Abstract
Fear conditioning studies in adults have found that posttraumatic stress disorder (PTSD) is associated with heightened fear responses and impaired discrimination. The objective of the current study was to examine the association between PTSD symptoms and fear conditioned responses in children from a highly traumatized urban population. Children between 8 and 13 years old participated in a fear conditioning study in addition to providing information about their trauma history and PTSD symptoms. Results showed that females showed less discrimination between danger and safety signals during conditioning compared to age-matched males. In boys, intrusive symptoms were predictive of fear responses, even after controlling for trauma exposure. However, in girls, conditioned fear to the danger cue was predictive of self-blame and fear of repeated trauma. This study suggests there are early sex differences in the patterns of fear conditioning and that these sex differences may translate to differential risk for trauma-related psychopathology.
Keywords: conditioning, sex differences, stress
INTRODUCTION
Dysregulated fear responses underlie some of the core symptoms of posttraumatic stress disorder (PTSD). According to the Diagnostic and Statistical Manual of Mental Disorders, 5th edition, DSM-5 (American Psychiatric Association, 2013), PTSD is characterized by four major symptom clusters following exposure to a life threatening event. The first category includes symptoms of intrusive thoughts about the trauma, nightmares, and flashbacks; phenomena that are often induced by reminders of the event. The second cluster is associated with avoidance of stimuli associated with the traumatic event. The third cluster involves negative beliefs, such as distorted feelings of guilt. The final category describes trauma-induced physiological alterations such as increased startle and hyper-arousal including tachycardia, elevated perspiration, and shortness of breath. Because fear-related symptoms are so prevalent in PTSD, and fear responses can be objectively indexed using physiological measures, fear conditioning methods offer biological approaches to the study of PTSD symptoms (Briscione, Jovanovic, & Norrholm, 2014; Lissek & van Meurs, 2014). In addition, PTSD is two to three times more prevalent in females compared to males; this sex difference is also observed in fear conditioned responses in PTSD (Inslicht et al., 2013), which may be associated with genetic risk (Ressler et al., 2011) or hormonal status (Glover et al., 2012).
Fear conditioning studies in adults have found that individuals with PTSD show heightened fear responses and an inability to control fear (Jovanovic & Norrholm, 2011; Lissek & van Meurs, 2014), as well as impaired discrimination between danger and safety signals which may be a result of hyperactive amygdala activation and deficient prefrontal cortex inhibition observed in these patients relative to controls (Shin, Rauch, & Pitman, 2006). However, very little is known about fear conditioning in children with PTSD symptoms. Given the prevalence of trauma in low-income, inner-city families, exposure to trauma may begin early in life; studies from such samples suggest that initial trauma exposure during childhood or adolescence is common (Beesdo, Knappe, & Pine, 2009; Wade, Shea, Rubin, & Wood, 2014). Childhood trauma exposure has long-term negative consequences on mental health: early-life stress is a predictor of adult depression, while childhood and adult trauma are both predictors of PTSD (Nemeroff et al., 2006). Taken together, these data emphasize the need to study fear conditioned responses in children at risk for PTSD.
The prevalence of anxiety disorders is known to increase during late childhood and early adolescence, suggesting that this period may be developmentally critical in identifying individuals at risk for adult PTSD (Cohen et al., 1993). Age of onset for PTSD is not as clear, since it is dependent on exposure to a traumatic event. Moreover, this diagnosis has been difficult to identify in children and is often misdiagnosed (Havens et al., 2012). Longitudinal studies have found a significant increase in anxiety disorders in girls relative to boys in adolescence (Velez, Johnson, & Cohen, 1989). A potential explanation for increased anxiety in girls compared to boys (Mian, Traenor, & Carter, 2008) may be that girls have more difficulty regulating negative emotion and emotion dysregulation is more predictive of anxiety in girls than boys (Bender, Reinholdt-Dunne, Esbjørn, & Pons, 2012).
Brain imaging studies indicate that anxiety disorders are associated with larger amygdala volume in children and adolescents (De Bellis et al., 2000), especially in those with early averse experiences (Tottenham et al., 2010). A recent review of development of brain structures in children at risk for anxiety reported dysfunction in amygdala and prefrontal regions associated with anxiety (Blackford & Pine, 2012). Given these neurobiological changes in areas that are a critical part of the fear neurocircuitry, it is surprising that very few studies have examined developmental modifications in fear conditioning (Britton, Lissek, Grillon, Norcross, & Pine, 2011). In human fear conditioning models, the two most commonly measured indices of fear are an increase in skin conductance response (SCR) and acoustic startle amplitude (Lang, Davis, & Ohman, 2000). Skin conductance, which reflects changes in sweat gland activity that alters the electrical conductivity of the skin, is a direct index of sympathetic nervous system activation, and thus is an excellent measure of arousal. Importantly, the magnitude of the SCR reliably increases during presentations of a conditioned stimulus (CS) that was previously paired with an aversive unconditioned stimulus (US), making it a good index of conditioned fear (LaBar, LeDoux, Spencer, & Phelps, 1995; Ohman & Soares, 1993; Orr et al., 2000; Phelps, Delgado, Nearing, & LeDoux, 2004). In fear-potentiated startle, the magnitude of the startle reflex increases during aversive CS presentations (Grillon & Davis, 1997; Hamm, Start, & Vaitl, 1990; Lang et al., 2000).
A small number of studies have examined the effect of anxiety on fear conditioned responses in children. Waters and colleagues included anxious and nonanxious children between 8 and 12 years of age in their study of fear conditioning, using a loud tone as the US and SCR as the measure of fear conditioning (Waters, Henry, & Neumann, 2009). The results indicated that anxious children showed greater fear responses to all CSs during conditioning compared to controls, and did not discriminate between danger (CS+, the conditioned stimulus followed by the aversive event) and safety (CS−, the conditioned stimulus that is never followed by the aversive event) signals on SCR. A similar fear conditioning study using the scream US found that pediatric anxiety was associated with higher ratings of fear to all CSs in the experiment (Lau et al., 2008). We found that increased fear-potentiated startle to the CS− (a safety signal) was associated with anxiety in children (Jovanovic et al., 2014).
The objective of this study was to investigate fear responses during a fear conditioning paradigm in school-age children growing up in high-trauma environments. Our laboratory has developed a fear conditioning paradigm which uses neutral stimuli (shapes appearing on a computer monitor) as CSs and an aversive airblast to the larynx as the US. This type of US has been used successfully with high-risk children and produces robust conditioning while being well tolerated (Grillon, Dierker, & Merikangas, 1998; Jovanovic et al., 2014). Given our previous findings in adults with PTSD, we hypothesized that PTSD symptoms in children would be associated with heightened responses to danger cues (CS+) and safety cues (CS−). Finally, given sex differences in fear conditioned responses in adults with PTSD (Inslicht et al., 2013), we looked at interactions of fear conditioning with sex, and the association of PTSD symptoms with fear conditioning separately in males and females.
METHODS
Participants
Study participants were 105 children (53 females, 52 males) between 8 and 13 years of age (mean = 9.9, SD = 1.6 years). The participants were recruited from the waiting rooms of primary care clinics in an urban hospital in Atlanta, GA. Eligible child participants were between 8 and 13 years of age willing to participate; exclusion criteria were a diagnosis of autism spectrum disorders, bipolar or psychotic disorders, or cognitive disability. Prior to their participation, all mothers signed informed consent as well as parental permission for their children, and the children provided study assent approved by the Emory University Institutional Review Board and the Grady Hospital Research Oversight Committee.
Clinical Assessments
Trauma exposure in children was assessed using the Violence Exposure-Revised (VEX-R) (Fox & Leavitt, 1995), and the Traumatic Events Screening Inventory-Parent Report Revised (TESI) (Ghosh-Ippen et al., 2002). The VEX-R is a 22-item cartoon self-report measure of children’s exposure to violence. The TESI is 24-item clinician-administered interview that assesses a child’s experience of a variety of potential traumatic events using parental report.
PTSD symptoms in the child were assessed using the UCLA Child PTSD Index; using the child report and parent report scales (Steinberg, Brymer, Decker, & Pynoos, 2004). The UCLA Child PTSD Index assesses child’s DSM-IV PTSD symptoms within the past month. This measure derives a total symptom score, as well as five sub-categories of symptoms as follows: (a) intrusive symptoms, (b) avoidance and numbing symptoms, (c) hyperarousal symptoms, (d) self-blame symptoms, and (e) fear of repeated traumatization.
Child pubertal status was assessed using the Pubertal Development Scale (Robertson et al., 1992), parent report, which allows girls and boys to be categorized into five pubertal stages (pre, beginning, mid, advanced, post). Pubertal status assessment using this approach is consistent with Tanner staging and has shown reliability and validity in samples that include urban and rural adolescents. Girls were asked about menarche and menstrual cycle information.
Psychophysiological Assessment
The psychophysiological data were collected using Biopac MP150 for Windows (Biopac Systems, Inc., Aero Camino, CA). Electromyographic (EMG) and skin conductance (SC) data were sampled at 1,000 Hz and amplified using the respective modules of the Biopac system. The acquired data were filtered, rectified, and smoothed in MindWare software (MindWare Technologies, Inc. Gahanna, OH) and exported for statistical analyses. EMG activity was recorded from two 5 mm Ag/AgCl electrodes placed over the orbicularis oculi muscle, approximately 1 cm under the pupil and 1 cm below the lateral canthus. The impedances for all participants were less than 6 kΩ. The EMG signal was filtered with low- and high-frequency cutoffs at 28 and 500 Hz, respectively. Startle magnitude was assessed as the peak amplitude of the EMG contraction 20 to 200 ms following the acoustic stimulus. SC was measured using two electrodes on the hypothenar surface of the non-dominant hand. The SCR was defined as the average increase (from a 1 s pre-CS onset baseline) from 3 to 6 s after the CS onset.
Fear Conditioning Paradigm
Participants were seated in a sound attenuated booth and asked to remain still and look at a computer monitor approximately 1 m in front of them. The startle probe (noise burst) was a 106 dB [A] SPL, 40 ms burst of broadband noise delivered binaurally through headphones. The unconditioned stimulus (US) was an aversive airblast directed to the larynx at an intensity of 50 psi and 100 ms in duration. This US has been used successfully in our lab to elicit fear conditioned responses in pediatric populations (Jovanovic et al., 2014). The conditioned stimuli (CSs) were colored shapes presented on a computer monitor using Superlab presentation software (Cedrus, Inc. San Pedro, CA) for 6,000 ms prior to the delivery of the startle probe, and co-terminated with the US 500 ms after the presentation of the startle stimulus. The CS+ was paired with the airblast 100% of the time, and the CS− was never paired with the airblast. The fear conditioning protocol consisted of two phases: habituation and fear acquisition. The habituation phase contained all the same trial types as the acquisition phase with the exception that the CSs were not reinforced. The acquisition phase consisted of three blocks, each with three CS+ trials, three CS− trials, and three noise alone (NA, no CS presented during startle probe) trials, for a total of 27 startle trials. In all phases of the experiment, inter-trial intervals will be randomized between 9 and 22 s. A response keypad (Cedrus, Inc.) was incorporated in the experiment: at the beginning and end of each block questions appeared on the screen asking if a shape was followed by an airblast (for example: “Was the purple triangle followed by an airblast?”). The child was asked to respond to the question be pressing “Yes,” “No,” of “I don’t know.” The same question was asked for each CS. The CS contingencies were counterbalanced across participants.
Data Analyses
Fear-potentiated startle (FPS) was indexed by calculating percent potentiation for each CS type, in order to account for individual differences in startle magnitude as well as startle habituation. This value was derived as follows: Percent Startle Potentiation = 100 × (startle magnitude during CS trials – NA startle)/(NA startle). As noted above, SCR was calculated as the average response during the 3–6 s following CS onset minus the pre-CS baseline. The SCR data for each individual were square root transformed in order to normalize the data. In cases where SCR was a negative number (when SCL during the CS was lower than prior to CS onset), the square root was derived from the absolute value and the negative sign was added back to the value after the transformation.
We used a repeated measures analysis of variance (RM-ANOVA) with the within-subject factors of Trial Type (CS+, CS−) and Block (four levels: habituation and three blocks of acquisition), with Sex as the between group variable. Block was included in order to capture; (1) changes due to fear learning through the session, and (2) habituation of physiological responses. The dependent variables were FPS and SCR, and the answers on the response keypad. We used bivariate correlations to examine associations between fear conditioned responses to the CS+ and CS− and PTSD symptoms. We also performed linear regression analyses to examine whether fear responses predicted PTSD symptoms after controlling for age and trauma exposure.
RESULTS
Participant Characteristics
Of the 105 children recruited for the study, 15 (seven males and eight females) discontinued the study during the fear conditioning session. Those that discontinued were on average younger than the completers (p = .004) but did not have higher symptoms. Of the remaining 90 participants, four had missing SC or EMG data due to data collection error, for a final sample size of 86 participants (45 males and 41 females). Boys and girls did not differ in age or PTSD symptom severity, but males had higher levels of trauma exposure, based on both maternal and child report (see Table 1). Regarding types of trauma exposure, boys were more likely to report being pushed by another person, p = .005 and were more likely to witness a person being stabbed, p = .014. Table 2 shows the rates of trauma exposure on VEX-R items (child report). Based on maternal report, boys (n = 11, 25.6%) received more verbal abuse than girls (n = 3, 7.1%) (eg., “repeatedly told they were no good”), p = .028. Twice as many mothers of girls (n = 8, 19.0%) compared to boys (n = 4, 9.3%) reported possible sexual abuse of the child, but this difference was not significant, p = .12. With regard to PTSD diagnosis, 14 children (seven male and seven female) met DSM-IV criteria for the disorder (16.7% of the 84 children with available data).
Table 1.
Trauma Exposure and PTSD Symptoms in Male and Female Participants
| Male | Female | F | p | |
|---|---|---|---|---|
| Age | 10.0 | 9.9 | .0 | ns |
| Trauma Exposure (VEX-R, Child report) | 20.3 | 16.3 | 4.1 | .047 |
| Trauma Exposure (TESI, Maternal report) | 7.3 | 5.6 | 5.8 | .018 |
| PTSD Total (UCLA, Child report) | 20.0 | 24.7 | 2.6 | ns |
| PTSD Intrusive (UCLA, Child report) | 4.9 | 6.6 | 2.4 | ns |
| PTSD Avoidance (UCLA, Child report) | 6.4 | 8.0 | 1.7 | ns |
| PTSD Hyperarousal (UCLA, Child report) | 7.1 | 8.4 | 2.1 | ns |
| PTSD Self-blame (UCLA, Child report) | .5 | .6 | .8 | ns |
| PTSD Fear of Repeat Trauma (UCLA, Child report) | .9 | .9 | .0 | ns |
| PTSD Total (UCLA, Maternal report) | 19.8 | 15.5 | 2.4 | ns |
| PTSD Intrusive (UCLA, Maternal report) | 5.8 | 4.2 | 2.5 | ns |
| PTSD Avoidance (UCLA, Maternal report) | 4.9 | 3.9 | 1.0 | ns |
| PTSD Hyperarousal (UCLA, Maternal report) | 8.0 | 6.7 | 1.9 | ns |
| PTSD Self-blame (UCLA, Maternal report) | .3 | .2 | 1.1 | ns |
| PTSD Fear of Repeat Trauma (UCLA, Maternal report) | .8 | .5 | 1.4 | ns |
Table 2.
List of Trauma Exposure Items Queried on the VEX-R
| VEX-R ITEM (Child Report) | Male (N = 42) | Female (N = 42) | p |
|---|---|---|---|
| observed a person yell at someone | 40 (95.2%) | 39 (92.9%) | .50 |
| experienced a person yelling at them | 37 (88.1%) | 32 (76.2%) | .13 |
| observed something thrown at a person | 27 (64.3%) | 26 (61.9%) | .50 |
| experienced a person throwing something at them | 19 (45.2%) | 14 (33.3%) | .19 |
| observed a person push or shove someone | 31 (73.8%) | 28 (66.7%) | .32 |
| experienced a person pushing or shoving them | 19 (45.2%) | 7 (16.7%) | .005** |
| observed a person chase a scared person | 22 (52.4%) | 19 (45.2%) | .33 |
| experienced an angry person chasing them | 14 (33.3%) | 10 (23.8%) | .23 |
| observed a person slap someone really hard | 20 (48.8%) | 15 (36.6%) | .19 |
| experienced a person slap them really hard | 12 (28.6%) | 6 (14.3%) | .09 |
| observed a person beat up someone | 33 (78.6%) | 31 (73.8%) | .40 |
| experienced a person beat them up | 6 (14.3%) | 6 (14.3%) | 1.00 |
| observed a person steal stuff from someone | 17 (40.5%) | 16 (38.1%) | .50 |
| experienced a person stealing stuff from them | 18 (42.9%) | 13 (31%) | .18 |
| observed a person point a knife or a gun at someone | 10 (23.8%) | 5 (11.9%) | .13 |
| experienced a person point a knife or a gun at them | 2 (4.8%) | 0 (0%) | .25 |
| observed a person stab someone with a knife | 8 (19.0%) | 1 (2.4%) | .014* |
| observed a person shoot someone with a gun | 3 (7.1%) | 3 (7.1%) | 1.00 |
| observed a person being arrested | 36 (85.7%) | 35 (83.3%) | .76 |
| observed a person dealing drugs | 7 (16.7%) | 9 (22.0%) | .54 |
| observed a kid getting spanked | 33 (78.6%) | 37 (88.1%) | .24 |
| experienced being spanked by another person | 34 (81.0%) | 32 (76.2%) | .60 |
| observed someone being bitten by a dog | 13 (35.1%) | 17 (43.6%) | .45 |
| experienced being bitten by a dog | 13 (35.1%) | 12 (30.8%) | .68 |
| known someone who was killed | 13 (35.1%) | 13 (35.1%) | 1.00 |
p < .05.
p < .01.
Fear Conditioning
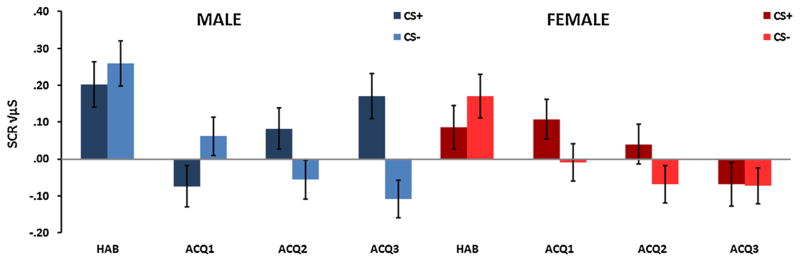
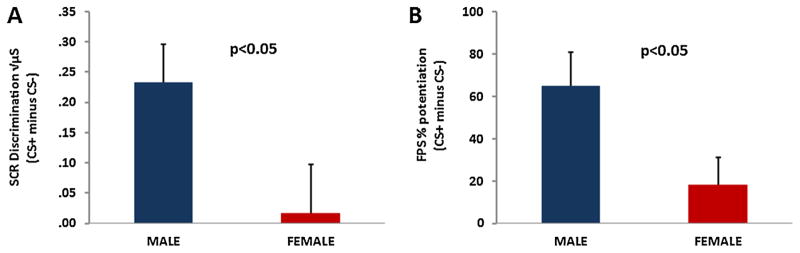
A RM-ANOVA of Block × Trial Type × Sex with SCR as the dependent variable revealed a significant three-way interaction, F(3,225) = 5.13, p = .002 (see Fig. 1). There was also a main effect of Block, F (3,225) = 11.36, p < .001, with SCR decreasing from the habituation phase to acquisition. A significant two-way interaction of Block × Trial Type, F(3,225) = 4.12, p = .007 reflected increased CS+ versus CS− discrimination across time. A comparison of Trial Type in the last block of acquisition with Sex as the between-groups variable demonstrated a significant main effect of Trial Type, F(1,83) = 6.06, p = .02, with higher SCR to the CS+ compared to the CS−. In addition there was an interaction between Trial Type and Sex, F (1,83) = 4.56, p = .04, in that males showed greater discrimination between the CS+ and the CS− than females (see Fig. 2A). Females habituated to the conditioning session faster, and no longer showed conditioned responses in the last block of conditioning (Fig. 1).
FIGURE 1.
Skin conductance responses (SCR) across Block (habituation, acquisition 1–3), Trial Type (CS+, CS−) and Sex (male, female). There was a significant three way interaction, p = .002, a significant interaction of Block × Trial Type, p = .007, and a main effect of Block, p < .001. There was a significant Trial Type × Sex interaction in the last block of acquisition, p = .04.
FIGURE 2.
Male participants (n = 45) showed greater discrimination between the reinforced conditioned stimulus (CS+) and the non-reinforced conditioned stimulus (CS−) than female participants (n = 41), in (A) skin conductance response and (B) fear-potentiated startle during the last block of fear acquisition.
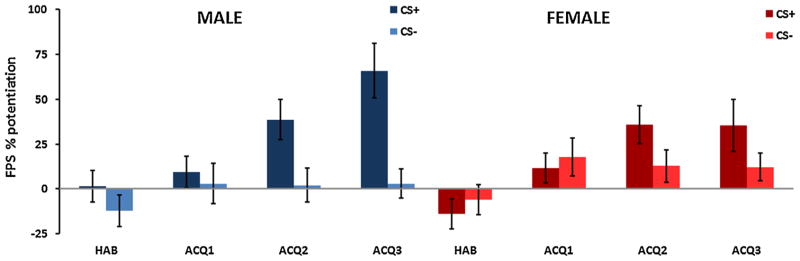
The same analysis with fear-potentiated startle as the dependent variable, revealed an interaction effect of Block and Trial Type, F(3,225) = 5.59, p = .001, with the difference between the CS+ and CS− increasing across Blocks (Fig. 3). In addition, there was a significant main effect of Block, F (3,225) = 9.94, p < .001. Unlike SCR, startle was not potentiated to the CSs during the habituation phase and FPS increased across time. There was a main effect of Trial Type, F(3,225) = 15.05, p < .001, with CS+ having higher FPS than CS, and an interaction of Type Type and Sex, F(3,225) = 4.95, p = .03. An analysis of Trial Type and Sex in the last block of acquisition showed that FPS to the CS+ was significantly greater than the CS−, F(1,83) = 16.88, p < .001. The significant intercept term for FPS, F (1,83) = 23.14, p < .001, indicated that both trial types were potentiated relative to baseline startle (i.e., percent potentiation from noise alone trials was greater than 0). Again, we observed an interaction of Trial Type with Sex, F(1,83) = 5.28, p = .02, in that males showed greater discrimination compared to females (see Fig. 2B). Analysis of response pad data also showed significantly higher US expectancy on CS+ trials compared to CS−, F(1,72) = 41.27, p < .001, but no interaction with Sex.
FIGURE 3.
Fear-potentiated startle (FPS) across Block (habituation, acquisition 1–3), Trial Type (CS+, CS−) and Sex (male, female). There was a significant two way interaction of Block and Trial Type, p = .001, a significant interaction of Trial Type × Sex, p = .03, a main effect of Block, p < .001, and a main effect of Trial Type, p < .001.
PTSD Symptoms
We examined the association between trauma exposure and total PTSD symptoms. We found that maternal report of the child’s trauma exposure was significantly correlated with her report of the child’s symptoms, r = .44, p < .001, whereas the child report of their trauma was positively correlated with their self-reported symptom severity, r = .39, p < .001, and somewhat less correlated with maternal report of the child’s symptoms, r = .23, p = .03. There were no significant correlations between maternal and child report of symptoms.
Next we examined the association between total PTSD symptoms and SCR and FPS measures of fear conditioning while controlling for trauma exposure. Child-reported PTSD symptom severity was positively correlated with SCR to the CS+, r = .24, p = .04, but not to the other measures. Maternally reported PTSD symptoms were not correlated with child fear conditioning measures. Interestingly, SCR (to either CS+ or CS−) was not correlated with FPS. In order to examine sex differences in the association between clusters of PTSD symptoms and SCR, we performed correlations between the five subset of symptoms and SCR to the CS+ within each sex. In order to correct for multiple tests, p values of ≤0.01 were considered significant. Table 3 shows the correlations for each symptom cluster within each sex. In males, SCR to the CS+ was significantly associated with intrusive symptoms, r = .37, p = .01. In females, SCR to the CS+ was associated with self-blame, r = .40, p = .01, and fear of repeated trauma, r = .43, p = .008. SCR discrimination (CS+ minus CS−) was correlated with avoidance symptoms in girls, r = .46, p = .004. We repeated the analysis after removing the 14 children who met criteria for PTSD in order to see whether these effects were driven by subthreshold symptoms. The SCR to the CS+ was still significantly associated with intrusive symptoms in males (p = .023), and fear of repeated trauma in girls (p = 0.017), albeit the p values were not low enough to survive correction. It is likely that removing the diagnostic children with the most severe symptoms reduced the range and power of the association.
Table 3.
Correlations Between Fear Conditioned Responses and PTSD Symptoms in Male and Female Participants
| Person’s correlations with SCR to CS+ | Male
|
Female
|
||
|---|---|---|---|---|
| r | p | r | p | |
| PTSD Intrusive (UCLA, Child report) | .37 | .01 | .13 | .43 |
| PTSD Avoidance (UCLA, Child report) | .17 | .28 | .33 | .04 |
| PTSD Hyperarousal (UCLA, Child report) | .10 | .50 | .18 | .29 |
| PTSD Self-blame (UCLA, Child report) | −.03 | .86 | .40 | .01 |
| PTSD Fear of Repeat Trauma (UCLA, Child report) | .17 | .27 | .43 | .008 |
| Person’s correlations with SCR to CS+ minus CS− | Male
|
Female
|
||
|---|---|---|---|---|
| r | p | r | p | |
| PTSD Intrusive (UCLA, Child report) | .23 | .15 | .08 | .62 |
| PTSD Avoidance (UCLA, Child report) | .04 | .79 | .46 | .004 |
| PTSD Hyperarousal (UCLA, Child report) | .20 | .22 | .24 | .15 |
| PTSD Self-blame (UCLA, Child report) | .00 | .99 | .29 | .08 |
| PTSD Fear of Repeat Trauma (UCLA, Child report) | .04 | .80 | .37 | .02 |
In order to test whether PTSD symptoms contributed to fear conditioned responses independently of age and trauma history, we conducted a stepwise linear regression analysis with age entered in the first step, trauma entered in the second step and PTSD symptoms in the final step. In males, intrusive symptoms predicted fear responses to the CS+ beyond age and trauma, F (1,40) = 5.82, p = .02, and accounted for 11% of the variance in SCR. In females, the same was true for self-blame and fear of repeated trauma, each accounting for 16 and 18% of the variance, respectively. Avoidance symptoms accounted for 18% of the variance in SCR discrimination after controlling for age and trauma exposure, F(1,33) = 7.65, p = .009.
Effects of Puberty
Female participants were much more likely to show signs of puberty onset, with 16 (39%) of the girls reporting a ≥3 score on the Pubertal Development Scale (at least mid-level puberty), compared to seven (15.6%) of the boys, Pearson χ2 = 6.03, p = .01. The observed interaction between Trial Type and Sex on CS+/CS− discrimination was still significant after removing those with a PDS ≥3 for SCR, p = .048 but dropped to trend levels for FPS, p = .067. In order to see whether the above sex differences in symptom associations were driven by the discrepancy in puberty onset, we repeated the above correlations after removing male and female pubertal participants. While the same correlations were observed as above, with the reduction of sample size, only the association between SCR to the CS+ and fear of repeated trauma, and SCR discrimination and avoidance symptoms remained significant in girls, r = .54, p = .01 and r = .56, p = .008, respectively.
DISCUSSION
The objective of the current study was to investigate the association between PTSD symptoms and fear conditioned responses in children with varying degrees of trauma exposure. As can be seen from Table 2, there were high rates of trauma exposure, with over 75% of the children witnessing physical violence and arrests, and over a third being confronted with murder. In this sample of children, 16.7% met criteria for PTSD, which is much higher than in other national adolescent samples, which report about 3.7% prevalence by age 14 (Merikangas et al., 2010). The aim of the study was to examine sex differences in the associations between fear conditioning and PTSD symptoms. We found that skin conductance responses to the CS+ were positively correlated with child-reported, but not mother-reported, PTSD symptoms, suggesting that the physiological responses tracked subjective reporting. The finding that the child-reported symptom severity was correlated with child report, but not mother report, of the child’s trauma exposure, suggests that child awareness and memory of the events may be necessary for symptom presentation (in some cases the maternal report included events that happened at a very young age, i.e., prior to 3 years).
Female children showed less discrimination between danger and safety signals during conditioning compared to age-matched males; this finding was observed using both skin conductance and startle responses. Skin conductance responses were highest during the habituation phase and declined as the experiment progressed, supporting findings that SCR is highly sensitive to novelty and habituates quickly (Glover et al., 2011; Lang, Bradley, & Cuthbert, 1998). In contrast, startle was not potentiated by the presentation of the neutral stimuli prior to conditioning and fear-potentiated startle increased during conditioning. Males had higher levels of exposure to trauma, but there were no sex differences in average PTSD symptoms. Interestingly, males and females had different profiles of the associations between fear conditioned responses and PTSD symptoms. In boys, intrusive symptoms were predictive of fear responses, even after controlling for trauma exposure. However, in girls, SCR to the CS+ was predictive of self-blame and fear of repeated trauma. On the other hand, differential SCR (CS+ minus CS−) was associated with avoidance symptoms in girls. These associations remained significant after excluding children with moderate signs of puberty from analyses.
Our previous studies in adults indicated an association between PTSD hyperarousal symptoms and fear-potentiated startle to the CS− (Jovanovic et al., 2010). In the current study with children, we did not see the same patterns as in adults, in that the associations with fear responses involved intrusive and avoidance rather than hyperarousal symptoms. Furthermore, SCR rather than FPS was correlated with PTSD symptoms. In a prior study of the effects of age and anxiety, we found that increased FPS to the CS− was associated with anxiety in children and youth (Jovanovic et al., 2014), which may be a risk factor for PTSD. It is possible that SCR is more related to the disorder than vulnerability. While we have not found SCR to fear conditioned cues (CS+) to be related to PTSD in our adult population (Glover et al., 2011), many other studies have found this relationship during fear acquisition (Inslicht et al., 2013; Orr et al., 2000). Furthermore, SCR in response to trauma-related or threatening imagery has repeatedly been found to be higher in PTSD compared to controls (McTeague et al., 2010).
The current study revealed sex differences in the pattern of fear-related PTSD symptoms, in that males showed an association between fear conditioned responses and intrusive symptoms, whereas females showed an association with symptoms of guilt, fear or repeated trauma, and avoidance. A study of adolescents who had early life stress found that adolescent females, but not males, had decreased functional connectivity between these circuits, and that this was correlated with higher anxiety symptoms (Burghy et al., 2012). Given that adult women are twice as likely to suffer from any anxiety disorder in general, and PTSD in particular, compared to men, understanding the emergence of these sex differences is even more crucial. Such differences may be driven by cultural or parental behavior, especially sex differences in the association between self-blame and arousal. However, some of the associations between fear-related symptoms and fear responses may have biological underpinnings. Recent evidence indicates that estrogen plays a significant role in severity of PTSD and the neurobiology of fear in women (Glover et al., 2012). Finally, women with PTSD have been found to have higher SCR to the CS+ compared to the CS− relative to men with PTSD (Inslicht et al., 2013). Taken together, these studies suggest that there are important sex differences in neurobiology of fear circuitry, and that these sex differences may translate to differential risk for trauma-related psychopathology. To our knowledge, this is the first study to examine the association between fear conditioning and PTSD in children, and the first to examine prepubertal sex differences.
A limitation of the current study is the lack of hormonal data in the children that would allow for precise tracking of pubertal endocrine changes. As mentioned above, estrogen levels have been implicated in fear responses in adult women with PTSD. It is unclear whether these differences emerge as a result of puberty. When we removed subjects that have started to undergo puberty based on maternal and self-report, the sex differences in symptom profiles remained. These results suggest that these may be related to sex differences during early development or genetic factors. We have found that a single nucleotide polymorphism of the gene coding for the pituitary adenylate cyclase-activating polypeptide receptor increases risk for PTSD and fear conditioned responses in adult women, but not men (Ressler et al., 2011). This same polymorphism is associated with increased anxiety-evoked startle responses in both boys and girls (Jovanovic et al., 2013). Future studies should include genetic and endocrine, in addition to parenting information to better determine the biological and environmental underpinnings of these sex differences.
Acknowledgments
We thank the research staff of the Mom and Kids study of the Grady Trauma Project. This work was supported by funding from NARSAD (TJ) and NIH (MH100122 to TJ; HD071982 to BB). The authors have no conflicts of interest. This material is the result of work supported with resources and the use of facilities at the Atlanta VA Medical Center, Decatur, GA. The contents of this manuscript do not represent the views of the U.S. Department of Veterans Affairs or the United States Government. The following author is employed by the Atlanta VAMC (Decatur, GA): Dr. Seth D. Norrholm (Program Analyst, Mental Health Service Line).
References
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders: DSM-5. Washington, D.C: American Psychiatric Association; 2013. [Google Scholar]
- Beesdo K, Knappe S, Pine DS. Anxiety and anxiety disorders in children and adolescents: Developmental issues and implications for DSM-V. Psychiatric Clinics of North America. 2009;32(3):483–524. doi: 10.1016/j.psc.2009.06.002. http://dx.doi.org/10.1016/j.psc.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bender PK, Reinholdt-Dunne ML, Esbjørn BH, Pons F. Emotion dysregulation and anxiety in children and adolescents: Gender differences. Personality and Individual Differences. 2012;53(3):284–288. http://dx.doi.org/10.1016/j.paid.2012.03.027. [Google Scholar]
- Blackford JU, Pine DS. Neural substrates of childhood anxiety disorders: A review of neuroimaging findings. Child and Adolescent Psychiatric Clinics of North America. 2012;21(3):501–525. doi: 10.1016/j.chc.2012.05.002. http://dx.doi.org/10.1016/j.chc.2012.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briscione MA, Jovanovic T, Norrholm SD. Conditioned fear associated phenotypes as robust, translational indices of trauma-, stressor-, and anxiety-related behaviors. Frontiers in Psychiatry. 2014;5:88. doi: 10.3389/fpsyt.2014.00088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britton JC, Lissek S, Grillon C, Norcross MA, Pine DS. Development of anxiety: The role of threat appraisal and fear learning. Depress Anxiety. 2011;28(1):5–17. doi: 10.1002/da.20733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burghy CA, Stodola DE, Ruttle PL, Molloy EK, Armstrong JM, Oler JA, … Birn RM. Developmental pathways to amygdala-prefrontal function and internalizing symptoms in adolescence. Nature Neuroscience. 2012;15(12):1736–1741. doi: 10.1038/nn.3257. http://www.nature.com/neuro/journal/v15/n12/abs/nn.3257.html#supplementary-information. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen P, Cohen J, Kasen S, Velez CN, Hartmark C, Johnson J, … Streuning EL. An epidemiological study of disorders in late childhood and adolescence?I. Age- and gender-specific prevalence. Journal Child Psychology Psychiatry. 1993;34(6):851–867. doi: 10.1111/j.1469-7610.1993.tb01094.x. [DOI] [PubMed] [Google Scholar]
- De Bellis MD, Casey BJ, Dahl RE, Birmaher B, Williamson DE, Thomas KM, … Ryan ND. A pilot study of amygdala volumes in pediatric generalized anxiety disorder. Biological Psychiatry. 2000;48(1):51–57. doi: 10.1016/s0006-3223(00)00835-0. http://dx.doi.org/10.1016/S0006-3223(00)00835-0. [DOI] [PubMed] [Google Scholar]
- Fox NA, Leavitt LA. The violence exposure scale for children-VEX (preschool version) College Park, MD: Department of Human Development, University of Maryland; 1995. [Google Scholar]
- Ghosh-Ippen C, Ford J, Racusin R, Acker M, Bosquet K, Rogers C, … Edwards J. Trauma events screening inventory- parent report revised. San Francisco: 2002. The child trauma research project of the early trauma network and the national centre for ptsd dartmouth child trauma research group. [Google Scholar]
- Glover EM, Jovanovic T, Mercer KB, Kerley K, Bradley B, Ressler KJ, Norrholm SD. Estrogen levels are associated with extinction deficits in women with posttraumatic stress disorder. Biological Psychiatry. 2012;72(1):19–24. doi: 10.1016/j.biop-sych.2012.02.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Glover EM, Phifer JE, Crain DF, Norrholm SD, Davis M, Bradley B, … Jovanovic T. Tools for translational neuroscience: PTSD is associated with heightened fear responses using acoustic startle but not skin conductance measures. Depression and Anxiety. 2011;28(12):1058–1066. doi: 10.1002/da.20880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grillon C, Davis M. Fear-potentiated startle conditioning in humans: Explicit and contextual cue conditioning following paired versus unpaired training. Psychophysiology. 1997;34(4):451–458. doi: 10.1111/j.1469-8986.1997.tb02389.x. [DOI] [PubMed] [Google Scholar]
- Grillon C, Dierker L, Merikangas KR. Fear-potentiated startle in adolescent offspring of parents with anxiety disorders. Biological Psychiatry. 1998;44(10):990–997. doi: 10.1016/s0006-3223(98)00188-7. [DOI] [PubMed] [Google Scholar]
- Hamm AO, Start O, Vaitl D. Classical fear conditioning and the startle probe reflex. Psychophysiology. 1990;27:S37. [Google Scholar]
- Havens JF, Gudiño OG, Biggs EA, Diamond UN, Weis JR, Cloitre M. Identification of trauma exposure and PTSD in adolescent psychiatric inpatients: An exploratory study. Journal of Traumatic Stress. 2012;25(2):171–178. doi: 10.1002/jts.21683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inslicht SS, Metzler TJ, Garcia NM, Pineles SL, Milad MR, Orr SP, … Neylan TC. Sex differences in fear conditioning in posttraumatic stress disorder. Journal of Psychiatric Research. 2013;47(1):64–71. doi: 10.1016/j.jpsychires.2012.08.027. http://dx.doi.org/10.1016/j.jpsychires.2012.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD. Neural mechanisms of impaired fear inhibition in posttraumatic stress disorder. Frontiers in Behavioral Neuroscience. 2011;5(44):1–8. doi: 10.3389/fnbeh.2011.00044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Blanding NQ, Davis M, Duncan E, Bradley B, Ressler KJ. Impaired fear inhibition is a biomarker of PTSD but not depression. Depression and Anxiety. 2010;27(3):244–251. doi: 10.1002/da.20663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Norrholm SD, Davis J, Mercer KB, Almli L, Nelson A, … Bradley B. PAC1 receptor (ADCYAP1R1) genotype is associated with dark-enhanced startle in children. Molecular Psychiatry. 2013;18(7):742–743. doi: 10.1038/mp.2012.98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jovanovic T, Nylocks KM, Gamwell KL, Smith A, Davis TA, Norrholm SD, Bradley B. Development of fear acquisition and extinction in children: Effects of age and anxiety. Neurobiology of Learning and Memory. 2014;113(0):135–142. doi: 10.1016/j.nlm.2013.10.016. http://dx.doi.org/10.1016/j.nlm.2013.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LaBar KS, LeDoux JE, Spencer DD, Phelps EA. Impaired fear conditioning following unilateral temporal lobectomy in humans. The Journal of Neuroscience. 1995;15(10):6848–6855. doi: 10.1523/JNEUROSCI.15-10-06846.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang PJ, Bradley MM, Cuthbert BN. Emotion, motivation, and anxiety: Brain mechanisms and psychophysiology. Biological Psychiatry. 1998;44:1248–1263. doi: 10.1016/s0006-3223(98)00275-3. [DOI] [PubMed] [Google Scholar]
- Lang PJ, Davis M, Ohman A. Fear and anxiety: Animal models and human cognitive psychophysiology. Journal of Affective Disorders. 2000;61(3):137–159. doi: 10.1016/s0165-0327(00)00343-8. [DOI] [PubMed] [Google Scholar]
- Lau JY, Lissek S, Nelson EE, Lee Y, Roberson-Nay R, Poeth K, … Pine DS. Fear conditioning in adolescents with anxiety disorders: Results from a novel experimental paradigm. Journal of the American Academy of Child and Adolescent Psychiatry. 2008;47(1):94–102. doi: 10.1097/chi.0b01e31815a5f01S0890-8567(09). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissek S, van Meurs B. Learning models of PTSD: Theoretical accounts and psychobiological evidence. International Journal of Psychophysiology, Epub. 2014 doi: 10.1016/j.ijpsycho.2014.11.006. doi: http://dx.doi.org/10.1016/j.ijpsy-cho.2014.11.006. [DOI] [PMC free article] [PubMed]
- McTeague LM, Lang PJ, Laplante MC, Cuthbert BN, Shumen JR, Bradley MM. Aversive imagery in posttraumatic stress disorder: Trauma recurrence, comorbidity, and physiological reactivity. Biological Psychiatry. 2010;67(4):346–356. doi: 10.1016/j.biopsych.2009.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merikangas KR, He J, Burstein M, Swanson SA, Avenevoli S, Cui L, … Swendsen J. Lifetime prevalence of mental disorders in U. S. adolescents: Results from the national comorbidity survey replication? adolescent supplement (NCS-A). Journal of the American Academy of Child &. Adolescent Psychiatry. 2010;49(10):980–989. doi: 10.1016/j.jaac.2010.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mian ND, Traenor M, Carter AS. Sex differences in parenting and child anxiety. Paper presented at the American Psychological Association Convention.2008. [Google Scholar]
- Nemeroff CB, Bremner JD, Foa EB, Mayberg HS, North CS, Stein MB. Posttraumatic stress disorder: A state-of-the-science review. Journal of Psychiatric Research. 2006;40(1):1–21. doi: 10.1016/j.jpsychires.2005.07.005. [DOI] [PubMed] [Google Scholar]
- Ohman A, Soares JJF. On the automatic nature of phobic fear: Conditioned electrodermal responses to masked fear-relevant stimuli. Journal of Abnormal Psychology. 1993;102:121–132. doi: 10.1037//0021-843x.102.1.121. [DOI] [PubMed] [Google Scholar]
- Orr SP, Metzger LJ, Lasko NB, Macklin ML, Peri T, Pitman RK. De novo conditioning in trauma-exposed individuals with and without posttraumatic stress disorder. Journal of Abnormal Psychology. 2000;109(2):290–298. [PubMed] [Google Scholar]
- Phelps EA, Delgado MR, Nearing KI, LeDoux JE. Extinction learning in humans: Role of the amygdala and vmPFC. Neuron. 2004;43(6):897–905. doi: 10.1016/j.neuron.2004.08.042. [DOI] [PubMed] [Google Scholar]
- Ressler KJ, Mercer KB, Bradley B, Jovanovic T, Mahan A, Kerley K, … May V. Posttraumatic stress disorder is associated with PACAP and the PAC1 receptor. Nature. 2011;470(7335):492–497. doi: 10.1038/nature09856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson EB, Skinner ML, Love MM, Elder GH, Conger RD, Dubas JS, Petersen AC. The pubertal development scale. The Journal of Early Adolescence. 1992;12(2):174–186. doi: 10.1177/0272431692012002003. [DOI] [Google Scholar]
- Shin LM, Rauch SL, Pitman RK. Amygdala, medial prefrontal cortex, and hippocampal function in PTSD. Annals of New York Academic Science. 2006;1071(1):67–79. doi: 10.1196/annals.1364.007. [DOI] [PubMed] [Google Scholar]
- Steinberg AM, Brymer MJ, Decker KB, Pynoos RS. The university of California at Los Angeles posttraumatic stress disorder reaction index. Current Psychiatry Reports. 2004;6(2):96–100. doi: 10.1007/s11920-004-0048-2. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Hare TA, Quinn BT, McCarry TW, Nurse M, Gilhooly T, … Casey BJ. Prolonged institutional rearing is associated with atypically large amygdala volume and difficulties in emotion regulation. Developmental Science. 2010;13(1):46–61. doi: 10.1111/j.1467-7687.2009.00852.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velez CN, Johnson JIM, Cohen P. A longitudinal analysis of selected risk factors for childhood psychopathology. Journal of the American Academy of Child & Adolescent Psychiatry. 1989;28(6):861–864. doi: 10.1097/00004583-198911000-00009. [DOI] [PubMed] [Google Scholar]
- Wade R, Shea JA, Rubin D, Wood J. Adverse childhood experiences of low-income urban youth. Pediatrics. 2014;134(1):e13–e20. doi: 10.1542/peds.2013-2475. [DOI] [PubMed] [Google Scholar]
- Waters AM, Henry J, Neumann DL. Aversive pavlovian conditioning in childhood anxiety disorders: Impaired response inhibition and resistance to extinction. Journal of Abnormal Psychology. 2009;118(2):311–321. doi: 10.1037/a0015635. [DOI] [PubMed] [Google Scholar]