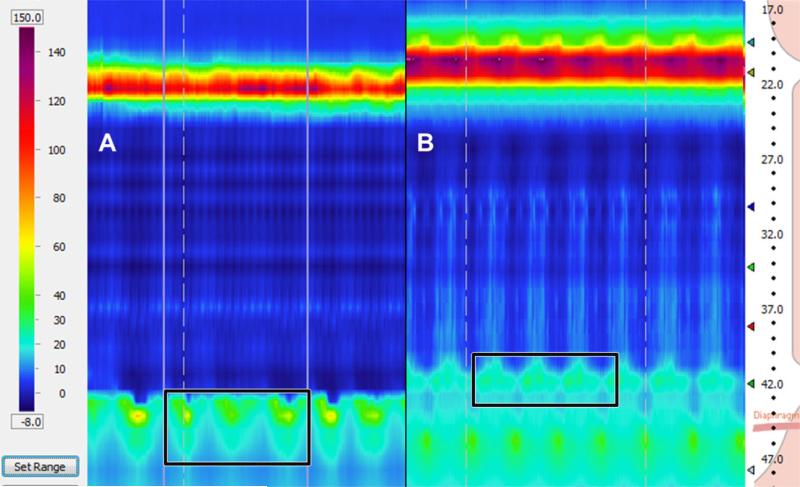
Fig. 1.
Calculation window for measurement of the esophagogastric junction contractile integral (EGJ-CI). (A) The software tool assessing contraction vigor (distal contractile vigor) is forced into a rectangle encompassing the proximal and distal extents of the EGJ, and covering exactly three respiratory cycles during a period of quiet rest and baseline breathing (landmark phase). EGJ-CI is recorded above the gastric baseline during the landmark phase. The value is divided by 3 to obtain EGJ-CI for a single respiratory cycle (EGJ-CI/SRC); division by the duration of the three respiratory cycles provides the EGJ-CI corrected for respiration (EGJ-CI/CRC). (B) In the presence of a measurable and consistent separation between the lower esophageal sphincter (LES) and the diaphragmatic crura, the distal contractile integral (DCI) rectangle is forced across the LES high-pressure zone for the duration of three respiratory cycles. The remainder of the calculations are exactly the same as described above.
Ambulatory pH monitoring
All pH studies were performed after an overnight fast, off all antisecretory medications for 5 to 7 days. All patients completed the pre-procedure symptom questionnaire prior to the study. The pH probe was placed at our outpatient endoscopy and motility facility by an experienced nurse, using the HRM localization of the proximal LES margin to position the pH sensor 5 cm proximal to this landmark. After discharge from the esophageal facility, all subjects were instructed to resume normal activity and diet, maintain a daily diary that included symptoms, activities, and meal periods, and activate the symptom indicator button of the pH recorder every time they experienced acid reflux symptoms.6
Analysis of pH data included quantification of total acid exposure time (AET), measured as the time the distal esophageal pH remained below 4 and expressed as a percentage. A total AET of ≥4.0% was considered abnormal for the purposes of this study per our institutional threshold; this threshold has been validated to have with good documented sensitivity (91%) and specificity (85%) for discriminating esophagitis.14 Our institutional threshold of ≥2.0% was considered abnormal for supine AET.
Symptom assessment
Prior to HRM and ambulatory pH monitoring and prospectively on follow-up, all subjects completed a symptom survey to characterize their dominant and secondary symptoms as well as their overall symptomatic status. In this symptom survey, symptom characteristics are individually rated, and overall GSS is recorded on a 100-point visual analog scale.15 GSS at follow-up formed the basis for determining response to GER therapy. The subjects were also asked about GER management (which was directed independent of this study by the subjects’ treating physicians using clinical data and clinical reports of esophageal HRM and pH monitoring provided to them), and segregated into medical management (proton pump inhibitor (PPI) therapy) and ARS cohorts based on their responses.
Data analysis
Continuous variables are reported as mean ± standard error of the mean (SEM) or median (interquartile range). Categorical data are reported using frequencies and proportions. Normative values for EGJ-CI were determined from analysis of data from normal controls; mean values and 95th percentile values were determined for both EGJ-CI/SRC and EGJ-CI/CRC. Receiver operating characteristic (ROC) analysis was performed to provide a best balance of sensitivity and specificity for the threshold EGJ-CI values (both SRC and CRC) predicting the abnormal acid exposure. Areas under the curves (AUC) were calculated. The performance of the EGJ-CI in patients undergoing ambulatory pH monitoring was determined using these normative and threshold values in predicting abnormal distal esophageal AET, as well as symptomatic improvement following GER management as measured by change in GSS, both medical management and ARS. Continuous data were compared using the two-tailed Student's t-test, and categorical data were compared using the chi-squared test or Fisher's exact test as appropriate. Correlation coefficients were calculated for the associations between EGJ-CI values calculated using the two described techniques, between EGJ-CI and existing conventional LES metrics, and between EGJ-CI and total AET. Univariate and multivariate analyses were performed to determine the predictive potential of the EGJ-CI in terms of AET and post-therapy GSS at follow-up, controlling for medical versus surgical therapy. In all cases, P < 0.05 was required for statistical significance. All statistical analyses were performed using IBM SPSS 22 (Chicago, IL) and SAS 9.3 (Cary, NC).
RESULTS
During the study period, 188 subjects (55.2 ± 0.9 years, 64% female) fulfilled all study inclusion criteria out of 744 patients undergoing pH studies off PPI in the institutional database. Presenting symptoms were dominated by heartburn (35.6%) and chest pain (11.2%). On ambulatory pH monitoring off antisecretory therapy, 90 subjects (47.9%) had total AET ≥4.0%, and an additional 6% had isolated elevation in supine AET >2.0%. On esophageal HRM, 44 subjects (23.4%) had a type III hernia; of the remainder, 113 (60.1%) had type I EGJ, and 31 (16.5%) had type II EGJ morphology. Medical management was pursued in 124 subjects, while the remaining 34.0% underwent ARS. The control group consisted of 21 subjects (27.6 ± 0.6 years, 52% female), none of whom had any gastrointestinal symptom, none were on any medications, and none had a hiatus hernia. Averaged conventional LES and EGJ parameters (LES length, LES basal pressure, IRP) were similar between controls (LES length: 2.9 ± 0.1 cm, mean LES basal pressure 21.4 ± 2.1 mmHg, end-expiratory LES pressure 10.9 ± 1.6 mmHg, IRP 7.1 ± 0.9 mmHg) and study subjects (LES length: 3.7 ± 0.3 cm, mean LES basal pressure 21.8 ± 1.0 mmHg, end-expiratory LES pressure 11.7 ± 0.7 mmHg, IRP 7.5 ± 0.4 mmHg), reflecting the heterogeneous nature of the EGJ in the study group.
EGJ-CI thresholds
Averaged EGJ-CI in normal controls and study subjects are described in Table 1. In normal controls, the EGJ-CI/SRC was 205.5 ± 35.6 mmHg.cm.s (5th–95th percentile 21–512 mmHg.cm.s), and EGJ-CI/ CRC was 46.2 ± 7.6 mmHg.cm (5th-95th percentile 6–125 mmHg.cm). The mean EGJ-CI was significantly higher in subjects with normal AET than in subjects with abnormal AET (Table 1, P ≤ 0.003). More striking, when a type III hiatus hernia was present, there was significant segregation between subjects with and without abnormal AET, with both EGJ-CI parameters being significantly lower in subjects with abnormal AET (Table 1). Values were similar with nonsignificant differences when a type III hiatus hernia was not identified.
Table 1.
EGJ-CI values in relationship to acid exposure and hiatus hernia
| EGJ-CI/SRC | EGJ-CI/CRC | |
|---|---|---|
| Parameters | mmHg.cm.s | mmHg.cm |
| Normal controls | 205.5 ± 35.6 | 46.2 ± 7.6 |
| All subjects | 167.3 ± 10.7 | 49.4 ± 2.8 |
| Normal acid exposure (AET < 4.0) | 197.2 ± 15.1 | 56.7 ± 3.6 |
| With type III hiatus hernia | 173.6 ± 25.5 | 50.4 ± 6.4 |
| Without type III hiatus hernia | 208.6 ± 18.6 | 59.7 ± 4.3 |
| Abnormal acid exposure (AET ≥4.0) | 134.9 ± 14.5* | 41.4 ± 4.2** |
| With type III hiatus hernia | 78.1 ± 16.8*** | 24.0 ± 4.7*** |
| Without type III hiatus hernia | 194.2 ± 20.4 | 59.6 ± 5.8 |
P = 0.003 and
P = 0.006 compared to cohort with normal acid exposure (AET < 4.0).
P < 0.05 compared cohorts with normal acid exposure (AET < 4.0) with hiatus hernia. CRC, corrected for respiratory cycle; EGJ-CI, esophagogastric junction contractile integral; SRC, single respiratory cycle.
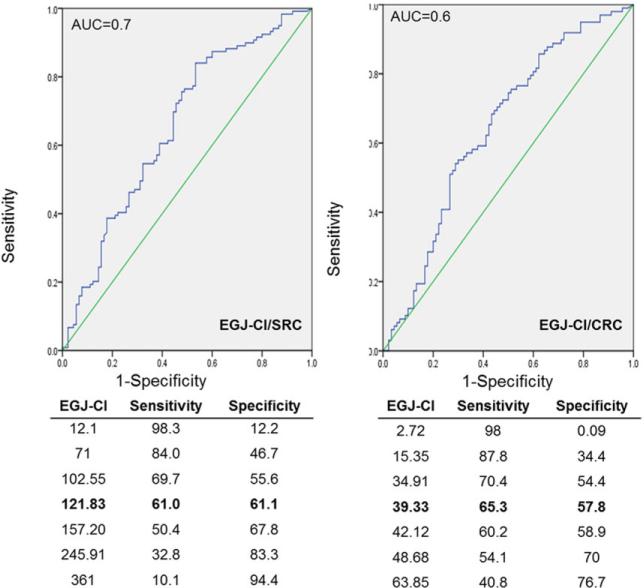
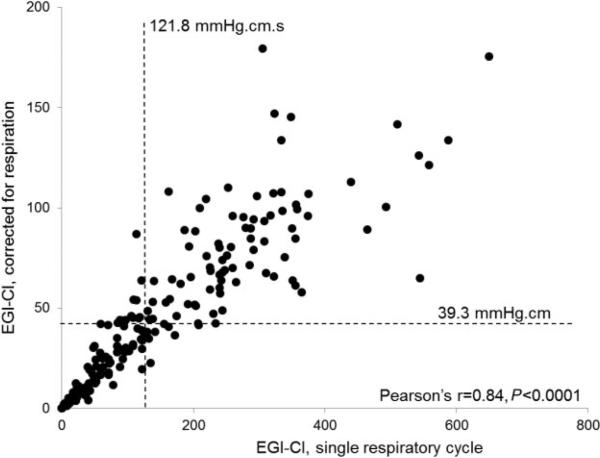
Using ROC analysis, EGJ-CI threshold values predicting abnormal AET were determined for both EGJ-CI parameters assessed (Fig. 2). A threshold EGJ-CI/SRC value of 121.8 mmHg.cm.s had a sensitivity of 61.0% and a specificity of 61.1% in predicting abnormal AET, with an AUC of 0.6. With EGJ-CI/CRC, a threshold value of 39.3 mmHg.cm had a sensitivity of 65.3% and a specificity of 57.8%, with an AUC of 0.7. These two parameters were concordant in 86.2% of patients (Pearson's r = 0.84, P < 0.0001); discordance was clustered around the identified thresholds for both parameters (Fig. 3). Both parameters only modestly correlated with total AET (Pearson's r = −0.21, P = 0.006), indicating mechanisms other than EGJ barrier function were contributing to abnormal AET.
Fig. 2.
Receiver operating characteristic (ROC) curves defining optimal esophagogastric junction contractile integral (EGJ-CI) thresholds for segregating subjects with normal and abnormal esophageal acid exposure. Both EGJ-CI calculated for a single respiratory cycle (EGJ-CI/SRC) and corrected for respiration (EGJ-CI/CRC) provided equivalent segregation of subjects with and without abnormal acid exposure times. Threshold values provided sensitivity of 61–65% range and specificity of 58–65%, with modest area under the curve (0.6–0.7).
Fig. 3.
Correlation between esophagogastric junction contractile integral (EGJ-CI) calculated for a single respiratory cycle (SRC) and corrected for respiration (CRC). The two metrics demonstrated excellent correlation of 86% (Pearson's r = 0.84, P < 0.0001), with most discordant values clustered around the identified thresholds for each value.
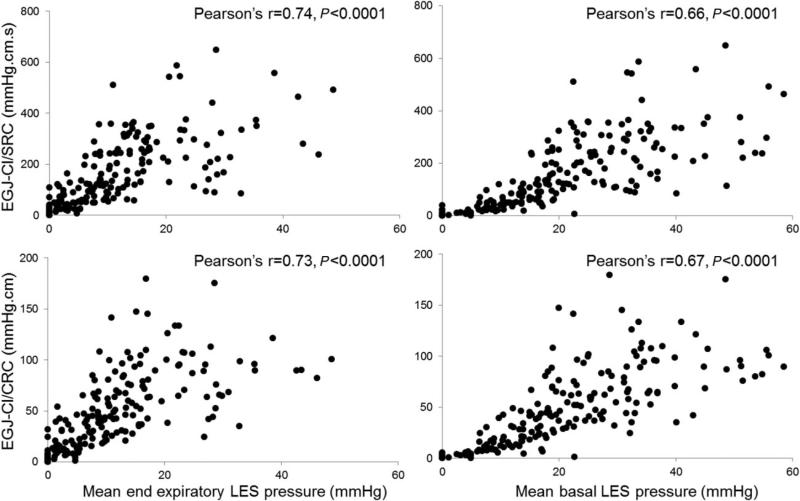
On univariate analysis, identified EGJ-CI/SRC and EGJ-CI/CRC thresholds significantly segregated subjects with abnormal reflux parameters and conventional LES metrics from those with normal metrics (Table 2). In particular, supine AET was significantly higher with low EGJ-CI compared to cohorts with normal EGJ-CI using identified thresholds (Table 2). The only exception was that mean upright AET was not significantly different between cohorts with normal and low EGJ-CI/SRC (5.2% vs. 6.3%, respectively, P = 0.2), while EGJ-CI/CRC readily segregated these cohorts (4.8% vs. 6.5%, P = 0.045, Table 2). Both EGJ-CI metrics correlated significantly with currently utilized metrics, mean basal LES pressure and end-expiratory LES pressure (Pearson's r = 0.7, P < 0.0001 for each correlation, Fig. 4), indicating that the EGJ-CI can adequately replace these metrics. The presence of a type III hiatus hernia (P = 0.01) and EGJ-CI, both SRC (P = 0.005) and CRC (P = 0.007) were significant predictors of abnormal AET. On multivariate analysis, only EGJ-CI (both SRC and CRC) was retained as an independent predictor of abnormal AET (P < 0.05).
Table 2.
EGJ-CI in relationship to acid exposure and EGJ metrics
| EGJ-CI/SRC |
EGJ-CI/CRC |
|||
|---|---|---|---|---|
| Parameters | ≤121.8 mmHg.cm.s n = 92 | >121.8 mmHg.cm.s n = 96 | ≤39.3 mmHg.cm n = 86 | >39.3 mmHg.cm n = 102 |
| Acid exposure | ||||
| Abnormal AET (%) | 55 (59.1%) | 35 (36.8%) | 52 (60.5%) | 38 (37.3%) |
| Mean total AET | 6.6% | 4.1% | 6.8% | 4.1% |
| Mean upright AET | 6.3% | 5.2% | 6.5% | 4.8% |
| Mean supine AET | 7.1% | 2.6% | 7.3% | 2.7% |
| DeMeester score | 24.0 ± 2.7 | 14.3 ± 1.6 | 24.9 ± 2.7 | 13.9 ± 1.6 |
| EGJ parameters | ||||
| Mean basal LES pressure (mmHg) | 12.8 ± 0.9 | 29.9 ± 1.1 | 12.1 ± 0.9 | 29.9 ± 1.2 |
| Mean end-expiratory LES pressure (mmHg) | 5.4 ± 0.7 | 17.7 ± 1.0 | 5.1 ± 0.7 | 17.2 ± 1.0 |
| Hiatus hernia† (%) | 36 (39.1%) | 8 (8.3%) | 36 (41.9%) | 8 (7.8%) |
P < 0.05 for comparisons between low and normal values for EGJ-CI/SRC and EGJ-CI/CRC in all instances except mean upright AET for EGJ-CI/SRC where P = 0.2.
Type III EGJ.
AET, acid exposure time; CRC, corrected for respiratory cycle; EGJ-CI, esophagogastric junction contractile integral; SRC, single respiratory cycle.
Fig. 4.
Correlation between esophagogastric junction contractile integral (EGJ-CI) and conventional lower esophageal sphincter (LES) metrics. Both EGJ-CI calculated for a single respiratory cycle (SRC) and corrected for respiration (CRC) correlated very well with mean basal LES pressure (Pearson's r = 0.73–0.74, P < 0.0001) and end-expiratory LES pressure (Pearson's r = 0.66–0.67, P < 0.0001) indicating that the EGJ-CI can adequately replace conventional metrics.
Symptomatic outcome
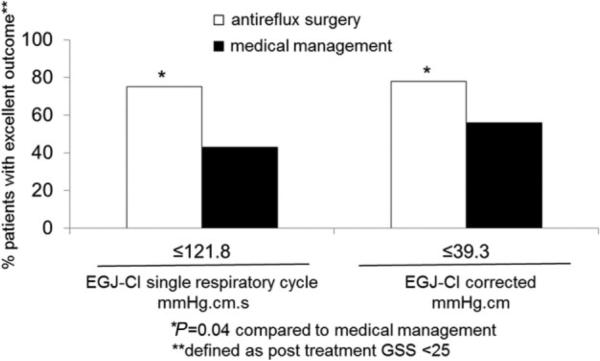
Baseline GSS declined from a mean of 62.6 to 25.8 upon follow-up assessment after 2.7 ± 0.1 years, an average 34.2% reduction overall. Decline in GSS was most marked after ARS (66.6% compared to 16.3% after medical management, P < 0.03). An excellent response to therapy (post-treatment GSS of <25) was achieved by three quarters of surgically managed patients when EGJ-CI was lower than the identified thresholds, but only by approximately half (43–56%) of medically managed patients (Fig. 5, P = 0.04 for each comparison with surgically managed patients). When the EGJ-CI/CRC was <39.3 mmHg.cm, ARS resulted in mean GSS decline of 67.5%, while medical management did not change mean GSS (0.1% increase in GSS, P = 0.01 compared to surgically managed patients); similar results were seen when calculations were performed using the EGJ-CI/SRC.
Fig. 5.
Symptomatic outcome segregated by esophagogastric junction contractile integral (EGJ-CI) thresholds after antireflux therapy. The likelihood of an excellent outcome (100-point visual analog score of <25 on follow-up) was significantly higher with antireflux surgery compared to medical management when EGJ-CI values were below the identified thresholds. This suggests that the EGJ-CI has values in predicting patients likely to improve following antireflux surgery in contrast to medical therapy.
DISCUSSION
In this study, we address the clinical utilization of a novel HRM metric addressing EGJ barrier function in patients with GER, which correlates well with and adds to information from currently used EGJ and LES metrics. Our report augments available data on EGJ-CI, and for the first time describes how this metric can help predict symptomatic response to treatment of GER, particularly the utilization of ARS in managing a structurally deficient EGJ barrier. We demonstrate that EGJ-CI can be assessed for a single respiratory cycle, or corrected for respiration with concordant values and similar prediction of symptomatic outcome. Our findings suggest that EGJ-CI provides better clinical direction than currently used parameters addressing EGJ barrier function.
The assessment of EGJ barrier function is clinically relevant in planning ARS. It is well known that patients with a structurally deficient EGJ barrier, especially those with a hiatus hernia are particularly prone to GER.3,4 To date, parameters derived from conventional esophageal manometry (LES endexpiratory pressure, LES basal pressure) continue to be utilized to assess LES basal function despite a worldwide trend toward use of HRM and the potential for novel software-driven LES measurements.16,17 In contrast, LES relaxation is addressed with an intuitive software tool that electronically interrogates the nadir LES pressure during expected swallow induced LES relaxation, the integrated relaxation pressure (IRP).18,19 Further, esophageal body motor function is analyzed with other HRM software tools and new metrics that efficiently utilize the increased data points recorded with esophageal pressure topography.6 These new esophageal metrics (IRP, DCI, distal latency) have changed our understanding of esophageal body motor function and have helped generate meaningful classification systems in categorizing esophageal body motor function.11,20 However, EGJ morphology at rest has not been subject to the same process of development of new metrics until recently.
The first attempt to define EGJ barrier function using an HRM-specific metric utilized the DCI tool to measure LES pressure integral over a 10-second swallow-free period.9 This metric did segregate patients with abnormal acid exposure from those with normal acid exposure, but did not control for respiratory rate, and did not reference values to the gastric baseline. An improvement on this metric was proposed by Nicodeme et al., where measurement for three respiratory cycles ensured uniformity in data collection, correction for length of the respiratory cycle was achieved by dividing the recorded value by the duration of the three respiratory cycles, and the value was referenced to 2 mmHg above the gastric baseline.10 This new metric, termed the EGJ-contractile integral, segregated patients with PPI-nonresponsive GER (as determined by pH impedance testing) from normal controls. Our report expands on the existing work by simplifying the measurement of the EGJ-CI. We demonstrate that referencing to the gastric baseline (rather than correction to a value above the gastric baseline) provides similar thresholds and normative values as reported by Nicodeme et al.10 Further, normalization to duration of respiratory cycle may be unnecessary if a consistent number of respiratory cycles are included in the measurement, as EGJ-CI over a single respiratory cycle correlated very well with EGJ-CI corrected for respiration. These simplifications may add to ease of incorporation of this metric into algorithms embedded within HRM analysis software.
In addition to its novelty and intuitive nature, the EGJ-CI takes into account physiologic details that define EGJ barrier function, including length of the LES high pressure zone, and inspiratory augmentation of EGJ pressure, both of which have been demonstrated to have key importance in assessments of the EGJ barrier.12 The fact that our measurements of the EGJ-CI were lower when the LES was isolated from the crural diaphragm (in the presence of a hiatus hernia) further support 3D-HRM reports that inspiratory diaphragmatic crural contraction dominates over the intrinsic LES tone in determining EGJ barrier function.21 These findings also establish that the EGJ-CI should be measured across the LES alone when measurable separation can be identified between the distal extent of the LES and diaphragmatic crura. We chose to utilize the existing morphologic HRM classification of the LES-crural diaphragm relationship in segregating settings where just the LES is interrogated, i.e. the type III relationship.12 While it can be argued that the EGJ-CI should be measured across the entire EGJ even when large hiatus hernias are present, this may incorporate pressure trapping within the hernia that may falsely elevate the recorded values. Further, there are instances where the catheter does not traverse the diaphragmatic crura, when only the LES can be interrogated by the EGJ-CI. Therefore, we chose to use a dichotomous approach, where just the LES is addressed when large (type III) separations exist between LES and diaphragmatic crura, and both LES and diaphragm are addressed when the two are together, or only demonstrate intermittent separation (types I and II).
Conventionally assessed LES and EGJ parameters correlated very well with EGJ-CI assessed by both methods (Fig. 4). Correlation with abnormal AET was modest at best, and understandably so, since transient LES relaxations are the most common mechanism of reflux, and this can occur despite an intact EGJ barrier. However, patients with continuing reflux symptoms despite medical therapy undergoing esophageal physiologic testing have a high likelihood of a structurally deficient EGJ.5 Consequently, we demonstrate that symptomatic outcome can be linked to abnormal EGJ-CI in these patients, since surgical management of a structurally deficient EGJ barrier as identified by low values resulted in significantly better symptomatic outcomes compared to medical management with PPI therapy alone. While further prospective studies are needed to understand the true clinical utility of EGJ-CI, our results and existing literature suggest that this metric may redefine assessment of EGJ barrier function. We propose that the EGJ-CI be assessed after correction for the respiratory cycle, since this has a potential to offer a more accurate profile in settings where respiratory variation skews recorded values, and especially since normative data exists from Nicodeme et al. that our study complements and concurs with. However, the EGJ-CI assessed for a single respiratory cycle could be easier to calculate, and appears to be as discriminative as EGJ-CI/CRC. In the future, we believe these metrics can be adapted for providing a measure of the post-fundoplication EGJ, and of LES after contraction in terms of contraction vigor, especially in defining and characterizing exaggerated LES after contraction that is sometimes seen in conjunction with esophageal body hypermotility.
A few limitations offset the strengths of this study. We recognize the limitations of the study cohort in that only subjects studied off antisecretory therapy who could be prospectively contacted for follow-up questionnaire survey were enrolled. Consecutive enrollment with prospective follow-up would potentially have provided more representative information. The control cohort was younger than the study cohort, and this reflects difficulties in identifying older patients without symptoms, comorbidities and on no medications, which were prerequisites for definition of our control cohort for obtaining institutional normative data. Of note, the fraction of study patients that fell into the same younger age range of the controls had similar SRC and CRC values to the remainder of the cohort. Nevertheless, normative values from these normal controls are likely representative, as shown by previous comparisons of motor function between these normal controls and younger cohorts with reflux disease.22 Patient management, both medical management and ARS, were not protocolized or randomized, and were left to treating physicians who ordered the esophageal physiologic studies, which has potential to bias outcome assessments; in particular, reasons for antireflux therapy in patients without abnormal AET could not be determined. At the time these pH studies were performed, our laboratory protocol was to hold antisecretory medications for 5–7 days for testing off therapy, which may have introduced the potential for rebound acid hypersecretion leading to unintended higher AET values. We did not use HRM studies with impedance, which could have further identified bolus transit mechanisms; we also used AET exclusively, and did not use impedance parameters in assessing weakly acidic or nonacid reflux. Finally, the DCI tool was forced into the EGJ-CI measurement domains for measurements; software algorithms specifically designed for this purpose could have provided more accurate data recording. Nevertheless, we believe the EGJ-CI makes intuitive sense, and as we have demonstrated, can provide information pertinent to management planning and patient counseling prior to discussion of therapeutic options in GER.
In conclusion, the EGJ-CI is a novel HRM metric that holds tremendous promise in changing the assessment of EGJ barrier function. This metric partially predicts abnormal esophageal AET as well as symptomatic outcome following antireflux therapy; further research needs to prospectively assess the role of esophageal body peristaltic function in conjunction with EGJ-CI in assessing similar reflux measures and outcomes. We believe the EGJ-CI has potential to complement or replace currently used basal LES and EGJ metrics, and further study is needed to duplicate and enhance our findings.
Acknowledgments
Financial support: This study was partially funded through NIH/NIDDK (5P30 DK052574-14 –AP) and through the Washington University Department of Medicine Mentors in Medicine (MIM) and Clinical Science Training and Research (CSTAR) programs.
Footnotes
Specific author contributions: P. Gor and Y. Li: Study design, data collection, manuscript preparation and review; S. Munigala: Data analysis, critical review of manuscript; A. Patel: Data collection, critical manuscript review; A. Bolkhir: Data collection, critical manuscript review; C. P. Gyawali: Study concept and design, data analysis, manuscript preparation, critical review and final approval of manuscript.
Conflicts of interest: None of the authors have any conflicts to declare.
References
- 1.Mittal RK, Balaban DH. The esophagogastric junction. N Engl J Med. 1997;336:924–32. doi: 10.1056/NEJM199703273361306. [DOI] [PubMed] [Google Scholar]
- 2.Shaker R, Dodds WJ, Kahrilas PJ, et al. Relationship of intraluminal pH and pressure within the lower esophageal sphincter. Am J Gastroenterol. 1991;86:812–6. [PubMed] [Google Scholar]
- 3.Bredenoord AJ, Weusten BL, Timmer R, et al. Intermittent spatial separation of diaphragm and lower esophageal sphincter favors acidic and weakly acidic reflux. Gastroenterology. 2006;130:334–40. doi: 10.1053/j.gastro.2005.10.053. [DOI] [PubMed] [Google Scholar]
- 4.Sloan S, Kahrilas PJ. Impairment of esophageal emptying with hiatal hernia. Gastroenterology. 1991;100:596–605. doi: 10.1016/0016-5085(91)80003-r. [DOI] [PubMed] [Google Scholar]
- 5.Chan WW, Haroian LR, Gyawali CP. Value of preoperative esophageal function studies before laparoscopic antireflux surgery. Surg Endosc. 2011;25:2943–9. doi: 10.1007/s00464-011-1646-9. [DOI] [PubMed] [Google Scholar]
- 6.Ghosh SK, Pandolfino JE, Zhang Q, et al. Quantifying esophageal peristalsis with high-resolution manometry: a study of 75 asymptomatic volunteers. Am J Physiol Gastrointest Liver Physiol. 2006;290:G988–97. doi: 10.1152/ajpgi.00510.2005. [DOI] [PubMed] [Google Scholar]
- 7.Xiao Y, Kahrilas PJ, Kwasny MJ, et al. High-resolution manometry correlates of ineffective esophageal motility. Am J Gastroenterol. 2012;107:1647–54. doi: 10.1038/ajg.2012.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Roman S, Pandolfino JE, Chen J, et al. Phenotypes and clinical context of hypercontractility in high-resolution esophageal pressure topography (EPT). Am J Gastroenterol. 2012;107:37–45. doi: 10.1038/ajg.2011.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hoshino M, Sundaram A, Mittal SK. Role of the lower esophageal sphincter on acid exposure revisited with high-resolution manometry. J Am Coll Surg. 2011;213:743–50. doi: 10.1016/j.jamcollsurg.2011.09.002. [DOI] [PubMed] [Google Scholar]
- 10.Nicodeme F, Pipa-Muniz M, Khanna K, et al. Quantifying esophagogastric junction contractility with a novel HRM topographic metric, the EGJ-Contractile Integral: normative values and preliminary evaluation in PPI non-responders. Neurogastroenterol Motil. 2014;26:353–60. doi: 10.1111/nmo.12267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bredenoord AJ, Fox M, Kahrilas PJ, et al. Chicago classification criteria of esophageal motility disorders defined in high resolution esophageal pressure topography. Neurogastroenterol Motil. 2012;24(Suppl 1):57–65. doi: 10.1111/j.1365-2982.2011.01834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pandolfino JE, Kim H, Ghosh SK, et al. High-resolution manometry of the EGJ: an analysis of crural diaphragm function in GERD. Am J Gastroenterol. 2007;102:1056–63. doi: 10.1111/j.1572-0241.2007.01138.x. [DOI] [PubMed] [Google Scholar]
- 13.Patel A, Ding A, Mirza F, et al. Optimizing the high-resolution manometry (HRM) study protocol. Neurogastroenterol Motil. 2015;27:300–4. doi: 10.1111/nmo.12494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kahrilas PJ, Quigley EM. Clinical esophageal pH recording: a technical review for practice guideline development. Gastroenterology. 1996;110:1982–96. doi: 10.1053/gast.1996.1101982. [DOI] [PubMed] [Google Scholar]
- 15.Patel A, Sayuk GS, Gyawali CP. Acid-based parameters on pH-impedance testing predict symptom improvement with medical management better than impedance parameters. Am J Gastroenterol. 2014;109:836–44. doi: 10.1038/ajg.2014.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sharata AM, Dunst CM, Pescarus R, et al. Peroral endoscopic myotomy (POEM) for esophageal primary motility disorders: analysis of 100 consecutive patients. J Gastrointest Surg. 2015;19:161–70. doi: 10.1007/s11605-014-2610-5. [DOI] [PubMed] [Google Scholar]
- 17.Tatum RP, Soares RV, Figueredo E, et al. High-resolution manometry in evaluation of factors responsible for fundoplication failure. J Am Coll Surg. 2010;210:611–7. 617–9. doi: 10.1016/j.jamcollsurg.2009.12.023. [DOI] [PubMed] [Google Scholar]
- 18.Ghosh SK, Pandolfino JE, Rice J, et al. Impaired deglutitive EGJ relaxation in clinical esophageal manometry: a quantitative analysis of 400 patients and 75 controls. Am J Physiol Gastrointest Liver Physiol. 2007;293:G878–85. doi: 10.1152/ajpgi.00252.2007. [DOI] [PubMed] [Google Scholar]
- 19.Gyawali CP, Bredenoord AJ, Conklin JL, et al. Evaluation of esophageal motor function in clinical practice. Neurogastroenterol Motil. 2013;25:99–133. doi: 10.1111/nmo.12071. [DOI] [PubMed] [Google Scholar]
- 20.Pandolfino JE, Roman S, Carlson D, et al. Distal esophageal spasm in high-resolution esophageal pressure topography: defining clinical phenotypes. Gastroenterology. 2011;141:469–75. doi: 10.1053/j.gastro.2011.04.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nicodeme F, Pandolfino JE, Lin Z, et al. Adding a radial dimension to the assessment of esophagogastric junction relaxation: validation studies of the 3D-eSleeve. Am J Physiol Gastrointest Liver Physiol. 2012;303:G275–80. doi: 10.1152/ajpgi.00063.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Porter RF, Kumar N, Drapekin JE, et al. Fragmented esophageal smooth muscle contraction segments on high resolution manometry: a marker of esophageal hypomotility. Neurogastroenterol Motil. 2012;24:763–8. doi: 10.1111/j.1365-2982.2012.01930.x. [DOI] [PubMed] [Google Scholar]