Abstract
Alcoholic fatty liver diseases (AFLD) and non-alcoholic fatty liver diseases (NAFLD) are two pathological conditions that are spreading worldwide. Both conditions are remarkably similar with regards to the pathophysiological mechanism and progression despite different causes. Oxidative stress-induced mitochondrial dysfunction through post-translational protein modifications and/or mitochondrial DNA damage has been a major risk factor in both AFLD and NAFLD development and progression. Cytochrome P450-2E1 (CYP2E1), a known important inducer of oxidative radicals in the cells, has been reported to remarkably increase in both AFLD and NAFLD. Interestingly, CYP2E1 isoforms expressed in both endoplasmic reticulum (ER) and mitochondria, likely lead to the deleterious consequences in response to alcohol or in conditions of NAFLD after exposure to high fat diet (HFD) and in obesity and diabetes. Whether CYP2E1 in both ER and mitochondria work simultaneously or sequentially in various conditions and whether mitochondrial CYP2E1 may exert more pronounced effects on mitochondrial dysfunction in AFLD and NAFLD are unclear. The aims of this review are to briefly describe the role of CYP2E1 and resultant oxidative stress in promoting mitochondrial dysfunction and the development or progression of AFLD and NAFLD, to shed a light on the function of the mitochondrial CYP2E1 as compared with the ER-associated CYP2E1. We finally discuss translational research opportunities related to this field.
Keywords: Alcoholic fatty liver disease, non-alcoholic fatty liver disease, mitochondria, CYP2E1, oxidative stress, lipid peroxidation, mitochondrial dysfunction, post-translational protein modification
INTRODUCTION
Cytochrome P450-2E1 (CYP2E1) has been reported to be expressed in hepatic and extrahepatic tissues in all mammalians that have been evaluated for its expression [1, 2]. CYP2E1 catalyzes the biotransformation of various compounds including: 1) small organic molecules such as ethanol, acetone, and pyrazole; 2) endogenous compounds such as fatty acids, ketone bodies, and glycerol; 3) clinically-used drugs such as salicylic acid, halothane, isoniazid, and isoflurane; 4) toxic chemicals including carbon tetrachloride and chloroform; 5) environmental contaminants such as nitrosamines, benzene, and acrylamide [1, 3–5].
CYP2E1 is well-established to be inducible by ethanol, pyrazole, acetone, and isoniazid both in living mammals and some cultured cells [1, 6]. In addition to alcohol exposure and alcoholic fatty liver diseases (AFLD), CYP2E1 was reportedly induced in other conditions related to alteration of metabolic homeostasis, especially lipid dyshomeostasis, such as fasting and non-alcoholic fatty liver disease (NAFLD) including obesity and diabetes [1, 7–12]. As reported in these studies and others, the increase of CYP2E1 is known to be regulated at the levels of transcription, post-transcription, translation, and post-translation [1, 13, 14]. However, both post-translational and pre-translational regulations seem to play a major role in CYP2E1 with ethanol and ketone bodies, respectively [1, 13–15].
Interestingly, when compared to other members of cytochrome P450 genes, CYP2E1 possesses a remarkably high NADPH oxidase activity, resulting in increased production of reactive oxygen species (ROS) such as hydrogen peroxide (H2O2), hydroxyl radical, and superoxide anion [16, 17], all of which can produce peroxynitrite through interaction with nitric oxide, or under toxic conditions, increase the levels of lipid peroxidation [18–20]. The increased ROS can exert deleterious effects on the cell functions and even survival mainly through damaging cellular and mitochondrial macromolecules including mitochondrial DNA.
AFLD, including alcoholic steatohepatitis (ASH) and NAFLD, including non-alcoholic steatohepatitis (NASH), are astonishingly similar in their pathophysiological processes and disease progression [21]. Indeed, Both AFLD and NAFLD exhibit a similar, wide range of liver abnormalities that can start with simple steatosis (micro-and macro-steatosis), but can advance to more serious conditions such as liver fibrosis, liver cirrhosis, and even liver carcinoma [22]. The “two-hit” hypothesis, which partly explains the progression of both diseases, suggests that steatosis (the first hit) primes the liver to the secondary hits, including reactive oxygen/nitrogen species (ROS/RNS), gut-derived endotoxins, tumor necrosis factor-α (TNF-α), and other proinflammatory cytokines, resulting in AFLD or NAFLD development and/or progression [22, 23]. CYP2E1 is considered one of the common pathogenic factors for both AFLD and NAFLD due to its inducibility under these conditions with the increased ROS, causing deleterious effects on mitochondrial dysfunction and hepatotoxicities [24–29] The majority of hepatic CYP2E1 is reported to be expressed in the endoplasmic reticulum (ER); however, CYP2E1 is also reported to be localized in mitochondria. The expression of hepatic CYP2E1 in different cell compartments may be important in the development and/or the progression of both AFLD and NAFLD. This might be due to possibly different regulations, substrates, and enzyme Km values between the two CYP2E1 isoforms in the ER and the mitochondria which may exert different .effects on mitochondrial dysfunction and ultimately cell demise.
In this paper, we briefly describe the role of CYP2E1 in the development of AFLD and NAFLD, with emphasis on exposure to high fat diet (HFD), obesity and diabetes, as examples of NAFLD and metabolic syndrome, mainly via the increased production of oxidative radicals. We also updated evidences for the presence of mitochondrial CYP2E1 and possible mechanism(s) of CYP2E1 involvement in mitochondrial dysfunction with emphasis on mitochondrial post-translational protein modifications (PTMs). Finally, we discuss potential areas of research for mitochondrial CYP2E1 as well as translational research.
AFLD and CYP2E1
Alcoholic liver disease (ALD) is a major worldwide problem and a major cause of both morbidity and mortality. AFLD is a disease that can start as simple steatosis but can advance to inflammatory steatohepatitis (ASH), fibrosis, cirrhosis, and carcinoma [30–33]. The two hit hypothesis described the possible pathogenesis of AFLD [22, 23]. Chronic exposure to alcohol seems to attract the majority of research as the time duration and the amount of alcohol consumed are the most important factors in determining the development and the progression of the simple AFLD to the more advanced hepatic diseases such as inflammation, fibrosis and cirrhosis [34, 35]. Several risk factors were suggested for the development and progression of AFLD such as smoking, obesity, NAFLD, female sex, human immunodeficiency virus (HIV), hepatitis B virus (HBV) and C virus (HCV), etc [36–39]. Although chronic exposure to alcohol caused serious hepatic injury that warrants many studies, acute exposure to alcohol also has recently been gaining more attention. Indeed, acute alcohol exposure (binge drinking), is the major pattern of drinking. Acute alcohol exposure, especially when achieving high blood alcohol concentration of 0.08% or more [40], may also cause alcoholic hepatitis, liver cirrhosis, and increased death rates which can be sudden [41, 42]. This suggests that binge alcohol exposure experiments are of great interest and can also be used as an indicator or predictor of the pathogenesis of AFLD.
The alcohol metabolism in the liver is interesting since it combines the involvement of both oxidative metabolism, by using the activities of alcohol dehydrogenase (ADH), mitochondrial aldehyde dehydrogenase 2 (ALDH2), and CYP2E1, and non-oxidative pathways using the activity of catalase [37, 43, 44]. Acetaldehyde, the main product of alcohol metabolism and elevated under the conditions of chronic alcohol exposure, overexposure of alcohol in short duration, and/or the deterioration of cellular defense mechanisms, can cause deleterious hepatic injury such as inflammatory, fibrogenic, and immune response, which is directly triggered by ethanol-induced adducts with proteins and DNA [45–47].
CYP2E1 has the highest catalytic activity among the members of cytochrome P450 enzymes in metabolizing alcohol (ethanol). The role of CYP2E1 becomes particularly important when CYP2E1 is upregulated following chronic and acute alcohol exposure in hepatocytes and in wild-type (WT) animals [1, 44, 48]. The usage of Cyp2e1-null mice or specific CYP2E1 inhibitors including chlormethiazole (CMZ) confirmed the harmful role of CYP2E1 since mice without CYP2E1 or pre-treated with CMZ were, at least partially, protected from AFLD. [24, 49–56]. Thus it is logical to study its deleterious effects on hepatic injury upon alcohol exposure. The role of CYP2E1 in mediating AFLD was also suggested in humans where CYP2E1 polymorphism, but neither ADH1B nor mitochondrial ALDH2 polymorphism, was associated with susceptibility and severity of alcohol-induced hepatic cirrhosis [57]. This effect was attributed mainly to increased oxidative stress levels caused by the specific CYP2E1 polymorphic form [57]. More clinical studies with a greater number of patients are needed to validate this data in humans.
The ethanol mediated-deleterious effects have been largely attributed to ethanol-induced oxidative stress and the subsequent damaging effects on mitochondria and other cellular compartments. Indeed, ROS/RNS-producing proteins for causing ethanol-mediated tissue injury include: CYP2E1, inducible nitric oxide synthase (iNOS), NADPH oxidase, xanthine oxidase, and mitochondrial complexes [25, 58–66]. These deleterious effects were prevented either by deletion and an inhibitor of a specific enzyme, or with the use of antioxidants, as described in those reviews. CYP2E1 is indeed suggested to induce its damaging effects to the liver following ethanol exposure due to its ability to produce oxidative radicals such as hydrogen peroxide and superoxide anion. There are several mechanisms by which elevated CYP2E1 exerts its pathological effects through increasing nitroxidative stress and lipid peroxidation, which stimulates inflammatory response and production of pro-inflammatory cytokines through activation of a redox-sensitive transcription factor nuclear factor kappa-B (NF-κB) and others [44, 67–69]. The oxidative stress-mediated hepatic injury has been largely attributed to direct damage by oxidative radicals to mitochondrial DNA and/or PTMs of many mitochondrial proteins, which will be discussed in the following sections.
Some new mechanisms of the CYP2E1-mediated hepatic injury in AFLD have also been suggested. For instance, the inhibition of autophagy by ethanol was suggested to be mediated by CYP2E1 in HepG2 hepatoma cells and WT mice but not in Cyp2e1-null mice through oxidative stress-mediated events [56, 65, 70]. Further, the inhibition of autophagy was also implied in increased CYP2E1-mediated hepatotoxicity and mitochondrial dysfunction following ethanol exposure, suggesting a vicious cycle among the inhibition of autophagy, induction of CYP2E1 and ROS production, contributing to AFLD. However, the response of autophagy to alcohol seems variable and can either increase or decrease, depending on the dose of ethanol, duration of exposure, anima strain, tissue examined, and so forth [24]. However, the protective effect of autophagy against ethanol-induced fatty liver and toxicity is consistently observed. For example, autophagy, at least partially, protects hepatocytes by removing damaged mitochondria (mitophagy) and excess lipids (lipophagy). Thus, inhibition of autophagy can result in oxidative stress and hepatic steatosis [2, 71–73]. Taken together, inhibition of autophagy can also be another mechanism by which alcohol exerts its mitochondrial damaging effects in a CYP2E1-dependent manner.
Ethanol-induced hepatic hypoxia also recently gained much attention due to its potential causal role in mediating mitochondrial damage mainly through the increased nitroxidative stress or hypoxia-inducible factor 1-α (HIF-α), which induces iNOS [74], following alcohol exposure. For instance, the induction of HIF-α can also promote apoptosis by increasing permeabilization of the mitochondrial membrane, resulting in the release of mitochondrial cytochrome c into the cytosol [75]. In addition, iNOS-null mice were partially protected from hypoxia-induced mitochondrial dysfunction, whereas WT mice exhibited increased mitochondrial dysfunction following ethanol exposure. Under hypoxia, HIF-1α also regulates iNOS [74, 76]. Further, CYP2E1 has been involved in the induction of hypoxia and the resultant nitroxidative stress, as evidenced by the results observed with Cyp2e1-null mice where they exhibited lower levels of hypoxia, HIF-1α, fatty liver, and apoptosis, implying a role of CYP2E1 in mediating ethanol-induced hypoxic liver damage [66, 77, 78]. Whether CYP2E1 is the main factor or merely plays a permissive role for iNOS to promote the hypoxia-mediated mitochondrial damage and apoptosis through nitroxidative damage still needs further investigation.
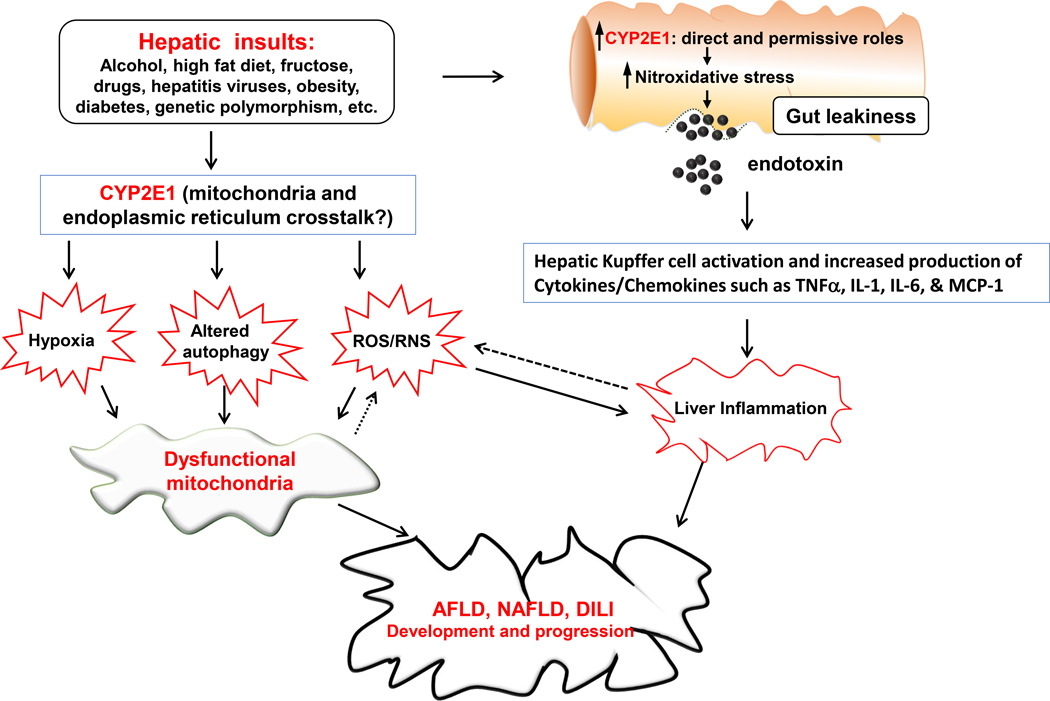
Gut-derived endotoxin is another important factor in ethanol-induced hepatic steatosis, inflammation, and fibrosis. Alcohol is known to decrease intestinal barrier integrity, leading to increased circulating endotoxin levels in rodents [79–85] and human alcoholics[86]. Alcohol-mediated gut leakage ultimately leads to increased levels of NF-κB and the release of inflammatory cytokines such as TNF-α. The increased TNF-α can cause a viscous cycle by worsening gut permeability and increasing hepatic damage [87–90]. Alcohol-induced gut leakiness and increased endotoxemia are caused by increased nitroxidative stress in intestinal epithelium, contributing to inflammatory liver injury. Similar to ethanol-induced hepatic hypoxia, CYP2E1 and iNOS in intestinal Caco-2 cells and mouse intestine seem to play a major role in ethanol-induced nitroxidative stress, gut leakiness and hepatotoxicity, since both proteins were induced in the mouse intestine following ethanol exposure and their inhibition led to the amelioration of gut leakiness, endotoxemia, and inflammatory liver injury [50, 91, 92]. Increased nitroxidative stress may significantly affect the cytoskeleton protein architecture [80] and/or intestinal tight-junction proteins, leading to alteration of the barrier function with increased permeability [91]. These results support at least a partial role of intestinal protein nitration in mediating the alcohol-induced gut leakiness and subsequent hepatic injury in a CYP2E1 dependent manner. Collectively, these studies suggest that CYP2E1 likely plays an important role in the pathogenesis of AFLD via different mechanisms, as illustrated in Figure 1.
Fig. 1. Proposed mechanisms of mitochondrial dysfunction, contributing to hepatotoxicity in AFLD, NAFLD and DILI.
Various hepatic insults including alcohol (ethanol), high fat diet, fructose, drugs, hepatitis virus, obesity, diabetes, genetic polymorphism as indicated in the Figure can negatively affect the liver by promoting ER stress and mitochondria dysfunction, contributing to fat accumulation and inflammatory liver injury in AFLD, NAFLD and DILI. These negative effects are largely mediated through increased ROS/RNS production, increased hypoxia and altered autophagy by the direct and/or permissive roles of CYP2E1, as discussed in the text. These agents or conditions can also upregulate intestinal CYP2E1 and promote gut leakiness through increased nitroxidative stress, contributing to endotoxemia and more severe inflammatory liver injury via activation of Kupffer cells and the production of cytokines/chemokines, which can also increase ROS/RNS production, resulting in a viscous cycle between inflammation and oxidative stress. Ultimately, this change in the redox state combined with the increased production of ROS/RNS promote the development and progression of mitochondrial dysfunction and various hepatic diseases. Uni-directional and bi-directional arrows indicate exclusive and mutual influences, respectively.
NAFLD AND CYP2E1
NAFLD has been recognized as one of the major health burdens that develop in the absence of alcohol abuse. Similar to AFLD, NAFLD was also suggested to follow the “two hit hypothesis” [22, 23], and ranges from relatively benign bland steatosis (more than 5–10% of liver weight is from fat content) to the more severe NASH. Approximately 10–20% of people with hepatic steatosis probably develop NASH in the long term [93]. Similar to ASH, liver steatosis in NAFLD can be progressed to liver necroinflammation, fibrosis, cirrhosis, and ultimately hepatocellular carcinoma and liver failure [94–96]. The prevalence of NAFLD in the general population is about 46% and the development of NASH may reach as high as 12% in the USA [97]. The estimates in other developed countries are similarly high since 20–30% of adults have excess fat accumulation in the liver [98].
Of the major risk factors for the development of NAFLD in developed countries are obesity and chronic over-nutrition [98]. Other factors include insulin resistance, type 2 diabetes, dysmetabolism, hyperlipidemia, genetic variation, ethnicity, and physical inactivity, all of which may increase the severity of the metabolic liver disease [99–101]. In fact, insulin resistance, in particular, is frequently detected in patients suffering from NAFLD, obesity, and diabetes mellitus [98], suggesting its major role in NAFLD development. It is important to mention though that intrahepatic fat accumulation such as lipodystrophy can occur independently from obesity [102], suggesting that intrahepatic fat accumulation may be a good indicator of the presence of metabolic complications independent from body weight [100]. However, the most common form ‘primary’ NASH is observed in obese individuals with insulin resistance and increased triglycerides levels often associated with diabetes [103]. NAFLD is also increasingly prevalent in the Asia-Pacific regions and is partially caused by metabolic syndrome accompanied with abdominal obesity, atherogenic dyslipidemia, increased blood pressure, insulin resistance and diabetes with or without glucose intolerance, pro-inflammatory state and pro-thrombotic state [104–106]. The prevalence of NAFLD in this area ranges from 5 −30% depending on the ethnic group studied [107, 108]. Thus there is increasing evidence for the relationship of NAFLD with the metabolic syndrome. It is noteworthy, however, to mention that NASH development may occur ‘secondary’ to other reasons than obesity and insulin resistance where CYP2E1 is upregulated, all of which are covered by other excellent reviews including [109] and not be the focus of this review.
Although several underlying mechanisms for the development of NAFLD/NASH and metabolic syndrome have been proposed, its pathophysiology is not completely unraveled. Accumulating evidence suggests that oxidative/nitrosative stress appears to play a critical role in the development and/or progression of NAFLD since CYP2E1, iNOS, and NADPH-oxidase have been increased and activated, as shown in various experimental models and people with NASH [9, 12, 110–119]. The Increased oxidative/nitrosative stress probably leads to covalent modifications of various proteins, lipids, and DNA, leading to their functional inactivation, which will be discussed in the following sections.
CYP2E1 is a critical player in elevating nitroxidative stress radicals in NAFLD, as similar to that in AFLD. As mentioned earlier, CYP2E1 has been reported to be markedly induced following HFD or under diabetes, and obesity whereas CYP2E1-null mice have been protected, at least partially but significantly, from the CYP2E1-mediated deleterious effects of NAFLD [38, 110, 111, 120–122]. In addition, over-expression of hepatic CYP2E1 both in vitro and in vivo promotes the development of hepatic insulin resistance, partly through the activation of c-Jun N-terminal protein kinase (JNK) [116, 123]. Together, the combined results of these studies largely agreed that CYP2E1-mediated increased nitroxidative radicals, lipid peroxidation, and protein PTMs are the main mechanisms by which CYP2E1 probably plays a prominent role in NAFLD development and progression. Studies in animal models mostly supported the role of CYP2E1 in mediating NAFLD, however, a few studies suggested otherwise. For instance, two studies suggested that CYP4A is another alternative and may be a more important source of oxidative stress particularly in the absence of CYP2E1, for the development of NAFLD. In mice fed a methionine-choline deficient diet, the deletion of CYP2E1 did not prevent development of NASH nor abrogated the increased microsomal NADPH-dependent lipid peroxidation. In addition, CYP4A antibody prevented the development of lipid peroxidation in CYP2E1-null mice, suggesting that CYP4A can become a compensatory factor in the absence of CYP2E1 [124, 125]. CYP4A, which also metabolizes long-chain fatty acids like CYP2E1, may be a major and more critical player than CYP2E1 in the development of insulin resistance and hepatic steatosis in the HFD-exposed diabetic mice since inhibition of CYP4A reduced hepatic ER stress, apoptosis, insulin resistance, and steatosis. In addition, the levels of CYP2E1 were down-regulated in these mice while CYP4A contents remained similar following HFD exposure for 10 weeks [126].
The clinical studies in humans, however, gave contradictory results with respect to the role of CYP2E1 in the development of NASH. On one hand, there are several reports supporting a positive role of CYP2E1 in mediating NAFLD. For instance, there was a remarkable increase of CYP2E1 hepatic activity in obese Type II diabetics and increased CYP2E1 mRNA levels in the peripheral mononuclear cells in Type I and Type II diabetes [10]. In addition, the hepatic liver content and activity of CYP2E1 were positively correlated with the liver carbonyl content and the development of NAFLD in obese women [127]. Further, Hepatic CYP2E1 content and activity were higher in the livers of patients with NAFLD and increased further in parallel with the progression to NASH [128]. In contrast, there was no correlation between the increased hepatic lipid peroxidation and CYP2E1 expression in people with NAFLD [129]. In addition, there were not many reports supporting the role of CYP2E1 in NASH development in non-diabetic patients despite the up-regulation of CYP2E1 activity in this group. Although more clinical studies are obviously needed, the interpretation of the results should be performed with caution since CYP2E1 levels tend to decrease with NAFLD progression [130] and thus a positive correlation may not be always observed. A negative correlation thus may not be sufficient to exclude the involvement of CYP2E1 since CYP2E1 may possibly play a permissive role for other genes or risk factors and that CYP2E1 may be important in the earlier stages, rather than the permanent or later stages during the progression of NAFLD/NASH. This careful interpretation should also be applied to animal studies where upregulated CYP2E1 protein may not be found and it can even be decreased especially at late time points following exposure to HFD. It should also be considered that especially in animal studies using HFD, the amount and composition of the fat can be critical for the modulation of CYP2E1 [131].
As mentioned in the previous section, CYP2E1 can possibly exert its deleterious effects via other mechanisms independent from direct oxidative damage to mitochondria and PTMs. For instance, the inhibition of autophagy, involved in fat accumulation [132] and thus the development of NAFLD, may be mediated at least partially via CYP2E1-mediated oxidative stress, as previously demonstrated with alcohol-exposed models [24, 49, 65]. This possibility can be supported by the increased CYP2E1 levels in response to HFD, diabetes and obesity. As with alcohol [50, 92], CYP2E1 may promote the progression of NAFLD, at least partially, is via increased gut-derived endotoxemia, which is critical in the development of hepatic steatosis, inflammation, and fibrosis possibly through activation of hepatic Kupffer cells [22]. The induction of hypoxia is another potential mechanism by which CYP2E1 promotes the progression of NAFLD as the increased levels of CYP2E1 seem to be positively correlated with the development of hypoxia in a specified mouse model with hepatic deficiency of PTEN and exposed to hypoxia for 7 days [133]. The deleterious effects by hypoxia on the progression of NAFLD may be mediated by up-regulation of HIF-1α. Indeed, the administration of HIF-1α antisense into mice subjected to diet-induced obesity for 8 weeks exhibited remarkable improvement of fasting blood glucose, plasma insulin, hepatic glucose output, and liver fat content [134]. Hence it is reasonable to suggest HIF-1α possible involvement of the progression of NAFLD, as with AFLD, partly through the induction of hypoxia, along with other mechanisms (Fig. 1).
Despite the discrepancy in some studies, many reports generally suggest the involvement of CYP2E1 in the development and progression of NAFLD. It is clear however that CYP2E1-induced oxidative stress seems to be important in the development and/or progression of NAFLD, since Cyp2e1-null mice were significantly protected from NASH development even after feeding a HFD [111, 120].
Potential role of CYP2E1 in Oxidative stress, mitochondria dysfunction and tissue injury in AFLD, NAFLD and DILI
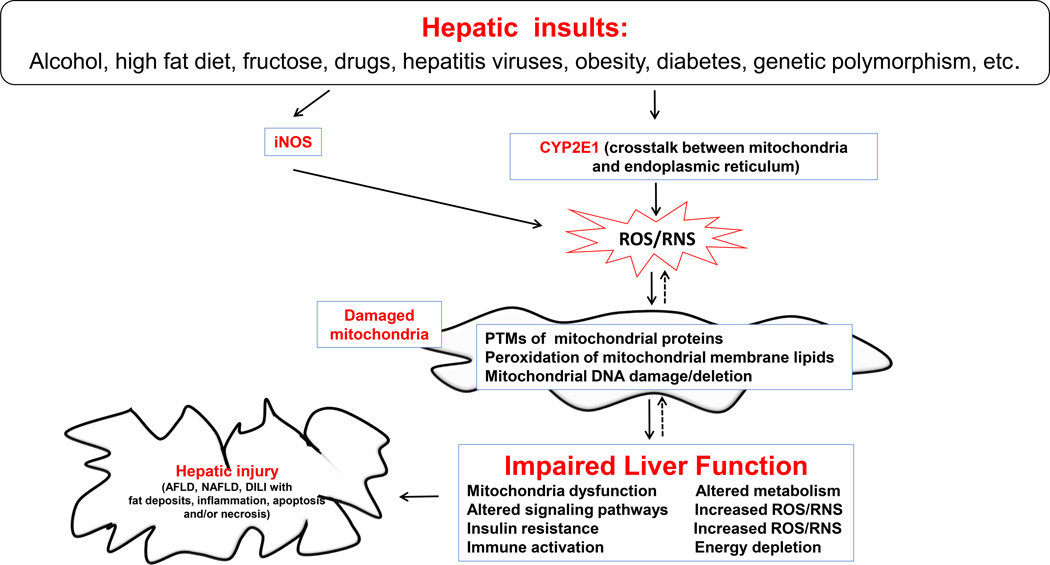
CYP2E1 is a major source of cellular and mitochondrial ROS/RNS. As mentioned earlier, the hepatic levels of CYP2E1, including mitochondrial CYP2E1, and iNOS markedly increase under conditions of AFLD and NAFLD, leading to increased production of ROS/RNS. Until recently, it has been believed that the members of cytochrome P450 1, 2 and 3 gene families are completely located in the ER. However, research in the last 20 years showed that several members of the cytochrome P450, including CYP2E1, are located in many cellular organelles including the mitochondria [29, 135–140]. There are several lines of evidence to support the location of CYP2E1 in the mitochondria both in vivo and in vitro models. It was first shown that CYP2E1 is also located in the mitochondria from rat livers in the area of inner mitochondrial membrane evaluated by electron microscopy and immunohistochemistry with the immunogold-labelled anti-CYP2E1 antibody [138]. The presence of CYP2E1 in the mitochondria was further confirmed by several other laboratories that also supported the presence and role of mitochondrial CYP2E1 in AFLD and NAFLD [20, 137, 141–143]. Figure 2 illustrates the CYP2E1-meditated oxidative stress on mitochondrial dysfunction.
Fig. 2. Direct and permissive roles of CYP2E1 in promoting mitochondrial dysfunction and hepatic injury in AFLD, NAFLD, and DILI.
Various hepatic insults as indicated can up-regulate CYP2E1 in the ER and mitochondria where CYP2E1 plays direct and/or permissive roles in promoting mitochondrial dysfunction and hepatotoxicity. Cross-talk between the ER and mitochondria CYP2E1 isoforms may exist to work either synergistically or independently. Nevertheless, the upregulated hepatic CYP2E1 along with iNOS can increase the cellular levels of ROS/RNS which likely modify mitochondrial proteins, lipids, and DNA. This will ultimately result in mitochondrial dysfunction with energy depletion, increased lipotoxicity and insulin resistance, as indicated in the Figure. Furthermore, persistent hepatic insults with mitochondrial dysfunction can ultimately contribute to organ failure and necroapoptosis of hepatic cells in AFLD, NAFLD, and DILI. Uni-directional and bi-directional arrows indicate exclusive and mutual influences, respectively
Mitochondrial CYP2E1 may be more stable with a longer half-life than the CYP2E1 isoform in the ER, possibly due to less degradation by the ubiquitin-proteasome pathway, as suggested [28, 144]. The presence of CYP2E1 isoforms in different cell compartments as illustrated by the elegant work of Avadhani group [135, 136, 138–140, 145, 146] triggers interesting questions some of which were previously raised by the same group: 1) Are microsomal and mitochondrial CYP2E1 work similarly to induce harmful effects or there is a distinctive function for each isoform?; 2) Is there any specific sequential events or specific conditions that are unique for the activation of microsomal and mitochondrial CYP2E1 or they are activated simultaneously by the same inducers, most of which are CYP2E1 substrates?; 3) Is it reasonable to assume that microsomal CYP2E1 possibly combined with mitochondrial isoform plays a part in the earlier disease process while mitochondrial CYP2E1 might be more important in later disease states due to its protection from degradation?; 4) Are microsomal and mitochondrial CYP2E1 always expressed together or regulated similarly in the same tissue known to express CYP2E1 or their expression is related to stimulus, cell, or other environmental conditions?; 5) What would be the endogenous and/or exogenous substrates of mitochondrial CYP2E1 compared to those of microsomal isoform?; and 6) Is the mitochondrial CYP2E1 important in promoting mitochondrial dysfunction and toxicities in hepatic as well as extra-hepatic tissues?
Many laboratories reported the deleterious effects of mitochondrial CYP2E1 different from the well-established effects of its microsomal counterpart in both AFLD and NAFLD, although additional studies are still needed to clearly address all of the aforementioned questions. For instance, ethanol increased the levels of both microsomal and mitochondrial CYP2E1 along with reduced levels of mitochondrial glutathione (GSH) in cultured rat hepatocytes and in the liver isolated from ethanol-treated lean mice [144]. The overexpression of catalase using adenoviral vectors either in the cytosol or mitochondrial compartments abolished the loss of mitochondrial membrane potential or damage to mitochondria observed in HepG2 cells over-expressed CYP2E1 following treatment with buthionine-(S,R)-sulfoximine (BSO) [147]. In addition, the loss of cell viability was prevented in catalase over-expressing cells. The catalase-protective effects were suggested to be due to the amelioration of increased levels of ROS and mitochondrial lipid peroxidation. The mitochondrial levels of 4-hydroxynonenal (4-HNE) paralleled the levels of mitochondrial CYP2E1 in rats fed ethanol with different ratios of long- or medium-chain triglycerides [20]. The harmful and distinctive regulatory effects of mitochondrial CYP2E1 were shown in in vivo studies using Swiss mice exposed to chronic ethanol [144]. In this study, up to one month, both microsomal and mitochondrial CYP2E1 were up-regulated. However, ethanol exposure for more than one month resulted in further increase of mitochondrial CYP2E1 while the elevated levels of microsomal CYP2E1 remained unchanged. Additionally the increased levels of mitochondrial CYP2E1 were accompanied by decreased levels of mitochondrial GSH. Taken together, these results suggest that the microsomal and mitochondrial CYP2E1 isoforms are distinctively regulated by prolonged ethanol treatment and possibly work at different stages of the liver disease, although the both contribute to the accumulative deleterious effects. A recent report showed that mitochondrion-targeted CYP2E1 markedly enhanced ethanol-mediated toxicity and oxidative stress production in COS-7 cells. In contrast, microsome-targeted CYP2E1 had only a marginal effect on alcohol-related toxicity [145]. Furthermore, the same group performed a very intriguing study using both COS-7 and HepG2 cell lines expressing predominantly mitochondria-targeted (Mt++) CYP2E1, WT CYP2E1-expressing, or ER+ (mainly microsome)-targeted cells (in vitro) and livers from alcohol-treated rats (in vivo) to evaluate the deleterious effects of mitochondrial CYP2E1 and their potential mechanism(s) [145]. This study suggested several interesting conclusions: 1) There was a decline in mitochondrial complex IV (cytochrome c oxidase) activity which was accompanied by a decline in the steady state levels of complex I and complex IV with increased protein carbonylation; 2) There was a reduction in the contents of mitochondrial DNA and mRNA. The effects of 1 and 2 were more prominent in Mt++ cells compared to ER+ cells. The results were further confirmed by using several mitochondria-specific antioxidants such as ubiquinol (mito-Q) conjugated to triphenyl phosphonium (TPP+) and TPP+-conjugated carboxyl proxyl (mito-CP). In addition, the CYP2E1 inhibitor diallyl sulfide reversed the loss of complex IV activity and its subunits, probably via inhibition of CYP2E1-mediated oxidative stress. The same group further confirmed these results by using COS-7 cells and HepG2 cells stably expressing either the W23/30R mutant, which favorably targets CYP2E1 to mitochondria rather than ER, or the L32N variant, which preferentially directs CYP2E1 to ER than mitochondria [146]. Upon exposure to alcohol, the cells expressing the W23/30R mutant showed markedly increased ROS production, respiratory dysfunction, and loss of cytochrome c oxidase subunits (complex IV) with decreased activity compared to the cells expressing the L32N variant. These results suggested that mutations in human CYP2E1 gene affect subcellular localization of CYP2E1 and hence influence the susceptibility to ethanol-induced toxicity in cellular models. In addition, another group evaluated the role of mitochondrial CYP2E1 in both ethanol- and acetaminophen (APAP)-induced oxidative stress and cytotoxicity using the cells over-expressing CYP2E1 in mitochondria only or in both ER and mitochondria compared to mock-transfected cells [148]. This study showed that the CYP2E1 substrates APAP or ethanol induce ROS overproduction, depletion of reduced GSH, increased expression of mitochondrial Hsp70, mitochondrial dysfunction and cytotoxicity when CYP2E1 was exclusively located in the mitochondria despite the lower cellular level and activity of CYP2E1, compared to the cells with over-expressed CYP2E1 in both ER and mitochondria. These results, especially with APAP, suggested that mitochondrial CYP2E1 may play a major role in drug-induced oxidative stress and cytotoxicity. Furthermore, the co-expression of hepatitis C virus (HCV) core protein and CYP2E1 in Huh-7 cells synergistically enhanced cell death induced by either tertiary butyl hydroperoxide or TNF-α [149]. Tertiary butyl hydroperoxide treatment also increased the ROS production by more than 3-fold, depleted the levels of reduced GSH and caused mitochondrial depolarization when compared with the cells without over-expressed HCV core protein and CYP2E1. In fact, mitochondria changes and cell death were significantly attenuated in the absence of HCV core/CYP2E1. When these cells were exposed to ethanol, greater production of ROS and further depletion of mitochondrial GSH were observed. All these effects were prevented by the antioxidant N-acetylcysteine. The authors concluded that sensitization of mitochondria to oxidative insults is thus a potential mechanism for alcohol-related exacerbation of liver injury in chronic HCV infection and that mitochondrial CYP2E1 may play a critical role in this process.
It has also been shown that there was a five- to eight-fold increase of mitochondrial CYP2E1 and GST A4-4 levels in mitochondria from streptozotocin (STZ)-treated diabetic rat tissues including the liver, kidney, pancreas and brain compared with those in non-diabetic rats, suggesting possible roles of mitochondrial CYP2E1 in the disease process [150]. In addition, transient transfection of COS cells with the CYP2E1 cDNA caused a similar accumulation of CYP2E1 and GST A4-4 in mitochondria besides the increased production of mitochondrial ROS. Taken together, these results implicate a direct role for mitochondrial CYP2E1 in the generation of intra-mitochondrial ROS [150]. Our laboratory also showed a significant increase in both cytosolic and mitochondrial CYP2E1 in both WT and peroxisome proliferator-activated receptor-alpha (Ppara)-null mice [143] when mice were fed a HFD (70% calories derived from fat) for 3 weeks. However, the resultant increase of oxidative stress and consequent mitochondrial dysfunction, as evidenced by the inhibition of the β-oxidation enzyme 3-ketoacyl-CoA thiolase (thiolase) seem more prominent in Ppara-null mice which exhibited higher levels of CYP2E1 than the corresponding WT mice. Together, these data suggested that mitochondrial CYP2E1 plays a critical role, at least partially, in mediating HFD-induced prominent hepatotoxicity and NASH development in Ppara-null mice. The mechanisms of targeting CYP2E1 to either ER or mitochondria would logically be distinctive. Indeed, ER-protein targeting that involves signal recognition particle (SRP) in delivering the nascent protein to translocon complex, whereas mitochondrial protein targeting is suggested to be a post-translational event and the contact between the protein and the inner and outer mitochondrial membrane transporters is deemed necessary for this targeting. In addition, the mitochondrial HSP70 is required to pull the proteins into the mitochondrial matrix and the various proposed mechanisms of CYP2E1 targeting to the mitochondria were eloquently reviewed elsewhere [29].
In contrast, there are a few studies that challenge the role of CYP2E1 (ER and/or mitochondrial), as a major player, in mediating the hepatic deleterious effects of ethanol or HFD. For instance, increased mitochondrial production of ROS/RNS and mitochondrial dysfunction, as evidenced by changes in the mitochondrial genome, mitochondrial DNA damage, and impaired efficiency of mitochondrial respiration, were all reversed when animals were treated with the anti-oxidant and anti-inflammatory S-adenosylmethionine (SAMe) [151]. SAMe-induced suppression of oxidative stress levels and mitochondrial protection was not accompanied by the inhibition of the markedly increased levels of CYP2E1 following ethanol exposure. Interestingly, the lack of protective effects of SAMe against inhibition of CYP2E1 activity was also observed in mice exposed to acute binge ethanol [152]. In this model, SAMe significantly alleviated the deleterious effects of ethanol, as evidenced by histological and biochemical measurements including the decreased mitochondrial GSH and increased mitochondrial lipid peroxidation, without the inhibition of the significantly elevated CYP2E1. In agreement, it was suggested that SAMe is a weak inhibitor of CYP2E1 due to its high IC50 value (1.5 – 5 mM) for the inhibition of p-nitrophenol (PNP) hydroxylation using an in vitro system [153]. However, the role of CYP2E1 cannot be excluded since mitochondrial CYP2E1 may play at least a partial or permissive role in mediating the ethanol-induced hepatic damage in these models and that the reversal of the hepatotoxicity was only partial, despite being significant. These results suggest a role of other factors in mediating the damaging effects. In addition, the induction of CYP2E1- or iNOS in rats exposed to chronic alcohol feeding was not ameliorated by treatment with the antioxidant mitochondria-targeted Mito-Q [154]. In contrast, Mito-Q significantly reduced the levels of ethanol-mediated protein nitration and lipid peroxidation and hepatic steatosis. Nonetheless, the role of mitochondrial CYP2E1 still cannot be excluded from this study since Mito-Q can interact directly with either peroxynitrite [155] or superoxide [156] and can also prevent the formation of lipid peroxides [157]. Thus, Mito-Q seems to work downstream to the deleterious effects of CYP2E1 and thus the role of mitochondrial CYP2E1 in mediating the ethanol-related hepatotoxicities still cannot be underestimated from this study.
Collectively, with the exception of a few studies, the aforementioned reports suggest that CYP2E1 can be expressed and regulated in different sub-cellular organelles of various tissues and that mitochondria-expressed CYP2E1 augments mitochondrial dysfunction in AFLD, NAFLD and drug-induced liver injury (DILI), possibly through increased production of ROS, depletion of mitochondrial reduced GSH and ultimately mitochondrial dysfunction.
Increased CYP2E1 levels and the resultant nitroxidative stress on mitochondrial function in AFLD, NAFLD and DILI
Although mitochondria represent one of the major sources of cellular ROS/RNS, mitochondria are major targets of nitroxidative damage. Indeed, mitochondria from tissues in animal disease models and/or human diseases characterized by increased oxidative stress levels show abnormal and irregular shapes with suppressed functions [158, 159]. In addition, upon exposure to nitrating conditions, isolated rat liver mitochondria exhibited a dramatically shorter turn-over rates from days to hours due to increased proteolytic degradation [160–163]. ATP, which is produced in mitochondria by oxidative phosphorylation, is essential for various cell functions. Most of the cellular energy provided by mitochondria are needed for numerous vital functions such as the fat oxidation, ammonia and glutamate metabolism, antioxidant defense, heme and steroid biosynthesis, and others [164]. The mitochondrial fat oxidation pathway becomes particularly important in cases of decreased glucose supply such as during fasting or compromised oxidative phosphorylation under major disease states [164]. Inhibition of mitochondrial respiration was also reported in AFLD and NAFLD [59, 165, 166]. Thus, it is conceivable that under these conditions and without sufficient energy supply mechanism(s) due to mitochondrial dysfunction, the hepatic cells would be more susceptible to cell death especially after simultaneous exposure to two potentially toxic substances such as ethanol and APAP. Healthy mitochondrial functions are maintained through proper redox balance. Under normal physiological conditions, approximately 1%-2% of ROS is leaked from the mitochondrial electron transport chain (ETC) [167]. In addition, transiently elevated ROS is essential to regulate cellular signaling pathways [168] and can be adequately balanced by the cellular, especially mitochondrial, anti-oxidant defense system including mitochondrial anti-oxidant enzymes such as superoxide dismutase-2 (SOD2) and glutathione peroxidase (Gpx) [168, 169]. Mitochondrial RNS can also be produced by the mitochondrial NOS or transferred from the cytosol since RNS including NO radicals can easily cross the mitochondrial membrane [170]. Taken together, the mitochondrial ETC is a major source of ROS/RNS in the mitochondria. In addition, as described earlier, CYP2E1 is involved in the production of ROS/RNS. CYP2E1 can thus play a significant role to alter the mitochondrial functions through nitroxidative or lipid peroxidation as observed in AFLD and NAFLD. This additional damage to the mitochondrial complexes is likely to produce more ROS/RNS and hence a vicious cycle can develop, ultimately leading to cellular apoptosis/necrosis. ROS such as superoxide anion and hydroxyl radical, and RNS, including NO, can also be produced from other cellular sources than the mitochondrial ETC and CYP2E1. These sources of ROS/RNS include: microsomal CYP4A, NADPH oxidase and myeloperoxidase in phagocytic immune cells, cytosolic xanthine oxidase, and nitric oxide synthase (NOS) isozymes [59, 124, 145, 171–174]. In addition, under increased ROS and RNS levels, a potently toxic peroxynitrite (ONOO−) can be formed through the interaction between superoxide anion and NO-containing compounds. Peroxynitrite can stimulate S-nitrosylation of Cys residues or nitration of tyrosine (Tyr) residue(s), ultimately contributing to alterations of the structure and function of many cellular proteins [63].
Mitochondria are particularly susceptible to nitroxidative damage including lipid peroxidation due to the low levels of antioxidants such as GSH as compared to cytosol [175]. The lower levels of mitochondrial GSH are due to the inability of mitochondria to synthesize GSH and hence a specific GSH transporter is needed to transfer GSH from cytosol to mitochondria. The combination of increased production of ROS/RNS and the low levels of mitochondrial GSH can cause accumulation of ROS/RNS in the mitochondria and damage various cells including hepatocytes and may ultimately lead to cell demise. This deleterious effect might even get worse in the presence of the various pro-oxidants which can further decrease the already low mitochondrial GSH, as observed with alcohol, HFD, obesity, and various drugs or toxic substances such as 3,4-methylendioxymethamphetamine (MDMA), APAP and in pathophysiological conditions such as ischemia reperfusion (I/R), hepatic steatosis and steatohepatitis, as reviewed [63, 64].
Under the conditions of elevated nitroxidative stress, mitochondrial proteins, DNA, and lipids can be covalently modified by oxidation, nitrosation, nitration, and other PTMs. The deletion and/or mutation through nitroxidative modifications of mitochondrial DNA can exert deleterious effects accompanied with increased amounts of ROS leakage, since mitochondrial DNA encode 13 polypeptides, all of which are subunits of the four mitochondrial ETC proteins [176, 177]. In addition, mitochondrial DNA and proteins are more susceptible to nitroxidative damage due to the absence of protective antioxidant protein catalase, the low activity of DNA repair enzymes, and the absence of histones and polyamines [178]. The damage of mitochondrial DNA, which is essential for normal formation of the ETC components, and inefficient repair are thought to be important in both alcohol- and HFD-induced hepatic cellular damage [179–182]. Enhanced mitochondrial generation of ROS has been reported in obese (ob/ob) mice [183] and a rat model of steatosis and steatohepatitis exposed to a choline-deficient diet [184, 185]. The high levels of 8-OH-dG, which indicates DNA lesion, have been reported in models of NAFLD as demonstrated with mice fed a methionine/choline deficient diet or in ob/ob mice [186]. Thus, it is safe to assume that oxidative stress and lipotoxicity are important factors in the modification of liver mitochondrial DNA that positively correlated with the severity of NASH in a clinical study with NAFLD patients in a case-control design consisting of 45 patients and 18 people with near-normal liver-histology [187]. Consistently, another clinical study analyzing patients with NASH, patients with simple steatosis, and healthy volunteers suggested that the progression of NAFLD is associated with the development of oxidative stress and mitochondrial dysfunction [188]. Further, mitochondrial DNA damage and/or PTMs of mitochondrial proteins by nitroxidative stress would result in decreased expression, assembly and activity of the mitochondrial ETC complexes. Indeed, under high levels of ROS/RNS in AFLD, NAFLD as observed with HFD or in obese (ob/ob) mice and in DILI by APAP, the activities of the hepatic mitochondrial ETC complexes were significantly compromised, ultimately contributing to more hepatocellular ROS production and the development of a vicious cycle of ROS/RNS production [19, 179–182, 189–192].
The presence of CYP2E1 affects the levs of nitroxidative stress in experimental models. In fact, the levels of S-nitrosylation of cysteine residues and/or nitration of tyrosine residues of mitochondrial proteins depend on the presence of CYP2E1 following exposure to alcohol and APAP, respectively, suggesting the important role of CYP2E1 in promoting various PTMs. The activities of modified mitochondrial proteins such as the ALDH2, thiolase and ATP synthase usually become inactivated, leading to further deterioration of the essential mitochondrial functions. PTMs of mitochondrial proteins represent one of the major mechanisms via which increased nitroxidative stress negatively modulates the function of the mitochondrial ETC and other enzyme activities, ultimately causing hepatotoxicities in AFLD, NAFLD and DILI. For instance, in cases of mitochondrial damage after exposure to alcohol, HFD, abused substances such as MDMA and cocaine and therapeutics like APAP at toxic doses or in obesity and I/R [176, 193, 194], markedly higher amounts of ROS and RNS could be produced from the mitochondrial ETC, possibly at the sites of Complex I (NADH ubiquinone oxidoreductase) and Complex III (ubiquinone cytochrome c oxidoreductase), as exemplified with hepatocytes subjected to ethanol [189, 195–197]. In addition, the activities of complexes I, III, IV (cytochrome c oxidase) and V (ATP synthase) in C57BL/6 obese mice fed a HFD for 16 weeks were significantly inhibited, possibly through various PTMs of mitochondrial proteins [198].
The systematic redox proteomics analysis of the oxidatively-modified mitochondrial proteins in various experimental models of acute liver disease caused by alcohol (acute or chronic), MDMA, or hepatic I/R injury detected all four enzymes involved in the β-oxidation of fatty acids namely medium-chain fatty acyl-CoA dehydrogenase, enoyl-CoA hydratase, 3-hydroxyacyl-CoA dehydrogenase, and thiolase [64, 189, 196, 197]. The activity measurement of thiolase revealed its inhibition under these conditions, implying similar oxidative suppression of the other three enzymes in the β-oxidation pathway. Since the inhibited activity of thiolase correlated with the degree of fat accumulation, it is reasonable to suggest that oxidative inhibition of the fatty acid metabolism contributes to the development of steatosis/steatohepatitis in AFLD and conceivably in NAFLD. The inactivation of the hepatic ATP synthase activity was also monitored in rodents exposed to alcohol, MDMA, APAP, and I/R injury [19, 64, 189, 196, 197]. Mass-spectral analysis revealed that nitration of the Tyr residues in the catalytic β subunit of ATP synthase seems to be the main reason for its inactivation in all of these conditions and that decreased ATP production could be expected in many pathological conditions, suggesting that nitroxidative stress plays a major role in the development and progression of NAFLD or DILI [199–202]. The suppression of ATP synthase would result in low levels of cellular ATP, leading to decreased cellular ability to perform many essential functions followed by cellular necrosis.
Anti-oxidant cellular enzymes represent another group of proteins whose activities were also compromised under increased nitroxidative stress conditions [19, 63, 64, 189, 196, 197]. Inactivation of the anti-oxidant enzymes such as mitochondrial Mn-dependent SOD2 and cytosolic Cu-Zn-dependent SOD1 (SOD1) were also observed in NAFLD and likely participated to the decreased anti-oxidant capacity and the disease progression [128, 203, 204]. Suppression of both SOD enzymes was reported to be mediated through tyrosine nitration [18, 63, 204]. The PTMs of mitochondrial SOD2 and the subsequent inactivation can also be deduced from a study using mice fed a methionine-choline deficient diet. In this model, however, the mRNA transcript of SOD2 was increased, possibly reflecting a compensatory mechanism against the suppressed SOD2 activity [205].
Mitochondrial ALDH2 is an enzyme which exhibits a low Km for the metabolism of reactive acetaldehyde and 4-HNE, produced from the metabolisms of ethanol and lipids, respectively. Mitochondrial ALDH2 is considered the major enzyme responsible for acetaldehyde metabolism in humans, although cytosolic ALDH1 may also be involved in rodents, due to a relative low Km value (11~18 µM for acetaldehyde) in comparison to that of human counterpart (> 180 µM) [206]. Consequently suppression of ALDH2, a protective enzyme, would lead to accumulation of reactive aldehydes including lipid peroxidation products such as 4-HNE and malondialdehyde (MDA) [207], leading to protein adduct formation and hepatic cell death. In addition, these lipid peroxides can be also involved in the development and progression of hepatic fibrogeneis due to the activation of immune system and hepatic stellate cells [43, 45, 208–210]. ALDH2 and other ALDH isoforms are generally expressed in the liver in large amounts. The dominant ALDH2 inactivation has been reported when a single nucleotide mutation (G to A nucleotide substitution) in human ALDH2 gene with a substitution of Glu487 with Lys487 result in marked suppression of NAD+ binding affinity [211–213]. This is particularly important in many East Asians where the frequency of individuals with ALDH2*2 mutant allele is abundant (30 ~ 50%). However, individuals with either heterozygous or homozygous ALDH2*2 seems to be protected from the harmful effects of alcohol drinking due to the development of facial flushing response accompanied with difficulty in breathing and disturbance in the cardiac rhythm possibly due to the increased acetaldehyde levels upon alcohol drinking [214–216]. Consequently, because of uncomfortable feeling, these individuals with the ALDH2*2 mutant gene tend to stop drinking alcohol [214–216]. The absence of alcohol drinking in people with the ALDH2*2 mutant gene is particularly true, as evidenced by genetic screening of more than 1,300 alcoholic Japanese individuals, where not a single person with ALDH2*2/*2 homozygous allele was reported [217]. On the other hand, if these individuals, choose to drink alcoholic beverage, they are more susceptible to alcohol- and acetaldehyde-related hepatic and extra-hepatic tissue injury and carcinogenesis, especially in the oral-esophagus-gastrointestinal track [218–220]. Thus, many pathological conditions can be mediated by the inhibition of ALDH2 and other ALDH isozymes with elevated DNA mutations [221] and protein modifications [208] by highly reactive carbonyl compounds such as acetaldehyde, 4-HNE, MDA, and ACR produced after alcohol intake and/or exposure to toxic substances including APAP and HFD. This harmful effects can be markedly increased particularly in combined cases of ALDH2 suppression and CYP2E1 upregulation through the massive formation of oxidative stress.
Indeed, under conditions of increased nitroxidative stress due to exposure to binge or chronic alcohol and APAP, ALDH2 activity was significantly inhibited [19, 63, 64, 189, 196, 197]. ALDH2 was also shown to be down-regulated following exposure to HFD for 16 weeks [222]. Although the mechanism for ALDH2 down-regulation was not studied, the importance of its PTMs under increased oxidative stress cannot be excluded. In this study, oxidative stress was higher in the mice fed a HFD than control after 8 weeks of feeding while it was decreased after 16 weeks, implying lower mitochondrial bioenergetics, possibly due to oxidative degradation of many mitochondrial proteins. It is also possible that ALDH2 was degraded following various PTMs, as previously discussed [63, 207]. Thus, the degradation of covalently modified proteins including those vital for cell maintenance and survival can also be detrimental to the well-being of the target cells, especially when the rates of degraded proteins, essential for energy production, antioxidant or anti-inflammatory defense, and urea metabolism exceed those of their regeneration or other compensatory mechanism(s) due to continuous exposure to toxic substances. Autophagy-dependent clearance of damaged mitochondria (mitophagy) and consequently decreased protein levels can also be another mechanism via which cells can remove damaged proteins [18, 223]. Taken together, in conditions with increased levels of the pro-oxidant CYP2E1, cytosolic and mitochondrial, with decreased levels anti-oxidants levels and inhibition of ALDH2 activities, as observed with AFLD and NAFLD, could result in massive increase in unchecked oxidative stress radicals including formation of reactively lipid peroxide radicals. Even under the conditions of massive oxidative stress without the correspondingly increased CYP2E1 levels, or with actually decreased CYP2E1, CYP2E1 could play a permissive role in the development of mitochondrial dysfunction and ultimate cell toxicity. This conclusion can be deduced from experiments with WT mice exposed to APAP and carbon tetrachloride, which actually decreased the CYP2E1 levels possibly through suicidal degradation pathways [224]. In addition, CYP2E1-null mice were fully protected from these toxic agents since both of them are CYP2E1 substances [18, 225].
As mentioned earlier, in addition to nitroxidative PTMs of many mitochondrial proteins, oxidation of lipids (lipid peroxidation) would also result in the production of potently cytotoxic lipid peroxides such as 4-HNE, MDA, 4-oxonon-2-enal (4-ONE), and acrolein (ACR), as reported with chronic alcohol drinking and/or smoking, HFD, or APAP [208, 226–228]. Lipid peroxidation can produce protein adducts with many amino acids such as Cys, His, and Lys [229, 230]. Through the protein adduct formation, the functional activities of many mitochondrial enzymes can be compromised in response to alcohol. For instance, cytochrome C oxidase (complex IV), ALDH2, Sirt3, the mitochondrial NAD+-dependent deacetylase, and many other enzymes can be suppressed through adduct formation with lipid peroxides [63, 64, 231–233]. These results were also confirmed with human specimens including patients with alcoholic cirrhosis and/or hepatitis, patients with nonalcoholic cirrhosis, heavy drinkers with fatty liver, and control [209]. The levels of albumin conjugated with MDA, 4-HNE, and oxidized fatty acids, but not with ACR, 2-hexenal, and methylglyoxal, were significantly higher in alcoholics than all other groups including patients with nonalcoholic cirrhosis or healthy controls. Furthermore, the plasma levels of anti-MDA and anti-HNE antibodies were significantly higher in cirrhotic or fibrotic patients than in those with fatty liver only. The authors suggested that the antigens derived from lipid peroxidation contribute to the development of immune responses associated with ALD. Indeed, HNE-protein adducts were reported to activate immune cells including hepatic macrophage Kupffer cells and/or infiltration of neutrophils into the liver. These immune-activating events hence stimulate increased production of inflammatory cytokines and activation of stellate cells, leading to advanced liver disease, as reported in ALD [208, 209, 226, 227]. The HNE-mediated immune reaction in ALD may also very well be true for patients with NASH since the serum levels of bioactive lipid peroxidation products positively correlates with the histologic features of NASH [234]. Although there was not much information about the identity of adduct proteins modified by lipid peroxides in NAFLD [63, 64], it is reasonable to assume that they are similar or close to those identified in AFLD since pathogenesis mechanisms and disease progression are very similar between AFLD and NAFLD [21].
Lipid peroxidation might also play a role, at least partially, in the degradation and depletion of mitochondrial DNA in hepatic and other organs in rodents. For instance, acute intragastric ethanol administration (5 g/kg) caused mitochondrial DNA depletion and replacement of its supercoiled form by linearized forms in the liver, brain, heart, and skeletal muscle of mice. However, there was a transient adaptive re-synthesis of DNA at earlier time point accompanied by an overshoot of mitochondria DNA re-synthesis at later time point following ethanol administration. In hepatic or extra-hepatic tissues, mitochondrial DNA degradation and depletion were prevented by an inhibitor of ethanol metabolism 4-methylpyrazole and attenuated by several anti-oxidants including vitamin E (lipid peroxidation inhibitor), melatonin, or coenzyme Q [235]. To avoid the adaptive re-synthesis which might hinder the evaluation of the ethanol effects on mitochondrial DNA following the single dose, ethanol (5 g/kg) was administered by daily gastric intubation for 4 consecutive days. Indeed, the mitochondrial DNA levels were decreased for 48 hours after the last ethanol dose, with no overshoot of re-synthesis phenomenon later. These events were accompanied by increased levels of CYP2E1 and mitochondrial levels of lipid peroxidation, suggesting a possible involvement of CYP2E1 and lipid peroxidation in the accumulation of unrepaired mitochondrial DNA lesions [236]. Furthermore, CYP2E1-related ROS but not from NADPH oxidase seems to be more important to cause oxidative DNA damage, leading to hepatic cancer in ethanol-exposed mice [237].
In addition to DNA depletion and/or damage, mitochondrial dysfunction can be mediated by various covalent modifications (e.g., oxidation, nitrosation, nitration, phosphorylation, acetylation, adduct formation, etc.) of various mitochondrial proteins. These PTMs can cause inactivation and compromise of the function of many mitochondrial proteins, as demonstrated in experimental models of alcohol exposure [189], APAP-mediated DILI [19], or I/R-mediated liver injury [197]. Several mitochondrial enzymes involved in energy production can be suppressed through various PTMs, leading to energy depletion. Oxidative inactivation of ALDH2 and other enzymes involved in the metabolism of reactive carbonyl compounds can result in increased levels of toxic lipid peroxides. Suppression of the enzymes involved in the fatty acid oxidation can lead to fat accumulation. Thus, it is logical to that oxidatively-modified mitochondrial proteins accompanied with their functional alterations cause mitochondrial dysfunction prior to full-blown tissue injury and that CYP2E1, including mitochondrial isoform, plays a critical role in promoting mitochondrial dysfunction through massive production of ROS/RNS with depletion of mitochondrial GSH. This is particularly true when the injurious agent is a substrate of CYP2E1 like APAP. Although we briefly focused on mitochondrial protein nitroxidation and lipid peroxidation, other forms of post-translational modifications such as phosphorylation, acetylation, glycation and ubiquitination can also occur in the cells, as recently reviewed [63, 64]. However, the role of CYP2E1 in promoting other PTMs of mitochondrial proteins and their functional consequences still need further investigations.
Future translational research opportunities including CYP2E1 inhibitors and mitochondria-targeted antioxidants
The harmful effects of CYP2E1 may result from either the activity of ER-associated CYP2E1 or mitochondrial isoform, although it is hard to dissect the role of each isozyme in promoting a specific disease development or progression. Interestingly, it is known that both mitochondria and ER are interconnected together via their network and their interaction is important for a variety of cellular functions and homeostasis such as lipid transport, energy homeostasis, and calcium signaling [238]. Thus, it would be interesting to determine the temporal effects along with a dose-response effect of different CYP2E1 inducers/substrates on both ER and mitochondrial CYP2E1.
The information about the functional role of CYP2E1 and underlying mechanisms of its definite harmful effects should be used logically for blocking or at least ameliorating its effects. These aims can be achieved either by directly trying to block the CYP2E1 pathway or indirectly by blocking its downstream injurious mediators such as using antioxidants or oxidative stress scavengers. The majority of these nitroxidative- and lipid peroxidation-mediated deleterious effects are associated with mitochondrial dysfunction, at least partially, through oxidative PTMs of mitochondrial proteins upon exposure to hepatotoxic agents. The impairment of normal mitochondrial functions by PTMs of mitochondrial proteins and other cellular proteins would lead to energy depletion, fat accumulation, altered metabolism, inflammation and necrotic/apoptotic tissue damage. Thus, the deleterious effects of CYP2E1 can be either mitochondria-dependent or independent, based on the identity of injurious agents, duration of exposure, dose of the agent, presence of other pathological conditions and/or other injurious agents simultaneously. Further, it is feasible to identify mitochondria as a major therapeutic target in various diseases including AFLD and NAFLD where CYP2E1 is a critical factor in mediating the disease, at least partially, by using anti-oxidants or CYP2E1 inhibitors. The most common method to directly counteract CYP2E1-deletrious effects could be the use of CYP2E1 inhibitors such as CMZ and polyenylphosphatidylcholine for ameliorating liver injury [50, 239, 240]. The effects of these CYP2E1 inhibitors were partial in these studies, suggesting many possibilities: 1) the dose of the inhibitors used could be insufficient; 2) the bioavailability of the inhibitors could be not high enough to counteract the injurious agent; 3) the mitochondrial CYP2E1 might play a major role than the microsomal isoform in these models and the levels of the inhibitors in the mitochondria were not sufficient to inhibit mitochondrial CYP2E1; 4) CYP2E1 may not be the only contributing factor in promoting the disease. Thus the determination of CYP2E1 inhibitor levels in the mitochondria following treatment will be beneficial, if technically feasible. However, the use of CYP2E1 inhibitors in clinical setting is still hampered by their high toxicities.
Another potential strategy is to interfere with the targeting of CYP2E1 to the mitochondria and or its induction within the mitochondria. In fact, there are several mechanisms for mitochondrial targeting of CYP2E1, as reviewed [29]. This approach will require the examination of the effects of various known CYP2E1 inducers and monitoration of the mitochondrial targeting and/or induction of CYP2E1. In addition, leptin and glucagon have been suggested to increase mitochondrial protein targeting, whereas our laboratory also reported that HFD (fatty acids) significantly increased levels of mitochondrial and cytosolic CYP2E1 isoforms. These results suggest a potential role of fatty acids in increasing mitochondrial protein targeting and/or induction [28, 143]. Thus, the factors that counteract the effects of leptin, glucagon and fatty acids are likely to decrease the levels of mitochondrial CYP2E1. The identification of additional factors and the mechanism(s) by which they increase CYP2E1 mitochondrial targeting and/or its induction would increase our understanding of the role and targeting mechanism of mitochondrial CYP2E1. This approach will subsequently improve the chances of the development of potentially better intervention therapeutics.
Another strategy to ameliorate CYP2E1-mediated mitochondrial damage and subsequent disease development and progression is to prevent and/or decrease the mitochondrial oxidative radicals. For this purpose, several natural [201, 241–245] and synthetic [246–253] anti-oxidants are available. It is believed, however, that the synthetic anti-oxidants including the mitochondria-targeted antioxidants generally work better and efficiently than the natural ones with poor purity, solubility, bioavailability and low transport to mitochondria [242, 243, 245, 246]. In contrast, synthetic anti-oxidants have been manipulated for better delivery to the mitochondria. These synthetic agents include Szeto-Schiller or SS-tetrapeptide, TPP+, a cell permeable lipophilic cation), SOD-catalase mimetics, Mito-Q, and Mito-CP[154, 247, 249, 251, 253, 254]. Mitochondria-targeted anti-oxidants would mostly work downstream to CYP2E1 or mitochondrial abnormalities possibly by scavenging the already performed oxidative radicals or interfering with its formation and/or affecting HIF-1α stabilization or protein nitration, since the elevated levels of CYP2E1 remained unchanged in cells treated with the mitochondria-targeted anti-oxidants [154].
ACKNOWLEDGEMENT
This research was supported by the Intramural Program Fund of the National Institute of Alcohol Abuse and Alcoholism. The authors thank Dr. Klaus Gawrisch for the support.
The abbreviations used are
- ACR
acrolein
- AFLD
alcoholic fatty liver disease
- APAP
acetaminophen
- ALD
alcoholic liver disease
- ALDH2
mitochondrial low-Km aldehyde dehydrogenase 2
- CMZ
chlormethiazole
- Complex I
NADH-dependent ubiquinone oxido-reductase
- Complex III
ubiquinone cytochrome bc1 oxidoreductase
- Complex IV
cytochrome c oxidase
- Complex V
ATP synthase
- CYP4A
cytochrome P450 4A
- CYP2E1
ethanol-inducible cytochrome P450 2E1
- DILI
drug induced liver injury
- ER
endoplasmic reticulum
- ETC
electron transport chain
- Gpx
glutathione peroxidase
- GSH
reduced glutathione
- HCV
hepatitis C virus
- HFD
high fat diet
- HIF
hypoxia-inducible factor
- 4-HNE
4-hydroxynonenal
- iNOS
inducible nitric oxide synthase
- I/R
ischemia-reperfusion
- JNK
c-Jun N-terminal protein kinase
- MDA
malondialdehyde
- MDMA
3,4-methylenedioxymethamphetamine
- mito-CP
mitochondria-targeted carboxy-proxyl
- mito-Q
mitochondria-targeted ubiquinone
- NAFLD
nonalcoholic fatty liver disease
- NF-kB
nuclear factor-kB
- NO
nitric oxide
- PPAR-α
peroxisome proliferator-activated receptor-α
- PTMs
post-translational protein modifications
- RNS
reactive nitrogen species
- ROS
reactive oxygen species
- SAMe
S-adenosyl-methionine
- SOD
superoxide dismutase
- Thiolase
3-ketoacyl-CoA thiolase
- TPP+
triphenylphosphonium cation
- TNF-α
tumor necrosis factor-α
- WT
wild-type
Footnotes
The authors do not have any conflict of interest.
References
- 1.Novak RF, Woodcroft KJ. The alcohol-inducible form of cytochrome P450 (CYP 2E1): role in toxicology and regulation of expression. Arch Pharm Res. 2000;23:267–282. doi: 10.1007/BF02975435. [DOI] [PubMed] [Google Scholar]
- 2.Retraction. ATP consumption by sarcoplasmic reticulum Ca2+ pumps accounts for 50% of resting metabolic rate in mouse fast and slow twitch skeletal muscle. Am J Physiol Cell Physiol. 2010 Mar;298(3):C521–C529. doi: 10.1152/ajpcell.00479.2009. Am J Physiol Cell Physiol, 2012; 303: C1000. [DOI] [PubMed] [Google Scholar]
- 3.Song BJ. Ethanol-inducible cytochrome P450 (CYP2E1): biochemistry, molecular biology and clinical relevance: 1996 update. Alcohol Clin Exp Res. 1996;20:138A–46A. doi: 10.1111/j.1530-0277.1996.tb01764.x. [DOI] [PubMed] [Google Scholar]
- 4.Gonzalez FJ. Role of cytochromes P450 in chemical toxicity and oxidative stress: studies with CYP2E1. Mutat Res. 2005;569:101–110. doi: 10.1016/j.mrfmmm.2004.04.021. [DOI] [PubMed] [Google Scholar]
- 5.Cederbaum AI. Alcohol metabolism. Clin Liver Dis. 2012;16:667–685. doi: 10.1016/j.cld.2012.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cederbaum AI, Lu Y, Wu D. Role of oxidative stress in alcohol-induced liver injury. Arch Toxicol. 2009;83:519–548. doi: 10.1007/s00204-009-0432-0. [DOI] [PubMed] [Google Scholar]
- 7.Raucy JL, Lasker JM, Kraner JC, Salazar DE, Lieber CS, Corcoran GB. Induction of cytochrome P450IIE1 in the obese overfed rat. Mol Pharmacol. 1991;39:275–280. [PubMed] [Google Scholar]
- 8.O’Shea D, Davis SN, Kim RB, Wilkinson GR. Effect of fasting and obesity in humans on the 6-hydroxylation of chlorzoxazone: a putative probe of CYP2E1 activity. Clin Pharmacol Ther. 1994;56:359–367. doi: 10.1038/clpt.1994.150. [DOI] [PubMed] [Google Scholar]
- 9.Chalasani N, Gorski JC, Asghar MS, Asghar A, Foresman B, Hall SD, Crabb DW. Hepatic cytochrome P450 2E1 activity in nondiabetic patients with nonalcoholic steatohepatitis. Hepatology. 2003;37:544–550. doi: 10.1053/jhep.2003.50095. [DOI] [PubMed] [Google Scholar]
- 10.Wang Z, Hall SD, Maya JF, Li L, Asghar A, Gorski JC. Diabetes mellitus increases the in vivo activity of cytochrome P450 2E1 in humans. Br J Clin Pharmacol. 2003;55:77–85. doi: 10.1046/j.1365-2125.2003.01731.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dong ZG, Hong JY, Ma QA, Li DC, Bullock J, Gonzalez FJ, Park SS, Gelboin HV, Yang CS. Mechanism of induction of cytochrome P-450ac (P-450j) in chemically induced and spontaneously diabetic rats. Arch Biochem Biophys. 1988;263:29–35. doi: 10.1016/0003-9861(88)90610-8. [DOI] [PubMed] [Google Scholar]
- 12.Weltman MD, Farrell GC, Hall P, Ingelman-Sundberg M, Liddle C. Hepatic cytochrome P450 2E1 is increased in patients with nonalcoholic steatohepatitis. Hepatology. 1998;27:128–133. doi: 10.1002/hep.510270121. [DOI] [PubMed] [Google Scholar]
- 13.Yun YP, Casazza JP, Sohn DH, Veech RL, Song BJ. Pretranslational activation of cytochrome P450IIE during ketosis induced by a high fat diet. Mol Pharmacol. 1992;41:474–479. [PubMed] [Google Scholar]
- 14.Song BJ, Veech RL, Park SS, Gelboin HV, Gonzalez FJ. Induction of rat hepatic N-nitrosodimethylamine demethylase by acetone is due to protein stabilization. J Biol Chem. 1989;264:3568–3572. [PubMed] [Google Scholar]
- 15.Song BJ, Matsunaga T, Hardwick JP, Park SS, Veech RL, Yang CS, Gelboin HV, Gonzalez FJ. Stabilization of cytochrome P450j messenger ribonucleic acid in the diabetic rat. Mol Endocrinol. 1987;1:542–547. doi: 10.1210/mend-1-8-542. [DOI] [PubMed] [Google Scholar]
- 16.Gorsky LD, Koop DR, Coon MJ. On the stoichiometry of the oxidase and monooxygenase reactions catalyzed by liver microsomal cytochrome P-450. Products of oxygen reduction. J Biol Chem. 1984;259:6812–6817. [PubMed] [Google Scholar]
- 17.Caro AA, Cederbaum AI. Oxidative stress, toxicology, and pharmacology of CYP2E1. Annu Rev Pharmacol Toxicol. 2004;44:27–42. doi: 10.1146/annurev.pharmtox.44.101802.121704. [DOI] [PubMed] [Google Scholar]
- 18.Abdelmegeed MA, Moon KH, Chen C, Gonzalez FJ, Song BJ. Role of cytochrome P450 2E1 in protein nitration and ubiquitin-mediated degradation during acetaminophen toxicity. Biochem Pharmacol. 2010;79:57–66. doi: 10.1016/j.bcp.2009.07.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Abdelmegeed MA, Jang S, Banerjee A, Hardwick JP, Song BJ. Robust protein nitration contributes to acetaminophen-induced mitochondrial dysfunction and acute liver injury. Free Radic Biol Med. 2013;60:211–222. doi: 10.1016/j.freeradbiomed.2013.02.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lieber CS, Cao Q, DeCarli LM, Leo MA, Mak KM, Ponomarenko A, Ren C, Wang X. Role of medium-chain triglycerides in the alcohol-mediated cytochrome P450 2E1 induction of mitochondria. Alcohol Clin Exp Res. 2007;31:1660–1668. doi: 10.1111/j.1530-0277.2007.00475.x. [DOI] [PubMed] [Google Scholar]
- 21.Lieber CS. CYP2E1: from ASH to NASH. Hepatol Res. 2004;28:1–11. doi: 10.1016/j.hepres.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 22.Sakaguchi S, Takahashi S, Sasaki T, Kumagai T, Nagata K. Progression of alcoholic and non-alcoholic steatohepatitis: common metabolic aspects of innate immune system and oxidative stress. Drug Metab Pharmacokinet. 2011;26:30–46. doi: 10.2133/dmpk.dmpk-10-rv-087. [DOI] [PubMed] [Google Scholar]
- 23.Adams LA, Lymp JF, St Sauver J, Sanderson SO, Lindor KD, Feldstein A, Angulo P. The natural history of nonalcoholic fatty liver disease: a population-based cohort study. Gastroenterology. 2005;129:113–121. doi: 10.1053/j.gastro.2005.04.014. [DOI] [PubMed] [Google Scholar]
- 24.Wu D, Cederbaum AI. Inhibition of autophagy promotes CYP2E1-dependent toxicity in HepG2 cells via elevated oxidative stress, mitochondria dysfunction and activation of p38 and JNK MAPK. Redox Biol. 2013;1:552–565. doi: 10.1016/j.redox.2013.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. 2009;29:141–154. doi: 10.1055/s-0029-1214370. [DOI] [PubMed] [Google Scholar]
- 26.Dey A, Cederbaum AI. Alcohol and oxidative liver injury. Hepatology. 2006;43:S63–S74. doi: 10.1002/hep.20957. [DOI] [PubMed] [Google Scholar]
- 27.Begriche K, Massart J, Robin MA, Bonnet F, Fromenty B. Mitochondrial adaptations and dysfunctions in nonalcoholic fatty liver disease. Hepatology. 2013;58:1497–1507. doi: 10.1002/hep.26226. [DOI] [PubMed] [Google Scholar]
- 28.Aubert J, Begriche K, Knockaert L, Robin MA, Fromenty B. Increased expression of cytochrome P450 2E1 in nonalcoholic fatty liver disease: mechanisms and pathophysiological role. Clin Res Hepatol Gastroenterol. 2011;35:630–637. doi: 10.1016/j.clinre.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 29.Knockaert L, Fromenty B, Robin MA. Mechanisms of mitochondrial targeting of cytochrome P450 2E1: physiopathological role in liver injury and obesity. FEBS J. 2011;278:4252–4260. doi: 10.1111/j.1742-4658.2011.08357.x. [DOI] [PubMed] [Google Scholar]
- 30.Tsukamoto H, Xi XP. Incomplete compensation of enhanced hepatic oxygen consumption in rats with alcoholic centrilobular liver necrosis. Hepatology. 1989;9:302–306. doi: 10.1002/hep.1840090223. [DOI] [PubMed] [Google Scholar]
- 31.Stewart S, Jones D, Day CP. Alcoholic liver disease: new insights into mechanisms and preventative strategies. Trends Mol Med. 2001;7:408–413. doi: 10.1016/s1471-4914(01)02096-2. [DOI] [PubMed] [Google Scholar]
- 32.Bertola A, Mathews S, Ki SH, Wang H, Gao B. Mouse model of chronic and binge ethanol feeding (the NIAAA model) Nat Protoc. 2013;8:627–637. doi: 10.1038/nprot.2013.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Brandon-Warner E, Schrum LW, Schmidt CM, McKillop IH. Rodent models of alcoholic liver disease: of mice and men. Alcohol. 2012;46:715–725. doi: 10.1016/j.alcohol.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kamper-Jorgensen M, Gronbaek M, Tolstrup J, Becker U. Alcohol and cirrhosis: dose--response or threshold effect? J Hepatol. 2004;41:25–30. doi: 10.1016/j.jhep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- 35.Ramstedt M. Per capita alcohol consumption and liver cirrhosis mortality in 14 European countries. Addiction. 2001;96(Suppl 1):S19–S33. doi: 10.1080/09652140020021152. [DOI] [PubMed] [Google Scholar]
- 36.Day CP. Who gets alcoholic liver disease: nature or nurture? J R Coll Physicians Lond. 2000;34:557–562. [PMC free article] [PubMed] [Google Scholar]
- 37.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cederbaum AI. CYP2E1 potentiates toxicity in obesity and after chronic ethanol treatment. Drug Metabol Drug Interact. 2012;27:125–144. doi: 10.1515/dmdi-2012-0014. [DOI] [PubMed] [Google Scholar]
- 39.Minato T, Tsutsumi M, Tsuchishima M, Hayashi N, Saito T, Matsue Y, Toshikuni N, Arisawa T, George J. Binge alcohol consumption aggravates oxidative stress and promotes pathogenesis of NASH from obesity induced simple steatosis. Mol Med. 2014 doi: 10.2119/molmed.2014.00048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Yu XQ, Maki M, Drew BT, Paton AJ, Li HW, Zhao JL, Conran JG, Li J. Phylogeny and historical biogeography of Isodon (Lamiaceae): rapid radiation in south-west China and Miocene overland dispersal into Africa. Mol Phylogenet Evol. 2014;77:183–194. doi: 10.1016/j.ympev.2014.04.017. [DOI] [PubMed] [Google Scholar]
- 41.Mathurin P, Lucey MR. Management of alcoholic hepatitis. J Hepatol. 2012;56(Suppl 1):S39–S45. doi: 10.1016/S0168-8278(12)60005-1. [DOI] [PubMed] [Google Scholar]
- 42.Mathurin P, Deltenre P. Effect of binge drinking on the liver: an alarming public health issue? Gut. 2009;58:613–617. doi: 10.1136/gut.2007.145573. [DOI] [PubMed] [Google Scholar]
- 43.Lieber CS. Role of oxidative stress and antioxidant therapy in alcoholic and nonalcoholic liver diseases. Adv Pharmacol. 1997;38:601–628. doi: 10.1016/s1054-3589(08)61001-7. [DOI] [PubMed] [Google Scholar]
- 44.Seth D, Haber PS, Syn WK, Diehl AM, Day CP. Pathogenesis of alcohol-induced liver disease: classical concepts and recent advances. J Gastroenterol Hepatol. 2011;26:1089–1105. doi: 10.1111/j.1440-1746.2011.06756.x. [DOI] [PubMed] [Google Scholar]
- 45.Mello T, Ceni E, Surrenti C, Galli A. Alcohol induced hepatic fibrosis: role of acetaldehyde. Mol Aspects Med. 2008;29:17–21. doi: 10.1016/j.mam.2007.10.001. [DOI] [PubMed] [Google Scholar]
- 46.Chen A. Acetaldehyde stimulates the activation of latent transforming growth factor-beta1 and induces expression of the type II receptor of the cytokine in rat cultured hepatic stellate cells. Biochem J. 2002;368:683–693. doi: 10.1042/BJ20020949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Niemela O. Distribution of ethanol-induced protein adducts in vivo: relationship to tissue injury. Free Radic Biol Med. 2001;31:1533–1538. doi: 10.1016/s0891-5849(01)00744-4. [DOI] [PubMed] [Google Scholar]
- 48.Crabb DW, Liangpunsakul S. Acetaldehyde generating enzyme systems: roles of alcohol dehydrogenase, CYP2E1 and catalase, and speculations on the role of other enzymes and processes. Novartis Found Symp. 2007;285:4–16. doi: 10.1002/9780470511848.ch2. discussion 16–22, 198–9. [DOI] [PubMed] [Google Scholar]
- 49.Wu D, Wang X, Zhou R, Yang L, Cederbaum AI. Alcohol steatosis and cytotoxicity: the role of cytochrome P4502E1 and autophagy. Free Radic Biol Med. 2012;53:1346–1357. doi: 10.1016/j.freeradbiomed.2012.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Abdelmegeed MA, Banerjee A, Jang S, Yoo SH, Yun JW, Gonzalez FJ, Keshavarzian A, Song BJ. CYP2E1 potentiates binge alcohol-induced gut leakiness, steatohepatitis, and apoptosis. Free Radic Biol Med. 2013;65:1238–1245. doi: 10.1016/j.freeradbiomed.2013.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Zhou R, Lin J, Wu D. Sulforaphane induces Nrf2 and protects against CYP2E1-dependent binge alcohol-induced liver steatosis. Biochim Biophys Acta. 2014;1840:209–218. doi: 10.1016/j.bbagen.2013.09.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Chen YY, Zhang CL, Zhao XL, Xie KQ, Zeng T. Inhibition of cytochrome P4502E1 by chlormethiazole attenuated acute ethanol-induced fatty liver. Chem Biol Interact. 2014;222C:18–26. doi: 10.1016/j.cbi.2014.08.009. [DOI] [PubMed] [Google Scholar]
- 53.Zeng T, Zhang CL, Song FY, Zhao XL, Xie KQ. CMZ reversed chronic ethanol-induced disturbance of PPAR-alpha possibly by suppressing oxidative stress and PGC-1alpha acetylation, and activating the MAPK and GSK3beta pathway. PLoS One. 2014;9:e98658. doi: 10.1371/journal.pone.0098658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ye Q, Wang X, Wang Q, Xia M, Zhu Y, Lian F, Ling W. Cytochrome P4502E1 inhibitor, chlormethiazole, decreases lipopolysaccharide-induced inflammation in rat Kupffer cells with ethanol treatment. Hepatol Res. 2013;43:1115–1123. doi: 10.1111/hepr.12063. [DOI] [PubMed] [Google Scholar]
- 55.Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. 2008;47:1483–1494. doi: 10.1002/hep.22222. [DOI] [PubMed] [Google Scholar]
- 56.Yang L, Rozenfeld R, Wu D, Devi LA, Zhang Z, Cederbaum A. Cannabidiol protects liver from binge alcohol-induced steatosis by mechanisms including inhibition of oxidative stress and increase in autophagy. Free Radic Biol Med. 2014;68:260–267. doi: 10.1016/j.freeradbiomed.2013.12.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garcia-Banuelos J, Panduro A, Gordillo-Bastidas D, Gordillo-Bastidas E, Munoz-Valle JF, Gurrola-Diaz CM, Sanchez-Enriquez S, Ruiz-Madrigal B, Bastidas-Ramirez BE. Genetic polymorphisms of genes coding to alcohol-metabolizing enzymes in western Mexicans: association of CYP2E1*c2/CYP2E1*5B allele with cirrhosis and liver function. Alcohol Clin Exp Res. 2012;36:425–431. doi: 10.1111/j.1530-0277.2011.01617.x. [DOI] [PubMed] [Google Scholar]
- 58.Lu Y, Cederbaum AI. CYP2E1 and oxidative liver injury by alcohol. Free Radic Biol Med. 2008;44:723–738. doi: 10.1016/j.freeradbiomed.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Venkatraman A, Shiva S, Wigley A, Ulasova E, Chhieng D, Bailey SM, Darley-Usmar VM. The role of iNOS in alcohol-dependent hepatotoxicity and mitochondrial dysfunction in mice. Hepatology. 2004;40:565–573. doi: 10.1002/hep.20326. [DOI] [PubMed] [Google Scholar]
- 60.Kim MJ, Nagy LE, Park PH. Globular adiponectin inhibits ethanol-induced reactive oxygen species production through modulation of NADPH oxidase in macrophages: involvement of liver kinase B1/AMP-activated protein kinase pathway. Mol Pharmacol. 2014;86:284–296. doi: 10.1124/mol.114.093039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Cederbaum AI. Nrf2 and antioxidant defense against CYP2E1 toxicity. Subcell Biochem. 2013;67:105–130. doi: 10.1007/978-94-007-5881-0_2. [DOI] [PubMed] [Google Scholar]
- 62.Cederbaum AI. Hepatoprotective effects of S-adenosyl-L-methionine against alcohol- and cytochrome P450 2E1-induced liver injury. World J Gastroenterol. 2010;16:1366–1376. doi: 10.3748/wjg.v16.i11.1366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Abdelmegeed MA, Song BJ. Functional roles of protein nitration in acute and chronic liver diseases. Oxid Med Cell Longev. 2014;2014:149627. doi: 10.1155/2014/149627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Song BJ, Abdelmegeed MA, Henderson LE, Yoo SH, Wan J, Purohit V, Hardwick JP, Moon KH. Increased nitroxidative stress promotes mitochondrial dysfunction in alcoholic and nonalcoholic fatty liver disease. Oxid Med Cell Longev. 2013;2013:781050. doi: 10.1155/2013/781050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Yang L, Wu D, Wang X, Cederbaum AI. Cytochrome P4502E1, oxidative stress, JNK, and autophagy in acute alcohol-induced fatty liver. Free Radic Biol Med. 2012;53:1170–1180. doi: 10.1016/j.freeradbiomed.2012.06.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Yun JW, Son MJ, Abdelmegeed MA, Banerjee A, Morgan TR, Yoo SH, Song BJ. Binge alcohol promotes hypoxic liver injury through a CYP2E1-HIF-1alpha-dependent apoptosis pathway in mice and humans. Free Radic Biol Med. 2014 doi: 10.1016/j.freeradbiomed.2014.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Parola M, Robino G. Oxidative stress-related molecules and liver fibrosis. J Hepatol. 2001;35:297–306. doi: 10.1016/s0168-8278(01)00142-8. [DOI] [PubMed] [Google Scholar]
- 68.Barnes MA, Roychowdhury S, Nagy LE. Innate immunity and cell death in alcoholic liver disease: role of cytochrome P4502E1. Redox Biol. 2014;2:929–935. doi: 10.1016/j.redox.2014.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lu Y, Cederbaum AI. CYP2E1 potentiation of LPS and TNFalpha-induced hepatotoxicity by mechanisms involving enhanced oxidative and nitrosative stress, activation of MAP kinases, and mitochondrial dysfunction. Genes Nutr. 2010;5:149–167. doi: 10.1007/s12263-009-0150-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Wu D, Wang X, Zhou R, Cederbaum A. CYP2E1 enhances ethanol-induced lipid accumulation but impairs autophagy in HepG2 E47 cells. Biochem Biophys Res Commun. 2010;402:116–122. doi: 10.1016/j.bbrc.2010.09.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Ding WX, Manley S, Ni HM. The emerging role of autophagy in alcoholic liver disease. Exp Biol Med (Maywood) 2011;236:546–556. doi: 10.1258/ebm.2011.010360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Amir M, Czaja MJ. Autophagy in nonalcoholic steatohepatitis. Expert Rev Gastroenterol Hepatol. 2011;5:159–166. doi: 10.1586/egh.11.4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Czaja MJ. Functions of autophagy in hepatic and pancreatic physiology and disease. Gastroenterology. 2011;140:1895–1908. doi: 10.1053/j.gastro.2011.04.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.He T, Ai M, Zhao XH, Xing YQ. Inducible nitric oxide synthase mediates hypoxia-induced hypoxia-inducible factor-1 alpha activation and vascular endothelial growth factor expression in oxygen-induced retinopathy. Pathobiology. 2007;74:336–343. doi: 10.1159/000110027. [DOI] [PubMed] [Google Scholar]
- 75.Greijer AE, van der Wall E. The role of hypoxia inducible factor 1 (HIF-1) in hypoxia induced apoptosis. J Clin Pathol. 2004;57:1009–1014. doi: 10.1136/jcp.2003.015032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Zelickson BR, Benavides GA, Johnson MS, Chacko BK, Venkatraman A, Landar A, Betancourt AM, Bailey SM, Darley-Usmar VM. Nitric oxide and hypoxia exacerbate alcohol-induced mitochondrial dysfunction in hepatocytes. Biochim Biophys Acta. 2011;1807:1573–1582. doi: 10.1016/j.bbabio.2011.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dupont I, Bodenez P, Berthou F, Simon B, Bardou LG, Lucas D. Cytochrome P-450 2E1 activity and oxidative stress in alcoholic patients. Alcohol Alcohol. 2000;35:98–103. doi: 10.1093/alcalc/35.1.98. [DOI] [PubMed] [Google Scholar]
- 78.Wang X, Wu D, Yang L, Gan L, Cederbaum AI. Cytochrome P450 2E1 potentiates ethanol induction of hypoxia and HIF-1alpha in vivo. Free Radic Biol Med. 2013;63:175–186. doi: 10.1016/j.freeradbiomed.2013.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Keshavarzian A, Choudhary S, Holmes EW, Yong S, Banan A, Jakate S, Fields JZ. Preventing gut leakiness by oats supplementation ameliorates alcohol-induced liver damage in rats. J Pharmacol Exp Ther. 2001;299:442–448. [PubMed] [Google Scholar]
- 80.Keshavarzian A, Fields J. Alcohol: "ice-breaker" yes, "gut barrier-breaker," maybe. Am J Gastroenterol. 2000;95:1124–1125. doi: 10.1111/j.1572-0241.2000.01998.x. [DOI] [PubMed] [Google Scholar]
- 81.Keshavarzian A, Fields J. Alcoholic liver disease: is it an "extraintestinal" complication of alcohol-induced intestinal injury? J Lab Clin Med. 2003;142:285–287. doi: 10.1016/S0022-2143(03)00140-9. [DOI] [PubMed] [Google Scholar]
- 82.Robinson GM, Orrego H, Israel Y, Devenyi P, Kapur BM. Low-molecular-weight polyethylene glycol as a probe of gastrointestinal permeability after alcohol ingestion. Dig Dis Sci. 1981;26:971–977. doi: 10.1007/BF01314757. [DOI] [PubMed] [Google Scholar]
- 83.Tang Y, Banan A, Forsyth CB, Fields JZ, Lau CK, Zhang LJ, Keshavarzian A. Effect of alcohol on miR-212 expression in intestinal epithelial cells and its potential role in alcoholic liver disease. Alcohol Clin Exp Res. 2008;32:355–364. doi: 10.1111/j.1530-0277.2007.00584.x. [DOI] [PubMed] [Google Scholar]
- 84.Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Prevention of alterations in intestinal permeability is involved in zinc inhibition of acute ethanol-induced liver damage in mice. J Pharmacol Exp Ther. 2003;305:880–886. doi: 10.1124/jpet.102.047852. [DOI] [PubMed] [Google Scholar]
- 85.Lambert JC, Zhou Z, Wang L, Song Z, McClain CJ, Kang YJ. Preservation of intestinal structural integrity by zinc is independent of metallothionein in alcohol-intoxicated mice. Am J Pathol. 2004;164:1959–1966. doi: 10.1016/S0002-9440(10)63756-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bode C, Kugler V, Bode JC. Endotoxemia in patients with alcoholic and non-alcoholic cirrhosis and in subjects with no evidence of chronic liver disease following acute alcohol excess. J Hepatol. 1987;4:8–14. doi: 10.1016/s0168-8278(87)80003-x. [DOI] [PubMed] [Google Scholar]
- 87.Lieber CS. Alcoholic fatty liver: its pathogenesis and mechanism of progression to inflammation and fibrosis. Alcohol. 2004;34:9–19. doi: 10.1016/j.alcohol.2004.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Hakucho A, Liu J, Liu X, Fujimiya T. Carvedilol improves ethanol-induced liver injury via modifying the interaction between oxidative stress and sympathetic hyperactivity in rats. Hepatol Res. 2014;44:560–570. doi: 10.1111/hepr.12143. [DOI] [PubMed] [Google Scholar]
- 89.Browning JD, Horton JD. Molecular mediators of hepatic steatosis and liver injury. J Clin Invest. 2004;114:147–152. doi: 10.1172/JCI22422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Liu J. Ethanol and liver: Recent insights into the mechanisms of ethanol-induced fatty liver. World J Gastroenterol. 2014;20:14672–14685. doi: 10.3748/wjg.v20.i40.14672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Tang Y, Forsyth CB, Farhadi A, Rangan J, Jakate S, Shaikh M, Banan A, Fields JZ, Keshavarzian A. Nitric oxide-mediated intestinal injury is required for alcohol-induced gut leakiness and liver damage. Alcohol Clin Exp Res. 2009;33:1220–1230. doi: 10.1111/j.1530-0277.2009.00946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Forsyth CB, Voigt RM, Shaikh M, Tang Y, Cederbaum AI, Turek FW, Keshavarzian A. Role for intestinal CYP2E1 in alcohol-induced circadian gene-mediated intestinal hyperpermeability. Am J Physiol Gastrointest Liver Physiol. 2013;305:G185–G195. doi: 10.1152/ajpgi.00354.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Angulo P. Nonalcoholic fatty liver disease. N Engl J Med. 2002;346:1221–1231. doi: 10.1056/NEJMra011775. [DOI] [PubMed] [Google Scholar]
- 94.Tiniakos DG, Vos MB, Brunt EM. Nonalcoholic fatty liver disease: pathology and pathogenesis. Annu Rev Pathol. 2010;5:145–171. doi: 10.1146/annurev-pathol-121808-102132. [DOI] [PubMed] [Google Scholar]
- 95.Sanyal AJ. American Gastroenterological A. AGA technical review on nonalcoholic fatty liver disease. Gastroenterology. 2002;123:1705–1725. doi: 10.1053/gast.2002.36572. [DOI] [PubMed] [Google Scholar]
- 96.Ioannou GN, Weiss NS, Kowdley KV, Dominitz JA. Is obesity a risk factor for cirrhosis-related death or hospitalization? A population-based cohort study. Gastroenterology. 2003;125:1053–1059. doi: 10.1016/s0016-5085(03)01200-9. [DOI] [PubMed] [Google Scholar]
- 97.Williams CD, Stengel J, Asike MI, Torres DM, Shaw J, Contreras M, Landt CL, Harrison SA. Prevalence of nonalcoholic fatty liver disease and nonalcoholic steatohepatitis among a largely middle-aged population utilizing ultrasound and liver biopsy: a prospective study. Gastroenterology. 2011;140:124–131. doi: 10.1053/j.gastro.2010.09.038. [DOI] [PubMed] [Google Scholar]
- 98.Zelber-Sagi S, Ratziu V, Oren R. Nutrition and physical activity in NAFLD: an overview of the epidemiological evidence. World J Gastroenterol. 2011;17:3377–3389. doi: 10.3748/wjg.v17.i29.3377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Di Rosa M, Malaguarnera L. Genetic variants in candidate genes influencing NAFLD progression. J Mol Med (Berl) 2012;90:105–118. doi: 10.1007/s00109-011-0803-x. [DOI] [PubMed] [Google Scholar]
- 100.Marchesini G, Brizi M, Morselli-Labate AM, Bianchi G, Bugianesi E, McCullough AJ, Forlani G, Melchionda N. Association of nonalcoholic fatty liver disease with insulin resistance. Am J Med. 1999;107:450–455. doi: 10.1016/s0002-9343(99)00271-5. [DOI] [PubMed] [Google Scholar]
- 101.Marchesini G, Marzocchi R, Agostini F, Bugianesi E. Nonalcoholic fatty liver disease and the metabolic syndrome. Curr Opin Lipidol. 2005;16:421–427. doi: 10.1097/01.mol.0000174153.53683.f2. [DOI] [PubMed] [Google Scholar]
- 102.Javor ED, Ghany MG, Cochran EK, Oral EA, DePaoli AM, Premkumar A, Kleiner DE, Gorden P. Leptin reverses nonalcoholic steatohepatitis in patients with severe lipodystrophy. Hepatology. 2005;41:753–760. doi: 10.1002/hep.20672. [DOI] [PubMed] [Google Scholar]
- 103.Begriche K, Igoudjil A, Pessayre D, Fromenty B. Mitochondrial dysfunction in NASH: causes, consequences and possible means to prevent it. Mitochondrion. 2006;6:1–28. doi: 10.1016/j.mito.2005.10.004. [DOI] [PubMed] [Google Scholar]
- 104.Yoon KH, Lee JH, Kim JW, Cho JH, Choi YH, Ko SH, Zimmet P, Son HY. Epidemic obesity and type 2 diabetes in Asia. Lancet. 2006;368:1681–1688. doi: 10.1016/S0140-6736(06)69703-1. [DOI] [PubMed] [Google Scholar]
- 105.Fan JG, Zhu J, Li XJ, Chen L, Li L, Dai F, Li F, Chen SY. Prevalence of and risk factors for fatty liver in a general population of Shanghai, China. J Hepatol. 2005;43:508–514. doi: 10.1016/j.jhep.2005.02.042. [DOI] [PubMed] [Google Scholar]
- 106.Grundy SM, Brewer HB, Jr, Cleeman JI, Smith SC, Jr, Lenfant C. American Heart A, National Heart L, Blood I. Definition of metabolic syndrome: Report of the National Heart, Lung, and Blood Institute/American Heart Association conference on scientific issues related to definition. Circulation. 2004;109:433–438. doi: 10.1161/01.CIR.0000111245.75752.C6. [DOI] [PubMed] [Google Scholar]
- 107.Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A. Asia-Pacific Working Party for N. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22:794–800. doi: 10.1111/j.1440-1746.2007.04952.x. [DOI] [PubMed] [Google Scholar]
- 108.Mohan V, Deepa M, Deepa R, Shanthirani CS, Farooq S, Ganesan A, Datta M. Secular trends in the prevalence of diabetes and impaired glucose tolerance in urban South India--the Chennai Urban Rural Epidemiology Study (CURES-17) Diabetologia. 2006;49:1175–1178. doi: 10.1007/s00125-006-0219-2. [DOI] [PubMed] [Google Scholar]
- 109.Pessayre D, Fromenty B. NASH: a mitochondrial disease. J Hepatol. 2005;42:928–940. doi: 10.1016/j.jhep.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 110.Leung TM, Nieto N. CYP2E1 and oxidant stress in alcoholic and non-alcoholic fatty liver disease. J Hepatol. 2013;58:395–398. doi: 10.1016/j.jhep.2012.08.018. [DOI] [PubMed] [Google Scholar]
- 111.Abdelmegeed MA, Banerjee A, Yoo SH, Jang S, Gonzalez FJ, Song BJ. Critical role of cytochrome P450 2E1 (CYP2E1) in the development of high fat-induced non-alcoholic steatohepatitis. J Hepatol. 2012;57:860–866. doi: 10.1016/j.jhep.2012.05.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ha SK, Chae C. Inducible nitric oxide distribution in the fatty liver of a mouse with high fat diet-induced obesity. Exp Anim. 2010;59:595–604. doi: 10.1538/expanim.59.595. [DOI] [PubMed] [Google Scholar]
- 113.Chatterjee S, Ganini D, Tokar EJ, Kumar A, Das S, Corbett J, Kadiiska MB, Waalkes MP, Diehl AM, Mason RP. Leptin is key to peroxynitrite-mediated oxidative stress and Kupffer cell activation in experimental non-alcoholic steatohepatitis. J Hepatol. 2013;58:778–784. doi: 10.1016/j.jhep.2012.11.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Traber PG, Zomer E. Therapy of experimental NASH and fibrosis with galectin inhibitors. PLoS One. 2013;8:e83481. doi: 10.1371/journal.pone.0083481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Wang W, Zhao C, Zhou J, Zhen Z, Wang Y, Shen C. Simvastatin ameliorates liver fibrosis via mediating nitric oxide synthase in rats with non-alcoholic steatohepatitis-related liver fibrosis. PLoS One. 2013;8:e76538. doi: 10.1371/journal.pone.0076538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Kathirvel E, Morgan K, French SW, Morgan TR. Overexpression of liver-specific cytochrome P4502E1 impairs hepatic insulin signaling in a transgenic mouse model of nonalcoholic fatty liver disease. Eur J Gastroenterol Hepatol. 2009;21:973–983. doi: 10.1097/MEG.0b013e328328f461. [DOI] [PubMed] [Google Scholar]
- 117.Kathirvel E, Chen P, Morgan K, French SW, Morgan TR. Oxidative stress and regulation of anti-oxidant enzymes in cytochrome P4502E1 transgenic mouse model of non-alcoholic fatty liver. J Gastroenterol Hepatol. 2010;25:1136–1143. doi: 10.1111/j.1440-1746.2009.06196.x. [DOI] [PubMed] [Google Scholar]
- 118.Fujita K, Nozaki Y, Yoneda M, Wada K, Takahashi H, Kirikoshi H, Inamori M, Saito S, Iwasaki T, Terauchi Y, Maeyama S, Nakajima A. Nitric oxide plays a crucial role in the development/progression of nonalcoholic steatohepatitis in the choline-deficient, l-amino acid-defined diet-fed rat model. Alcohol Clin Exp Res. 2010;34(Suppl 1):S18–S24. doi: 10.1111/j.1530-0277.2008.00756.x. [DOI] [PubMed] [Google Scholar]
- 119.Seth RK, Das S, Kumar A, Chanda A, Kadiiska MB, Michelotti G, Manautou J, Diehl AM, Chatterjee S. CYP2E1-dependent and leptin-mediated hepatic CD57 expression on CD8+ T cells aid progression of environment-linked nonalcoholic steatohepatitis. Toxicol Appl Pharmacol. 2014;274:42–54. doi: 10.1016/j.taap.2013.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Zong H, Armoni M, Harel C, Karnieli E, Pessin JE. Cytochrome P-450 CYP2E1 knockout mice are protected against high-fat diet-induced obesity and insulin resistance. Am J Physiol Endocrinol Metab. 2012;302:E532–E539. doi: 10.1152/ajpendo.00258.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Seth RK, Kumar A, Das S, Kadiiska MB, Michelotti G, Diehl AM, Chatterjee S. Environmental toxin-linked nonalcoholic steatohepatitis and hepatic metabolic reprogramming in obese mice. Toxicol Sci. 2013;134:291–303. doi: 10.1093/toxsci/kft104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Seth RK, Das S, Pourhosseini S, Dattaroy D, Igwe S, Basuray J, Fan D, Michelotti GA, Diehl AM, Chatterjee S. M1 Polarization bias and subsequent NASH progression is attenuated by nitric oxide donor DETA NONOate via inhibition of CYP2E1 induced oxidative stress in obese mice. J Pharmacol Exp Ther. 2014 doi: 10.1124/jpet.114.218131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Schattenberg JM, Wang Y, Singh R, Rigoli RM, Czaja MJ. Hepatocyte CYP2E1 overexpression and steatohepatitis lead to impaired hepatic insulin signaling. J Biol Chem. 2005;280:9887–9894. doi: 10.1074/jbc.M410310200. [DOI] [PubMed] [Google Scholar]
- 124.Leclercq IA, Farrell GC, Field J, Bell DR, Gonzalez FJ, Robertson GR. CYP2E1 and CYP4A as microsomal catalysts of lipid peroxides in murine nonalcoholic steatohepatitis. J Clin Invest. 2000;105:1067–1075. doi: 10.1172/JCI8814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Robertson G, Leclercq I, Farrell GC. Nonalcoholic steatosis and steatohepatitis. II. Cytochrome P-450 enzymes and oxidative stress. Am J Physiol Gastrointest Liver Physiol. 2001;281:G1135–G1139. doi: 10.1152/ajpgi.2001.281.5.G1135. [DOI] [PubMed] [Google Scholar]
- 126.Park EC, Kim SI, Hong Y, Hwang JW, Cho GS, Cha HN, Han JK, Yun CH, Park SY, Jang IS, Lee ZW, Choi JS, Kim S, Kim GH. Inhibition of CYP4A reduces hepatic endoplasmic reticulum stress and features of diabetes in mice. Gastroenterology. 2014;147:860–869. doi: 10.1053/j.gastro.2014.06.039. [DOI] [PubMed] [Google Scholar]
- 127.Orellana M, Rodrigo R, Varela N, Araya J, Poniachik J, Csendes A, Smok G, Videla LA. Relationship between in vivo chlorzoxazone hydroxylation, hepatic cytochrome P450 2E1 content and liver injury in obese non-alcoholic fatty liver disease patients. Hepatol Res. 2006;34:57–63. doi: 10.1016/j.hepres.2005.10.001. [DOI] [PubMed] [Google Scholar]
- 128.Videla LA, Rodrigo R, Orellana M, Fernandez V, Tapia G, Quinones L, Varela N, Contreras J, Lazarte R, Csendes A, Rojas J, Maluenda F, Burdiles P, Diaz JC, Smok G, Thielemann L, Poniachik J. Oxidative stress-related parameters in the liver of non-alcoholic fatty liver disease patients. Clin Sci (Lond) 2004;106:261–268. doi: 10.1042/CS20030285. [DOI] [PubMed] [Google Scholar]
- 129.Bell LN, Molleston JP, Morton MJ, Klipsch A, Saxena R, Vuppalanchi R, Chalasani N. Hepatic lipid peroxidation and cytochrome P-450 2E1 in pediatric nonalcoholic fatty liver disease and its subtypes. J Clin Gastroenterol. 2011;45:800–807. doi: 10.1097/MCG.0b013e31821377e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Fisher CD, Lickteig AJ, Augustine LM, Ranger-Moore J, Jackson JP, Ferguson SS, Cherrington NJ. Hepatic cytochrome P450 enzyme alterations in humans with progressive stages of nonalcoholic fatty liver disease. Drug Metab Dispos. 2009;37:2087–2094. doi: 10.1124/dmd.109.027466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Osabe M, Sugatani J, Fukuyama T, Ikushiro S, Ikari A, Miwa M. Expression of hepatic UDP-glucuronosyltransferase 1A1 and 1A6 correlated with increased expression of the nuclear constitutive androstane receptor and peroxisome proliferator-activated receptor alpha in male rats fed a high-fat and high-sucrose diet. Drug Metab Dispos. 2008;36:294–302. doi: 10.1124/dmd.107.017731. [DOI] [PubMed] [Google Scholar]
- 132.Singh R, Kaushik S, Wang Y, Xiang Y, Novak I, Komatsu M, Tanaka K, Cuervo AM, Czaja MJ. Autophagy regulates lipid metabolism. Nature. 2009;458:1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Piguet AC, Stroka D, Zimmermann A, Dufour JF. Hypoxia aggravates non-alcoholic steatohepatitis in mice lacking hepatocellular PTEN. Clin Sci (Lond) 2010;118:401–410. doi: 10.1042/CS20090313. [DOI] [PubMed] [Google Scholar]
- 134.Shin MK, Drager LF, Yao Q, Bevans-Fonti S, Yoo DY, Jun JC, Aja S, Bhanot S, Polotsky VY. Metabolic consequences of high-fat diet are attenuated by suppression of HIF-1alpha. PLoS One. 2012;7:e46562. doi: 10.1371/journal.pone.0046562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Addya S, Anandatheerthavarada HK, Biswas G, Bhagwat SV, Mullick J, Avadhani NG. Targeting of NH2-terminal-processed microsomal protein to mitochondria: a novel pathway for the biogenesis of hepatic mitochondrial P450MT2. J Cell Biol. 1997;139:589–599. doi: 10.1083/jcb.139.3.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Sangar MC, Anandatheerthavarada HK, Tang W, Prabu SK, Martin MV, Dostalek M, Guengerich FP, Avadhani NG. Human liver mitochondrial cytochrome P450 2D6--individual variations and implications in drug metabolism. FEBS J. 2009;276:3440–3453. doi: 10.1111/j.1742-4658.2009.07067.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Bai J, Cederbaum AI. Overexpression of CYP2E1 in mitochondria sensitizes HepG2 cells to the toxicity caused by depletion of glutathione. J Biol Chem. 2006;281:5128–5136. doi: 10.1074/jbc.M510484200. [DOI] [PubMed] [Google Scholar]
- 138.Anandatheerthavarada HK, Addya S, Dwivedi RS, Biswas G, Mullick J, Avadhani NG. Localization of multiple forms of inducible cytochromes P450 in rat liver mitochondria: immunological characteristics and patterns of xenobiotic substrate metabolism. Arch Biochem Biophys. 1997;339:136–150. doi: 10.1006/abbi.1996.9855. [DOI] [PubMed] [Google Scholar]
- 139.Robin MA, Anandatheerthavarada HK, Fang JK, Cudic M, Otvos L, Avadhani NG. Mitochondrial targeted cytochrome P450 2E1 (P450 MT5) contains an intact N terminus and requires mitochondrial specific electron transfer proteins for activity. J Biol Chem. 2001;276:24680–24689. doi: 10.1074/jbc.M100363200. [DOI] [PubMed] [Google Scholar]
- 140.Anandatheerthavarada HK, Biswas G, Mullick J, Sepuri NB, Otvos L, Pain D, Avadhani NG. Dual targeting of cytochrome P4502B1 to endoplasmic reticulum and mitochondria involves a novel signal activation by cyclic AMP-dependent phosphorylation at ser128. EMBO J. 1999;18:5494–5504. doi: 10.1093/emboj/18.20.5494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Neve EP, Ingelman-Sundberg M. A soluble NH(2)-terminally truncated catalytically active form of rat cytochrome P450 2E1 targeted to liver mitochondria(1) FEBS Lett. 1999;460:309–314. doi: 10.1016/s0014-5793(99)01361-7. [DOI] [PubMed] [Google Scholar]
- 142.Neve EP, Ingelman-Sundberg M. Identification and characterization of a mitochondrial targeting signal in rat cytochrome P450 2E1 (CYP2E1) J Biol Chem. 2001;276:11317–11322. doi: 10.1074/jbc.M008640200. [DOI] [PubMed] [Google Scholar]
- 143.Abdelmegeed MA, Yoo SH, Henderson LE, Gonzalez FJ, Woodcroft KJ, Song BJ. PPARalpha expression protects male mice from high fat-induced nonalcoholic fatty liver. J Nutr. 2011;141:603–610. doi: 10.3945/jn.110.135210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Robin MA, Sauvage I, Grandperret T, Descatoire V, Pessayre D, Fromenty B. Ethanol increases mitochondrial cytochrome P450 2E1 in mouse liver and rat hepatocytes. FEBS Lett. 2005;579:6895–6902. doi: 10.1016/j.febslet.2005.11.029. [DOI] [PubMed] [Google Scholar]
- 145.Bansal S, Liu CP, Sepuri NB, Anandatheerthavarada HK, Selvaraj V, Hoek J, Milne GL, Guengerich FP, Avadhani NG. Mitochondria-targeted cytochrome P450 2E1 induces oxidative damage and augments alcohol-mediated oxidative stress. J Biol Chem. 2010;285:24609–24619. doi: 10.1074/jbc.M110.121822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bansal S, Anandatheerthavarada HK, Prabu GK, Milne GL, Martin MV, Guengerich FP, Avadhani NG. Human cytochrome P450 2E1 mutations that alter mitochondrial targeting efficiency and susceptibility to ethanol-induced toxicity in cellular models. J Biol Chem. 2013;288:12627–12644. doi: 10.1074/jbc.M113.452367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Mari M, Bai J, Cederbaum AI. Adenovirus-mediated overexpression of catalase in the cytosolic or mitochondrial compartment protects against toxicity caused by glutathione depletion in HepG2 cells expressing CYP2E1. J Pharmacol Exp Ther. 2002;301:111–118. doi: 10.1124/jpet.301.1.111. [DOI] [PubMed] [Google Scholar]
- 148.Knockaert L, Descatoire V, Vadrot N, Fromenty B, Robin MA. Mitochondrial CYP2E1 is sufficient to mediate oxidative stress and cytotoxicity induced by ethanol and acetaminophen. Toxicol In Vitro. 2011;25:475–484. doi: 10.1016/j.tiv.2010.11.019. [DOI] [PubMed] [Google Scholar]
- 149.Otani K, Korenaga M, Beard MR, Li K, Qian T, Showalter LA, Singh AK, Wang T, Weinman SA. Hepatitis C virus core protein, cytochrome P450 2E1, and alcohol produce combined mitochondrial injury and cytotoxicity in hepatoma cells. Gastroenterology. 2005;128:96–107. doi: 10.1053/j.gastro.2004.10.045. [DOI] [PubMed] [Google Scholar]
- 150.Raza H, Prabu SK, Robin MA, Avadhani NG. Elevated mitochondrial cytochrome P450 2E1 and glutathione S-transferase A4-4 in streptozotocin-induced diabetic rats: tissue-specific variations and roles in oxidative stress. Diabetes. 2004;53:185–194. doi: 10.2337/diabetes.53.1.185. [DOI] [PubMed] [Google Scholar]
- 151.Bailey SM, Robinson G, Pinner A, Chamlee L, Ulasova E, Pompilius M, Page GP, Chhieng D, Jhala N, Landar A, Kharbanda KK, Ballinger S, Darley-Usmar V. S-adenosylmethionine prevents chronic alcohol-induced mitochondrial dysfunction in the rat liver. Am J Physiol Gastrointest Liver Physiol. 2006;291:G857–G867. doi: 10.1152/ajpgi.00044.2006. [DOI] [PubMed] [Google Scholar]
- 152.Song Z, Zhou Z, Chen T, Hill D, Kang J, Barve S, McClain C. S-adenosylmethionine (SAMe) protects against acute alcohol induced hepatotoxicity in mice. J Nutr Biochem. 2003;14:591–597. doi: 10.1016/s0955-2863(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 153.Caro AA, Cederbaum AI. Inhibition of CYP2E1 catalytic activity in vitro by S-adenosyl-L-methionine. Biochem Pharmacol. 2005;69:1081–1093. doi: 10.1016/j.bcp.2005.01.007. [DOI] [PubMed] [Google Scholar]
- 154.Chacko BK, Srivastava A, Johnson MS, Benavides GA, Chang MJ, Ye Y, Jhala N, Murphy MP, Kalyanaraman B, Darley-Usmar VM. Mitochondria-targeted ubiquinone (MitoQ) decreases ethanol-dependent micro and macro hepatosteatosis. Hepatology. 2011;54:153–163. doi: 10.1002/hep.24377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.James AM, Cocheme HM, Smith RA, Murphy MP. Interactions of mitochondria-targeted and untargeted ubiquinones with the mitochondrial respiratory chain and reactive oxygen species. Implications for the use of exogenous ubiquinones as therapies and experimental tools. J Biol Chem. 2005;280:21295–21312. doi: 10.1074/jbc.M501527200. [DOI] [PubMed] [Google Scholar]
- 156.Maroz A, Anderson RF, Smith RA, Murphy MP. Reactivity of ubiquinone and ubiquinol with superoxide and the hydroperoxyl radical: implications for in vivo antioxidant activity. Free Radic Biol Med. 2009;46:105–109. doi: 10.1016/j.freeradbiomed.2008.09.033. [DOI] [PubMed] [Google Scholar]
- 157.James AM, Smith RA, Murphy MP. Antioxidant and prooxidant properties of mitochondrial Coenzyme Q. Arch Biochem Biophys. 2004;423:47–56. doi: 10.1016/j.abb.2003.12.025. [DOI] [PubMed] [Google Scholar]
- 158.Caldwell SH, de Freitas LA, Park SH, Moreno ML, Redick JA, Davis CA, Sisson BJ, Patrie JT, Cotrim H, Argo CK, Al-Osaimi A. Intramitochondrial crystalline inclusions in nonalcoholic steatohepatitis. Hepatology. 2009;49:1888–1895. doi: 10.1002/hep.22851. [DOI] [PubMed] [Google Scholar]
- 159.Campello S, Scorrano L. Mitochondrial shape changes: orchestrating cell pathophysiology. EMBO Rep. 2010;11:678–684. doi: 10.1038/embor.2010.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Elfering SL, Haynes VL, Traaseth NJ, Ettl A, Giulivi C. Aspects, mechanism, and biological relevance of mitochondrial protein nitration sustained by mitochondrial nitric oxide synthase. Am J Physiol Heart Circ Physiol. 2004;286:H22–H29. doi: 10.1152/ajpheart.00766.2003. [DOI] [PubMed] [Google Scholar]
- 161.Davies KJ, Shringarpure R. Preferential degradation of oxidized proteins by the 20S proteasome may be inhibited in aging and in inflammatory neuromuscular diseases. Neurology. 2006;66:S93–S96. doi: 10.1212/01.wnl.0000192308.43151.63. [DOI] [PubMed] [Google Scholar]
- 162.Pickering AM, Davies KJ. Differential roles of proteasome and immunoproteasome regulators Pa28alphabeta, Pa28gamma and Pa200 in the degradation of oxidized proteins. Arch Biochem Biophys. 2012;523:181–190. doi: 10.1016/j.abb.2012.04.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Pickering AM, Davies KJ. Degradation of damaged proteins: the main function of the 20S proteasome. Prog Mol Biol Transl Sci. 2012;109:227–248. doi: 10.1016/B978-0-12-397863-9.00006-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Soboll S. Regulation of energy metabolism in liver. J Bioenerg Biomembr. 1995;27:571–582. doi: 10.1007/BF02111655. [DOI] [PubMed] [Google Scholar]
- 165.Chan B, Parker G. Some recommendations to assess depression in Chinese people in Australasia. Aust N Z J Psychiatry. 2004;38:141–147. doi: 10.1080/j.1440-1614.2004.01321.x. [DOI] [PubMed] [Google Scholar]
- 166.Annie-Jeyachristy S, Geetha A, Surendran R, Sundaram A, Lavanya K, Kumar SJ, Prakash SA. Level of nitrated proteins in the plasma, platelets and liver of patients with liver cirrhosis. Redox Rep. 2009;14:259–266. doi: 10.1179/135100009X12525712409616. [DOI] [PubMed] [Google Scholar]
- 167.Boveris A, Chance B. The mitochondrial generation of hydrogen peroxide. General properties and effect of hyperbaric oxygen. Biochem J. 1973;134:707–716. doi: 10.1042/bj1340707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Hamanaka RB, Chandel NS. Mitochondrial reactive oxygen species regulate cellular signaling and dictate biological outcomes. Trends Biochem Sci. 2010;35:505–513. doi: 10.1016/j.tibs.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Pessayre D, Mansouri A, Fromenty B. Nonalcoholic steatosis and steatohepatitis. V. Mitochondrial dysfunction in steatohepatitis. Am J Physiol Gastrointest Liver Physiol. 2002;282:G193–G199. doi: 10.1152/ajpgi.00426.2001. [DOI] [PubMed] [Google Scholar]
- 170.Giulivi C. Characterization and function of mitochondrial nitric-oxide synthase. Free Radic Biol Med. 2003;34:397–408. doi: 10.1016/s0891-5849(02)01298-4. [DOI] [PubMed] [Google Scholar]
- 171.Purohit V, Rapaka R, Kwon OS, Song BJ. Roles of alcohol and tobacco exposure in the development of hepatocellular carcinoma. Life Sci. 2013;92:3–9. doi: 10.1016/j.lfs.2012.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Purohit V, Gao B, Song BJ. Molecular mechanisms of alcoholic fatty liver. Alcohol Clin Exp Res. 2009;33:191–205. doi: 10.1111/j.1530-0277.2008.00827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Cohen JI, Chen X, Nagy LE. Redox signaling and the innate immune system in alcoholic liver disease. Antioxid Redox Signal. 2011;15:523–534. doi: 10.1089/ars.2010.3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.McKim SE, Gabele E, Isayama F, Lambert JC, Tucker LM, Wheeler MD, Connor HD, Mason RP, Doll MA, Hein DW, Arteel GE. Inducible nitric oxide synthase is required in alcohol-induced liver injury: studies with knockout mice. Gastroenterology. 2003;125:1834–1844. doi: 10.1053/j.gastro.2003.08.030. [DOI] [PubMed] [Google Scholar]
- 175.Fernandez-Checa JC, Kaplowitz N, Garcia-Ruiz C, Colell A. Mitochondrial glutathione: importance and transport. Semin Liver Dis. 1998;18:389–401. doi: 10.1055/s-2007-1007172. [DOI] [PubMed] [Google Scholar]
- 176.Wallace KB. Mitochondrial off targets of drug therapy. Trends Pharmacol Sci. 2008;29:361–366. doi: 10.1016/j.tips.2008.04.001. [DOI] [PubMed] [Google Scholar]
- 177.Chandra D, Singh KK. Genetic insights into OXPHOS defect and its role in cancer. Biochim Biophys Acta. 2011;1807:620–625. doi: 10.1016/j.bbabio.2010.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Wan J, Bae MA, Song BJ. Acetoaminophen-induced accumulation of 8-oxodeoxyguanosine through reduction of Ogg1 DNA repair enzyme in C6 glioma cells. Exp Mol Med. 2004;36:71–77. doi: 10.1038/emm.2004.10. [DOI] [PubMed] [Google Scholar]
- 179.Mansouri A, Fromenty B, Berson A, Robin MA, Grimbert S, Beaugrand M, Erlinger S, Pessayre D. Multiple hepatic mitochondrial DNA deletions suggest premature oxidative aging in alcoholic patients. J Hepatol. 1997;27:96–102. doi: 10.1016/s0168-8278(97)80286-3. [DOI] [PubMed] [Google Scholar]
- 180.Mansouri A, Gaou I, De Kerguenec C, Amsellem S, Haouzi D, Berson A, Moreau A, Feldmann G, Letteron P, Pessayre D, Fromenty B. An alcoholic binge causes massive degradation of hepatic mitochondrial DNA in mice. Gastroenterology. 1999;117:181–190. doi: 10.1016/s0016-5085(99)70566-4. [DOI] [PubMed] [Google Scholar]
- 181.Venkatraman A, Landar A, Davis AJ, Chamlee L, Sanderson T, Kim H, Page G, Pompilius M, Ballinger S, Darley-Usmar V, Bailey SM. Modification of the mitochondrial proteome in response to the stress of ethanol-dependent hepatotoxicity. J Biol Chem. 2004;279:22092–22101. doi: 10.1074/jbc.M402245200. [DOI] [PubMed] [Google Scholar]
- 182.Garcia-Ruiz I, Solis-Munoz P, Fernandez-Moreira D, Grau M, Colina F, Munoz-Yague T, Solis-Herruzo JA. High-fat diet decreases activity of the oxidative phosphorylation complexes and causes nonalcoholic steatohepatitis in mice. Dis Model Mech. 2014;7:1287–1296. doi: 10.1242/dmm.016766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 183.Yang S, Zhu H, Li Y, Lin H, Gabrielson K, Trush MA, Diehl AM. Mitochondrial adaptations to obesity-related oxidant stress. Arch Biochem Biophys. 2000;378:259–268. doi: 10.1006/abbi.2000.1829. [DOI] [PubMed] [Google Scholar]
- 184.Koteish A, Diehl AM. Animal models of steatosis. Semin Liver Dis. 2001;21:89–104. doi: 10.1055/s-2001-12932. [DOI] [PubMed] [Google Scholar]
- 185.Rao MS, Papreddy K, Musunuri S, Okonkwo A. Prevention/reversal of choline deficiency-induced steatohepatitis by a peroxisome proliferator-activated receptor alpha ligand in rats. In Vivo. 2002;16:145–152. [PubMed] [Google Scholar]
- 186.Gao D, Wei C, Chen L, Huang J, Yang S, Diehl AM. Oxidative DNA damage and DNA repair enzyme expression are inversely related in murine models of fatty liver disease. Am J Physiol Gastrointest Liver Physiol. 2004;287:G1070–G1077. doi: 10.1152/ajpgi.00228.2004. [DOI] [PubMed] [Google Scholar]
- 187.Pirola CJ, Gianotti TF, Burgueno AL, Rey-Funes M, Loidl CF, Mallardi P, Martino JS, Castano GO, Sookoian S. Epigenetic modification of liver mitochondrial DNA is associated with histological severity of nonalcoholic fatty liver disease. Gut. 2013;62:1356–1363. doi: 10.1136/gutjnl-2012-302962. [DOI] [PubMed] [Google Scholar]
- 188.Kojima H, Sakurai S, Uemura M, Fukui H, Morimoto H, Tamagawa Y. Mitochondrial abnormality and oxidative stress in nonalcoholic steatohepatitis. Alcohol Clin Exp Res. 2007;31:S61–S66. doi: 10.1111/j.1530-0277.2006.00288.x. [DOI] [PubMed] [Google Scholar]
- 189.Moon KH, Hood BL, Kim BJ, Hardwick JP, Conrads TP, Veenstra TD, Song BJ. Inactivation of oxidized and S-nitrosylated mitochondrial proteins in alcoholic fatty liver of rats. Hepatology. 2006;44:1218–1230. doi: 10.1002/hep.21372. [DOI] [PubMed] [Google Scholar]
- 190.Solis-Munoz P, Solis-Herruzo JA, Fernandez-Moreira D, Gomez-Izquierdo E, Garcia-Consuegra I, Munoz-Yague T, Garcia Ruiz I. Melatonin improves mitochondrial respiratory chain activity and liver morphology in ob/ob mice. J Pineal Res. 2011;51:113–123. doi: 10.1111/j.1600-079X.2011.00868.x. [DOI] [PubMed] [Google Scholar]
- 191.Sudheesh NP, Ajith TA, Janardhanan KK. Hepatoprotective effects of DL-alpha-lipoic acid and alpha-Tocopherol through amelioration of the mitochondrial oxidative stress in acetaminophen challenged rats. Toxicol Mech Methods. 2013;23:368–376. doi: 10.3109/15376516.2013.769289. [DOI] [PubMed] [Google Scholar]
- 192.McGill MR, Staggs VS, Sharpe MR, Lee WM, Jaeschke H. Acute Liver Failure Study G. Serum mitochondrial biomarkers and damage-associated molecular patterns are higher in acetaminophen overdose patients with poor outcome. Hepatology. 2014;60:1336–1345. doi: 10.1002/hep.27265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Fromenty B, Pessayre D. Impaired mitochondrial function in microvesicular steatosis. Effects of drugs, ethanol, hormones and cytokines. J Hepatol. 1997;26(Suppl 2):43–53. doi: 10.1016/s0168-8278(97)80496-5. [DOI] [PubMed] [Google Scholar]
- 194.Song BJ, Moon KH, Upreti VV, Eddington ND, Lee IJ. Mechanisms of MDMA (ecstasy)-induced oxidative stress, mitochondrial dysfunction, and organ damage. Curr Pharm Biotechnol. 2010;11:434–443. doi: 10.2174/138920110791591436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 195.Bailey SM, Pietsch EC, Cunningham CC. Ethanol stimulates the production of reactive oxygen species at mitochondrial complexes I and III. Free Radic Biol Med. 1999;27:891–900. doi: 10.1016/s0891-5849(99)00138-0. [DOI] [PubMed] [Google Scholar]
- 196.Moon KH, Upreti VV, Yu LR, Lee IJ, Ye X, Eddington ND, Veenstra TD, Song BJ. Mechanism of 3,4-methylenedioxymethamphetamine (MDMA, ecstasy)-mediated mitochondrial dysfunction in rat liver. Proteomics. 2008;8:3906–3918. doi: 10.1002/pmic.200800215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Moon KH, Hood BL, Mukhopadhyay P, Rajesh M, Abdelmegeed MA, Kwon YI, Conrads TP, Veenstra TD, Song BJ, Pacher P. Oxidative inactivation of key mitochondrial proteins leads to dysfunction and injury in hepatic ischemia reperfusion. Gastroenterology. 2008;135:1344–1357. doi: 10.1053/j.gastro.2008.06.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Xu J, Cao K, Li Y, Zou X, Chen C, Szeto IM, Dong Z, Zhao Y, Shi Y, Wang J, Liu J, Feng Z. Bitter gourd inhibits the development of obesity-associated fatty liver in C57BL/6 mice fed a high-fat diet. J Nutr. 2014;144:475–483. doi: 10.3945/jn.113.187450. [DOI] [PubMed] [Google Scholar]
- 199.Lu H, Koshkin V, Allister EM, Gyulkhandanyan AV, Wheeler MB. Molecular and metabolic evidence for mitochondrial defects associated with beta-cell dysfunction in a mouse model of type 2 diabetes. Diabetes. 2010;59:448–459. doi: 10.2337/db09-0129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 200.Menzies KJ, Robinson BH, Hood DA. Effect of thyroid hormone on mitochondrial properties and oxidative stress in cells from patients with mtDNA defects. Am J Physiol Cell Physiol. 2009;296:C355–C362. doi: 10.1152/ajpcell.00415.2007. [DOI] [PubMed] [Google Scholar]
- 201.Zou X, Yan C, Shi Y, Cao K, Xu J, Wang X, Chen C, Luo C, Li Y, Gao J, Pang W, Zhao J, Zhao F, Li H, Zheng A, Sun W, Long J, Szeto IM, Zhao Y, Dong Z, Zhang P, Wang J, Lu W, Zhang Y, Liu J, Feng Z. Mitochondrial dysfunction in obesity-associated nonalcoholic fatty liver disease: the protective effects of pomegranate with its active component punicalagin. Antioxid Redox Signal. 2014;21:1557–1570. doi: 10.1089/ars.2013.5538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Teodoro JS, Duarte FV, Gomes AP, Varela AT, Peixoto FM, Rolo AP, Palmeira CM. Berberine reverts hepatic mitochondrial dysfunction in high-fat fed rats: a possible role for SirT3 activation. Mitochondrion. 2013;13:637–646. doi: 10.1016/j.mito.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 203.Alvarez B, Demicheli V, Duran R, Trujillo M, Cervenansky C, Freeman BA, Radi R. Inactivation of human Cu,Zn superoxide dismutase by peroxynitrite and formation of histidinyl radical. Free Radic Biol Med. 2004;37:813–822. doi: 10.1016/j.freeradbiomed.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 204.Surmeli NB, Litterman NK, Miller AF, Groves JT. Peroxynitrite mediates active site tyrosine nitration in manganese superoxide dismutase. Evidence of a role for the carbonate radical anion. J Am Chem Soc. 2010;132:17174–17185. doi: 10.1021/ja105684w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.von Montfort C, Matias N, Fernandez A, Fucho R, Conde de la Rosa L, Martinez-Chantar ML, Mato JM, Machida K, Tsukamoto H, Murphy MP, Mansouri A, Kaplowitz N, Garcia-Ruiz C, Fernandez-Checa JC. Mitochondrial GSH determines the toxic or therapeutic potential of superoxide scavenging in steatohepatitis. J Hepatol. 2012;57:852–859. doi: 10.1016/j.jhep.2012.05.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Klyosov AA, Rashkovetsky LG, Tahir MK, Keung WM. Possible role of liver cytosolic and mitochondrial aldehyde dehydrogenases in acetaldehyde metabolism. Biochemistry. 1996;35:4445–4456. doi: 10.1021/bi9521093. [DOI] [PubMed] [Google Scholar]
- 207.Song BJ, Abdelmegeed MA, Yoo SH, Kim BJ, Jo SA, Jo I, Moon KH. Post-translational modifications of mitochondrial aldehyde dehydrogenase and biomedical implications. J Proteomics. 2011;74:2691–2702. doi: 10.1016/j.jprot.2011.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Fritz KS, Petersen DR. An overview of the chemistry and biology of reactive aldehydes. Free Radic Biol Med. 2013;59:85–91. doi: 10.1016/j.freeradbiomed.2012.06.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Mottaran E, Stewart SF, Rolla R, Vay D, Cipriani V, Moretti M, Vidali M, Sartori M, Rigamonti C, Day CP, Albano E. Lipid peroxidation contributes to immune reactions associated with alcoholic liver disease. Free Radic Biol Med. 2002;32:38–45. doi: 10.1016/s0891-5849(01)00757-2. [DOI] [PubMed] [Google Scholar]
- 210.Sutti S, Rigamonti C, Vidali M, Albano E. CYP2E1 autoantibodies in liver diseases. Redox Biol. 2014;3:72–78. doi: 10.1016/j.redox.2014.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Yoshida A, Huang IY, Ikawa M. Molecular abnormality of an inactive aldehyde dehydrogenase variant commonly found in Orientals. Proc Natl Acad Sci U S A. 1984;81:258–261. doi: 10.1073/pnas.81.1.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Farres J, Wang X, Takahashi K, Cunningham SJ, Wang TT, Weiner H. Effects of changing glutamate 487 to lysine in rat and human liver mitochondrial aldehyde dehydrogenase. A model to study human (Oriental type) class 2 aldehyde dehydrogenase. J Biol Chem. 1994;269:13854–13860. [PubMed] [Google Scholar]
- 213.Sheikh S, Ni L, Hurley TD, Weiner H. The potential roles of the conserved amino acids in human liver mitochondrial aldehyde dehydrogenase. J Biol Chem. 1997;272:18817–18822. doi: 10.1074/jbc.272.30.18817. [DOI] [PubMed] [Google Scholar]
- 214.Peng GS, Chen YC, Wang MF, Lai CL, Yin SJ. ALDH2*2 but not ADH1B*2 is a causative variant gene allele for Asian alcohol flushing after a low-dose challenge: correlation of the pharmacokinetic and pharmacodynamic findings. Pharmacogenet Genomics. 2014;24:607–617. doi: 10.1097/FPC.0000000000000096. [DOI] [PubMed] [Google Scholar]
- 215.Day CP, Bashir R, James OF, Bassendine MF, Crabb DW, Thomasson HR, Li TK, Edenberg HJ. Investigation of the role of polymorphisms at the alcohol and aldehyde dehydrogenase loci in genetic predisposition to alcohol-related end-organ damage. Hepatology. 1991;14:798–801. doi: 10.1002/hep.1840140509. [DOI] [PubMed] [Google Scholar]
- 216.Pompili M, Rapaccini GL, Covino M, Pignataro G, Caturelli E, Siena DA, Villani MR, Cedrone A, Gasbarrini G. Prognostic factors for survival in patients with compensated cirrhosis and small hepatocellular carcinoma after percutaneous ethanol injection therapy. Cancer. 2001;92:126–135. doi: 10.1002/1097-0142(20010701)92:1<126::aid-cncr1300>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- 217.Higuchi S, Matsushita S, Imazeki H, Kinoshita T, Takagi S, Kono H. Aldehyde dehydrogenase genotypes in Japanese alcoholics. Lancet. 1994;343:741–742. doi: 10.1016/s0140-6736(94)91629-2. [DOI] [PubMed] [Google Scholar]
- 218.Brooks PJ, Enoch MA, Goldman D, Li TK, Yokoyama A. The alcohol flushing response: an unrecognized risk factor for esophageal cancer from alcohol consumption. PLoS Med. 2009;6:e50. doi: 10.1371/journal.pmed.1000050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Seitz HK, Cho CH. Contribution of alcohol and tobacco use in gastrointestinal cancer development. Methods Mol Biol. 2009;472:217–241. doi: 10.1007/978-1-60327-492-0_9. [DOI] [PubMed] [Google Scholar]
- 220.Setshedi M, Wands JR, Monte SM. Acetaldehyde adducts in alcoholic liver disease. Oxid Med Cell Longev. 2010;3:178–185. doi: 10.4161/oxim.3.3.3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 221.Minko IG, Kozekov ID, Harris TM, Rizzo CJ, Lloyd RS, Stone MP. Chemistry and biology of DNA containing 1,N(2)-deoxyguanosine adducts of the alpha,beta-unsaturated aldehydes acrolein, crotonaldehyde, and 4-hydroxynonenal. Chem Res Toxicol. 2009;22:759–778. doi: 10.1021/tx9000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 222.Eccleston HB, Andringa KK, Betancourt AM, King AL, Mantena SK, Swain TM, Tinsley HN, Nolte RN, Nagy TR, Abrams GA, Bailey SM. Chronic exposure to a high-fat diet induces hepatic steatosis, impairs nitric oxide bioavailability, and modifies the mitochondrial proteome in mice. Antioxid Redox Signal. 2011;15:447–459. doi: 10.1089/ars.2010.3395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Ni HM, Bockus A, Boggess N, Jaeschke H, Ding WX. Activation of autophagy protects against acetaminophen-induced hepatotoxicity. Hepatology. 2012;55:222–232. doi: 10.1002/hep.24690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Sohn DH, Yun YP, Park KS, Veech RL, Song BJ. Post-translational reduction of cytochrome P450IIE by CCl4, its substrate. Biochem Biophys Res Commun. 1991;179:449–454. doi: 10.1016/0006-291x(91)91391-o. [DOI] [PubMed] [Google Scholar]
- 225.Wong FW, Chan WY, Lee SS. Resistance to carbon tetrachloride-induced hepatotoxicity in mice which lack CYP2E1 expression. Toxicol Appl Pharmacol. 1998;153:109–118. doi: 10.1006/taap.1998.8547. [DOI] [PubMed] [Google Scholar]
- 226.Jaeschke H, Gores GJ, Cederbaum AI, Hinson JA, Pessayre D, Lemasters JJ. Mechanisms of hepatotoxicity. Toxicol Sci. 2002;65:166–176. doi: 10.1093/toxsci/65.2.166. [DOI] [PubMed] [Google Scholar]
- 227.Galligan JJ, Smathers RL, Fritz KS, Epperson LE, Hunter LE, Petersen DR. Protein carbonylation in a murine model for early alcoholic liver disease. Chem Res Toxicol. 2012;25:1012–1021. doi: 10.1021/tx300002q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Kheradpezhouh E, Ma L, Morphett A, Barritt GJ, Rychkov GY. TRPM2 channels mediate acetaminophen-induced liver damage. Proc Natl Acad Sci U S A. 2014;111:3176–3181. doi: 10.1073/pnas.1322657111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 229.Esterbauer H, Schaur RJ, Zollner H. Chemistry and biochemistry of 4-hydroxynonenal, malonaldehyde and related aldehydes. Free Radic Biol Med. 1991;11:81–128. doi: 10.1016/0891-5849(91)90192-6. [DOI] [PubMed] [Google Scholar]
- 230.Berlett BS, Stadtman ER. Protein oxidation in aging, disease, and oxidative stress. J Biol Chem. 1997;272:20313–20316. doi: 10.1074/jbc.272.33.20313. [DOI] [PubMed] [Google Scholar]
- 231.Chen J, Robinson NC, Schenker S, Frosto TA, Henderson GI. Formation of 4-hydroxynonenal adducts with cytochrome c oxidase in rats following short-term ethanol intake. Hepatology. 1999;29:1792–1798. doi: 10.1002/hep.510290611. [DOI] [PubMed] [Google Scholar]
- 232.Doorn JA, Hurley TD, Petersen DR. Inhibition of human mitochondrial aldehyde dehydrogenase by 4-hydroxynon-2-enal and 4-oxonon-2-enal. Chem Res Toxicol. 2006;19:102–110. doi: 10.1021/tx0501839. [DOI] [PubMed] [Google Scholar]
- 233.Fritz KS, Galligan JJ, Smathers RL, Roede JR, Shearn CT, Reigan P, Petersen DR. 4-Hydroxynonenal inhibits SIRT3 via thiol-specific modification. Chem Res Toxicol. 2011;24:651–662. doi: 10.1021/tx100355a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 234.Alkhouri N, Berk M, Yerian L, Lopez R, Chung YM, Zhang R, McIntyre TM, Feldstein AE, Hazen SL. OxNASH score correlates with histologic features and severity of nonalcoholic fatty liver disease. Dig Dis Sci. 2014;59:1617–1624. doi: 10.1007/s10620-014-3031-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 235.Mansouri A, Demeilliers C, Amsellem S, Pessayre D, Fromenty B. Acute ethanol administration oxidatively damages and depletes mitochondrial dna in mouse liver, brain, heart, and skeletal muscles: protective effects of antioxidants. J Pharmacol Exp Ther. 2001;298:737–743. [PubMed] [Google Scholar]
- 236.Demeilliers C, Maisonneuve C, Grodet A, Mansouri A, Nguyen R, Tinel M, Letteron P, Degott C, Feldmann G, Pessayre D, Fromenty B. Impaired adaptive resynthesis and prolonged depletion of hepatic mitochondrial DNA after repeated alcohol binges in mice. Gastroenterology. 2002;123:1278–1290. doi: 10.1053/gast.2002.35952. [DOI] [PubMed] [Google Scholar]
- 237.Bradford BU, Kono H, Isayama F, Kosyk O, Wheeler MD, Akiyama TE, Bleye L, Krausz KW, Gonzalez FJ, Koop DR, Rusyn I. Cytochrome P450 CYP2E1, but not nicotinamide adenine dinucleotide phosphate oxidase, is required for ethanol-induced oxidative DNA damage in rodent liver. Hepatology. 2005;41:336–344. doi: 10.1002/hep.20532. [DOI] [PubMed] [Google Scholar]
- 238.Rowland AA, Voeltz GK. Endoplasmic reticulum-mitochondria contacts: function of the junction. Nat Rev Mol Cell Biol. 2012;13:607–625. doi: 10.1038/nrm3440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 239.Aleynik MK, Leo MA, Aleynik SI, Lieber CS. Polyenylphosphatidylcholine opposes the increase of cytochrome P-4502E1 by ethanol and corrects its iron-induced decrease. Alcohol Clin Exp Res. 1999;23:96–100. [PubMed] [Google Scholar]
- 240.Gouillon Z, Lucas D, Li J, Hagbjork AL, French BA, Fu P, Fang C, Ingelman-Sundberg M, Donohue TM, Jr, French SW. Inhibition of ethanol-induced liver disease in the intragastric feeding rat model by chlormethiazole. Proc Soc Exp Biol Med. 2000;224:302–308. doi: 10.1046/j.1525-1373.2000.22435.x. [DOI] [PubMed] [Google Scholar]
- 241.Cheshchevik VT, Lapshina EA, Dremza IK, Zabrodskaya SV, Reiter RJ, Prokopchik NI, Zavodnik IB. Rat liver mitochondrial damage under acute or chronic carbon tetrachloride-induced intoxication: protection by melatonin and cranberry flavonoids. Toxicol Appl Pharmacol. 2012;261:271–279. doi: 10.1016/j.taap.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 242.Pallauf K, Giller K, Huebbe P, Rimbach G. Nutrition and healthy ageing: calorie restriction or polyphenol-rich "MediterrAsian" diet? Oxid Med Cell Longev. 2013;2013:707421. doi: 10.1155/2013/707421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 243.Basli A, Soulet S, Chaher N, Merillon JM, Chibane M, Monti JP, Richard T. Wine polyphenols: potential agents in neuroprotection. Oxid Med Cell Longev. 2012;2012:805762. doi: 10.1155/2012/805762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 244.Kim HS, Quon MJ, Kim JA. New insights into the mechanisms of polyphenols beyond antioxidant properties; lessons from the green tea polyphenol, epigallocatechin 3-gallate. Redox Biol. 2014;2:187–195. doi: 10.1016/j.redox.2013.12.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 245.Davinelli S, Sapere N, Zella D, Bracale R, Intrieri M, Scapagnini G. Pleiotropic protective effects of phytochemicals in Alzheimer’s disease. Oxid Med Cell Longev. 2012;2012:386527. doi: 10.1155/2012/386527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 246.Rodrigues CF, Ascencao K, Silva FA, Sarmento B, Oliveira MB, Andrade JC. Drug-delivery systems of green tea catechins for improved stability and bioavailability. Curr Med Chem. 2013;20:4744–4757. doi: 10.2174/09298673113209990158. [DOI] [PubMed] [Google Scholar]
- 247.Szeto HH. Cell-permeable, mitochondrial-targeted, peptide antioxidants. AAPS J. 2006;8:E277–E283. doi: 10.1007/BF02854898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 248.Chacko BK, Reily C, Srivastava A, Johnson MS, Ye Y, Ulasova E, Agarwal A, Zinn KR, Murphy MP, Kalyanaraman B, Darley-Usmar V. Prevention of diabetic nephropathy in Ins2(+/)(-)(AkitaJ) mice by the mitochondria-targeted therapy MitoQ. Biochem J. 2010;432:9–19. doi: 10.1042/BJ20100308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Reily C, Mitchell T, Chacko BK, Benavides G, Murphy MP, Darley-Usmar V. Mitochondrially targeted compounds and their impact on cellular bioenergetics. Redox Biol. 2013;1:86–93. doi: 10.1016/j.redox.2012.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 250.Jauslin ML, Meier T, Smith RA, Murphy MP. Mitochondria-targeted antioxidants protect Friedreich Ataxia fibroblasts from endogenous oxidative stress more effectively than untargeted antioxidants. FASEB J. 2003;17:1972–1974. doi: 10.1096/fj.03-0240fje. [DOI] [PubMed] [Google Scholar]
- 251.Melov S, Doctrow SR, Schneider JA, Haberson J, Patel M, Coskun PE, Huffman K, Wallace DC, Malfroy B. Lifespan extension and rescue of spongiform encephalopathy in superoxide dismutase 2 nullizygous mice treated with superoxide dismutase-catalase mimetics. J Neurosci. 2001;21:8348–8353. doi: 10.1523/JNEUROSCI.21-21-08348.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 252.Sheu SS, Nauduri D, Anders MW. Targeting antioxidants to mitochondria: a new therapeutic direction. Biochim Biophys Acta. 2006;1762:256–265. doi: 10.1016/j.bbadis.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 253.Murphy MP. Antioxidants as therapies: can we improve on nature? Free Radic Biol Med. 2014;66:20–23. doi: 10.1016/j.freeradbiomed.2013.04.010. [DOI] [PubMed] [Google Scholar]
- 254.Mukhopadhyay P, Horvath B, Zsengeller Z, Batkai S, Cao Z, Kechrid M, Holovac E, Erdelyi K, Tanchian G, Liaudet L, Stillman IE, Joseph J, Kalyanaraman B, Pacher P. Mitochondrial reactive oxygen species generation triggers inflammatory response and tissue injury associated with hepatic ischemia-reperfusion: therapeutic potential of mitochondrially targeted antioxidants. Free Radic Biol Med. 2012;53:1123–1138. doi: 10.1016/j.freeradbiomed.2012.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]