Abstract
Purpose
To explore the relationship between chronic kidney disease (CKD) and diabetic retinopathy (DR) in a representative population of type 2 diabetes mellitus (DM2) patients in Catalonia (Spain).
Methods
This was a population-based, cross-sectional study. A total of 28,344 patients diagnosed with DM2 who had recorded ophthalmologic and renal functional examinations were evaluated. Data were obtained from a primary healthcare electronic database of medical records. CKD was defined as an estimated glomerular filtration ratio (eGFR) of <60 ml/min/1.73m2 and/or urine albumin to creatinine ratio (UACR) ≥30 mg/g. DR was categorized as non-vision threatening diabetic retinopathy and vision threatening diabetic retinopathy.
Results
CKD was associated with a higher rate of DR [OR], 95% confidence interval [CI], 1.5 (1.4–1.7). When we analyzed the association between different levels of UACR and DR prevalence observed that DR prevalence rose with the increase of UACR levels, and this association was significant from UACR values ≥10 mg/g, and increased considerably with UACR values ≥300mg/g (Odds ratio [OR], 95% confidence interval [CI], 2.0 (1.6–2.5). This association was lower in patients with eGFR levels 44 to 30 mL/min/1.73m2 [OR], 95% confidence interval [CI], 1.3 (1.1–1.6).
Conclusions
These results show that CKD, high UACR and/or low eGFR, appear to be associated with DR in this DM2 population.
Introduction
Chronic kidney disease (CKD) and diabetes retinopathy (DR) are two major microvascular complications found in long-standing type 1 (DM1) and type 2 diabetic (DM2) patients. In addition to sharing risk factors, such as poor glycaemia control and systolic hypertension, CKD and DR are reflected in the clinical manifestations of similar microvascular lesions in the glomerular and retinal vessels [1–3]. UACR and eGFR are clinically markers for evaluate renal function.
Urine albumin to creatinine ratio (UACR) and low estimated glomerular filtration rate (eGFR) are clinical markers of renal function. CKD is often associated with DR in DM1 [4, 5], and in some studies in DM2 [6–8]. However, this relationship has not been properly investigated in DM2, and there are few data on which of the two markers of CKD, eGFR or UACR, is more closely related to DR.
High UACR is a marker of endothelial dysfunction and may influence the microvasculature of the kidney and retina. However some studies [9–10] found a relationship between higher UACR levels and DR in DM1 patients but not in DM2 patients. Sabanayagam et al. [11] observed that CKD was associated with DR only in the presence of albuminuria, suggesting that CKD is more likely related to diabetes in the presence of albuminuria. Previous studies have shown that UACR not only is an important clinical marker for CKD, but also is closely associated with the progression of DR [12]. Moreover, the relationship between eGFR with DR remains unclear, particularly in DM2. Man et al. [13] observed that lower levels of eGFR were associated with the presence and severity of DR. A recent report showed that both an elevated UACR and decreased eGFR predicted the development of DR in DM2 patients with the former having a greater impact [14].
The aim of this study was to investigate the association between CKD, UACR and/or eGFR, and DR in a representative Catalonian (Spain) DM2 population.
Materials and Methods
A population-based, cross-sectional study was performed in Catalonia (Spain) with patients aged between 30 and 90 years (at 31st December, 2012), diagnosed with DM2 prior to retinal photography screening, and whose DR category was recorded in their medical records. The most recent retinal photography registered was selected and employed as the index date. The criteria for the diagnosis of type DM2 were those established by the American Diabetes Association [15] at the time of registration in the electronic database.
Data were obtained from the SIDIAP (System for Research and Development in Primary Care) electronic database. The SIDIAP includes data from the primary healthcare electronic medical records (e-CAP) on demographic information, appointment dates with doctors and nurses, clinical diagnoses, clinical variables, prescriptions written, referrals to specialists and hospitals, results from laboratory tests, and medication sold by pharmacies. The quality of the SIDIAP data has been previously documented, and the database has been widely used to study the epidemiology of a number of health outcomes [16, 17].
Measures of Kidney Diabetic Disease
Serum creatinine levels and UACR were determined. CKD was defined as UACR >30 mg/g and/or eGFR <60 mL/min/1.73m2.
Normoalbuminuria was defined as UACR <30 mg/g; microalbuminuria as UACR 30–299 mg/g; and macroalbuminuria as UACR ≥300 mg/g. The Chronic Kidney Disease Epidemiology Collaboration equation was employed to measure eGFR [18].
Assessment of Retinopathy
In a large, community-based screening programmer, such as that employed in our study, fundus photography is considered to be the preferred option [19]. Photographs are captured from each eye and the severity of DR is categorized according to the international clinical diabetic retinopathy severity scales recommended by the Global Diabetic Retinopathy Project Group [20] as: non-vision threatening DR (non-VTDR) and vision threatening DR (VTDR). The former includes no apparent retinopathy (no DR); mild non-proliferative DR (mild NPDR); and moderate non-proliferative DR (moderate NPDR). The latter is composed of severe non-proliferative DR (severe NPDR); proliferative DR (PDR); and diabetic macular edema (DME). In our study, retinal photography was performed by skilled personnel using a non-midriatic camera. Subsequently, in the primary health care center, a family physician trained in reading eye fundus photographs registered the result in the patient’s medical records. Each eye was given a DR grade according to the worst result. In the case of patients having more than one fundus photograph during this period, the most recent was employed for analysis. In the study we only included retinal photographs from patients undergoing diabetic retinopathy screening performed at primary health care centers.
Clinical variables
The following data were obtained from each patient: age, age at diagnosis of diabetes, duration of diabetes, gender, and glycated hemoglobin levels (A1C). Cardiovascular risk factors including body mass index (BMI), total cholesterol, low-density (LDL) cholesterol, high density lipoprotein (HDL) cholesterol, non-HDL cholesterol, systolic and diastolic blood pressure (SBP and DBP), pulse pressure (PP), and smoking status according to the last condition registered before the index date were collected. Data for clinical variables were gathered from the 15 months prior to the index date with the exception of blood pressure, PP, and BMI, which were obtained from the previous 12 months. Additional data were gathered on medication.
The study was approved by the Ethics Committee of the Primary Health Care University Research Institute Jordi Gol (protocol number 13/137). All patient records and information was anonymized and de-identified prior to analysis.
Statistical analysis
A descriptive analysis stratified by renal function (according to eGFR and UACR) and retinography result was performed. The absolute and relative frequencies of the qualitative variables, and their mean and standard deviation (or median and interquartile range according to the characteristics of the variable) were calculated.
In order to study whether the clinical variables of interest depended on renal function, Pearson’s chi square test and analysis of variance (ANOVA) were employed for means and proportions, respectively. The hypothesis contrast was bilateral in all cases with a 0.05 level of significance.
In addition, the relationship between renal function values (according to eGFR and UACR) and the presence of retinopathy was assessed by logistic regression models adjusted by age, sex, age at retinography, duration of DM2, systolic blood pressure, A1C ≤7%, smoking, BMI, and cardiovascular disease.
Analyses were performed with Stata/SE version 13 for Windows (Stata Corp., College Station, Texas, USA).
Results
Prior to retinography 28,344 DM2 patients had their eGFR and UACR values registered. From the total sample, 14.6% had an eGFR <60 mL/min/1.73m2, women had lower rates than men (p<0.001). As can be observed in Table 1, diabetes duration, hypertension, cardiovascular disease (CVD), and treatment with insulin displayed a direct association with decreased eGFR.
Table 1. Patients’ characteristics with respect to glomerular filtration rate (eGFR).
| Variable | Global | eGFR ≥60 | eGFR 59–45 | eGFR 44–30 | eGFR <30 | p-valuea |
|---|---|---|---|---|---|---|
| Female, n (%) | 12,520 (44.2) | 10,363 (42.8) | 1,478(51.2) | 568 (53.9) | 111 (52.6) | <0.001 |
| Hypertension, n (%) | 22,971 (81.0) | 19,001 (78.5) | 2,737 (94.8) | 1,026 (97.4) | 207 (98.1) | <0.001 |
| Any cardiovascular diseaseb, n (%) | 3,969 (14.0) | 3,026 (12.5) | 590 (20.4) | 286 (27.2) | 67 (31.8) | <0.001 |
| Coronary Heart disease, n (%) | 3,048 (10.8) | 2,326 (9.6) | 446 (15.5) | 221 (21.0) | 55 (26.1) | <0.001 |
| Stroke, n (%) | 1,148 (4.1) | 854 (3.5) | 186 (6.4) | 90 (8.5) | 18 (8.5) | <0.001 |
| Insulin treatment, n (%) | 4,737 (16.7) | 3,781 (15,6) | 567 (19.6) | 302 (28.7) | 87 (41.2) | <0.001 |
| Age T2DM (years), mean (SD) | 58.8(10.5) | 57.5(10.2) | 65.6 (9.0) | 67.2 (9.7) | 65.0(10.1) | <0.001 |
| Age at retinography (years), mean (SD) | 65.7(10.7) | 64.3(10.4) | 73.7(7.9) | 76.1 (7.8) | 74.6(8.9) | <0.001 |
| Diabetes duration (years), mean (SD) | 7.0 (5.2) | 6.8 (5.0) | 8.2 (5.6) | 9.0 (6.0) | 9.7(6.1) | <0.001 |
| A1C (%), mean (SD) | 7.4 (1.4) | 7.4(1.4) | 7.2 (1.2) | 7.3 (1.4) | 7.2 (1.3) | <0.001 |
| Non-HDL cholesterol (mg/dL), mean (SD) | 137.3(35.2) | 137.8(35.2) | 135.4(34.6) | 132.5(35.2) | 132.5(38.2) | <0.001 |
| Hemoglobin (g/dL), mean (SD) | 14.0 (1.5) | 14.1 (1.4) | 13.3 (1.6) | 12.7 (1.6) | 12.0 (1.5) | <0.001 |
| SBP (mmHg), mean (SD) | 134.9(12.6) | 134.8(12.5) | 136.1(13.1) | 135.8(13.5) | 133.9(14.0) | <0.001 |
| DBP (mmHg), mean (SD) | 76.4 (8.3) | 76.9 (8.2) | 74.2 (8.2) | 71.9 (8.3) | 70.4 (8.3) | <0.001 |
| Heart Rate (bpm), mean (SD) | 76.1 (12.1) | 76.3 (12.0) | 75 (12.5) | 74.2 (12.5) | 72.5 (12.0) | <0.001 |
aP value for comparison of groups by glomerular filtration rate with Pearson’s chi-square test for qualitative variables and t-test for quantitative ones.
bAny cardiovascular disease includes coronary heart disease and/or stroke
A1C, glycated hemoglobin; bpm, beats per minute; DBP, Diastolic Blood Pressure; DR: diabetic retinopathy.
In contrast, an inverse relationship between eGFR and non-HDL cholesterol, plasma hemoglobin, heart rate, and DBP was reported. With respect to UACR values (Table 2), 16.0% were ≥30 mg/g with higher levels being found in men (p<0.001). DM2 duration, poor glycemic control (glycated hemoglobin; A1C >7%), SBP, increased heart rate, hypertension, CVD, and treatment with insulin showed a direct relationship with an increase in UACR levels. An inverse relationship, however, was observed between UACR and plasma hemoglobin levels.
Table 2. Patients’ characteristics with respect to urine albumin to creatinine ratio (UACR).
| Variable | Global | UACR <30 mg/g | UACR = 30–299 mg/g | UACR ≥300 mg/g | p-valuea |
|---|---|---|---|---|---|
| Female, n (%) | 12,520 (44.2) | 10,886 (45.7) | 1,443 (36.4) | 191 (33.7) | <0.001 |
| Hypertension, n (%) | 22,971 (81.0) | 18,857 (79.2) | 3,573 (90.2) | 541 (95.4) | <0.001 |
| Any cardiovascular diseaseb, n (%) | 3,969 (14.0) | 2,996 (12.6) | 830 (21.0) | 143 (25.2) | <0.001 |
| Coronary Heart disease, n (%) | 3,048 (10.8) | 2,321 (9.7) | 616 (15.6) | 111 (19.6) | <0.001 |
| Stroke, n (%) | 1,148 (4.1) | 842 (3.5) | 268 (6.8) | 38 (6.7) | <0.001 |
| Insulin treatment, n (%) | 4,737 (20.0) | 3,512 (17,9) | 1,028 (29.0) | 197 (39.4) | <0.001 |
| Age T2DM (years), mean (SD) | 58.8(10.5) | 58.6(10.4) | 59.8(10.9) | 59.1(10.5) | <0.001 |
| Age at retinography (years), mean (SD) | 65.7(10.7) | 65.4(10.6) | 67.6(11.0) | 67.6(11.0) | <0.001 |
| Diabetes duration (years), mean (SD) | 7.0 (5.2) | 6.8 (5.1) | 7.8 (5.4) | 8.6 (5.8) | <0.001 |
| A1C (%), mean (SD) | 7.4 (1.4) | 7.3(1.3) | 7.7 (1.6) | 7.8 (1.7) | <0.001 |
| Non-HDL cholesterol (mg/dL), mean (SD) | 137.3(35.2) | 137.4 (34.7) | 136.5(37.6) | 136.6(37.3) | 0.320 |
| Hemoglobin (g/dL), mean (SD) | 14.0 (1.5) | 14.0 (1.5) | 13.8 (1.7) | 13.5 (1.9) | <0.001 |
| SBP (mmHg), mean (SD) | 134.9(12.6) | 134.2(12.2) | 138.3(13.7) | 141.8(15.8) | <0.001 |
| DBP (mmHg), mean (SD) | 76.4 (8.3) | 76.4 (8.1) | 76.4 (9.0) | 76.7 (10.0) | 0.736 |
| Heart Rate (bpm), mean (SD) | 76.1 (12.1) | 76.0(12.0) | 76.6 (12.8) | 77.5 (13.2) | 0.002 |
aP value for comparison of groups by urine albumin to creatinine ratio (ACR) with Pearson’s chi-square test for qualitative variables and t-test for the quantitative ones.
bAny cardiovascular disease includes coronary heart disease and/or stroke
A1C, glycated hemoglobin; DBP, diastolic blood pressure; SBP, systolic blood pressure.
In Table 3 the characteristics of the patients with respect to the presence or absence of DR can be observed. Duration of diabetes, A1C levels, SBP, heart rate, CVD, and insulin treatment all displayed a direct relationship with DR. An inverse relationship, however, was observed for DR with non-HDL cholesterol, plasma hemoglobin, and DBP.
Table 3. Patients’ characteristics with respect to diabetic retinopathy.
| Variable | Global | Non DR | Non-VTDR | VTDR | p-valuea |
|---|---|---|---|---|---|
| Female, n (%) | 12,520 (44.2) | 11,089 (44.3) | 1,316 (43.8) | 115 (40.2) | 0.345 |
| Hypertension, n (%) | 22,971 (81.0) | 20,141 (80.4) | 2,589 (86.1) | 241 (84.3) | <0.001 |
| Any cardiovascular disease, b n (%) | 3,969 (14.0) | 3,347 (13.4) | 553 (18.4) | 69 (24.1) | <0.001 |
| Coronary heart disease, n (%) | 3,048 (10.8) | 2,602 (10.4) | 396 (13.2) | 50 (17.5) | <0.001 |
| Stroke, n (%) | 1,148 (4.1) | 935 (3.7) | 187 (6.2) | 26 (9.1) | <0.001 |
| Insulin treatment, n (%) | 4,737 (20.0) | 3,529 (17.1) | 1,067 (38.8) | 141 (52.8) | <0.001 |
| Age DM2 (years), mean (SD) | 58.8(10.5) | 58.8 (10.4) | 58.1 (11.2) | 58.4 (11.6) | <0.001 |
| Age at retinography (years), mean (SD) | 65.7(10.7) | 65.6 (10.6) | 67.1 (10.8) | 67.9 (10.9) | <0.001 |
| Diabetes duration (years), mean (SD) | 7.0 (5.2) | 6.7 (5.0) | 9.0 (5.8) | 9.5 (5.8) | <0.001 |
| A1C (%), mean (SD) | 7.4 (1.4) | 7.3 (1.3) | 7.9 (1.6) | 8.2 (1.7) | <0.001 |
| Non-HDL cholesterol (mg/dL), mean (SD) | 137.3(35.2) | 137.8 (35.0) | 133.0 (35.5) | 133.9 (40.1) | <0.001 |
| Hemoglobin (g/dL), mean (SD) | 14.0 (1.5) | 14.0 (1.5) | 13.7 (1.6) | 13.7 (1.8) | <0.001 |
| SBP (mmHg), mean (SD) | 134.9(12.6) | 134.6 (12.5) | 137.4 (13.6) | 138.2 (12.7) | <0.001 |
| DBP (mmHg), mean (SD) | 76.4 (8.3) | 76.5 (8.3) | 75.7 (8.5) | 75.0 (8.3) | <0.001 |
| Heart Rate (bpm), mean (SD) | 76.1 (12.1) | 76.0 (12.1) | 76.9 (12.1) | 78.2 (12.3) | <0.001 |
a P value for comparison of groups by glomerular filtration rate with Pearson’s chi-square test for qualitative variables and t-test for the quantitative ones.
b Any cardiovascular disease includes coronary heart disease and/or stroke
A1C, glycated hemoglobin; DBP, diastolic blood pressure; DR: diabetic retinopathy; SBP, systolic blood pressure; VTDR, Vision Threatening Diabetic Retinopathy.
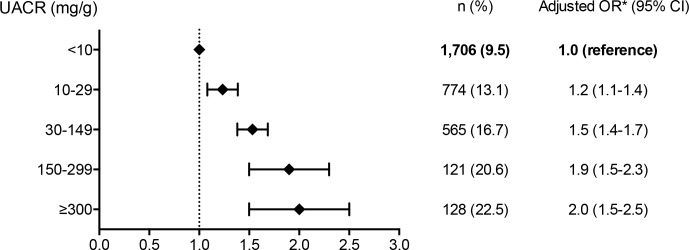
Table 4 shows that CKD was associated with a higher rate of DR (Odds ratio [OR], 1.5; 95% confidence interval [CI], 1.4–1.7). Patients with elevated UACR had higher rates of DR, particularly if UACR values were ≥300 mg/g (OR, 2.0; 95% CI, 1.6–2.5); this rate was lower in patients with eGFR levels 44 to 30 mL/min/1.73m2 (OR, 1.3; 95% CI, 1.1–1.6). Patients with UACR values 30 to 299 mg/gr had DR rate slightly higher than those with eGFR values of 30 to 44 mL/min/1.73m2 (OR, 1.5; 95% CI, 1.4–1.7, and OR, 1.3; 95% CI 1.1–1.6, respectively). When we analyzed the association between different levels of UACR and DR prevalence (Fig 1) we observed that DR prevalence rose with the increase of UACR levels, and this association was significant from UACR values 10 to 29 mg/g, (OR, 1.2; CI, 1.1–1.4), and increased considerably with UACR values ≥300 mg/g (OR, 2.0; 95% CI, 1.5–2.5).
Table 4. Types of chronic kidney disease and diabetic retinopathy.
| OR | (95% CI) | p-value | |
|---|---|---|---|
| UACR (mg/g)a | |||
| <30 | 1.0 | ||
| 30 to 299 | 1.5 | (1.4, 1.7) | <0.001 |
| ≥300 | 2.0 | (1.6, 2.5) | <0.001 |
| eGFR (mL/min / 1.73m2)a | |||
| ≥90 | 1.0 | ||
| [60, 89] | 1.0 | (0.9, 1.1) | 0.674 |
| [45, 59] | 1.2 | (1.0, 1.3) | 0.142 |
| [30, 44] | 1.3 | (1.1, 1.6) | 0.009 |
| <30 | 1.3 | (0.9, 1.9) | 0.193 |
aAdjusted by age, sex, age at retinography, duration type 2 diabetes, systolic blood pressure <140 mmHg, A1C ≤ 7%, smoking, cardiovascular disease, BMI, UACR and/or eGFR.
Model Diabetic Retinopathy (DR): without DR vs. any type DR (n = 26,897 patients)
DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; OR, odds ratio; UACR, urine albumin to creatinine ratio.
Fig 1. Urine albumin to creatinine ratio and prevalence of diabetic retinopathy.
*Adjusted by age, sex, age at retinography, duration type 2 diabetes, systolic blood pressure <140 mmHg, A1C ≤ 7%, smoking, cardiovascular disease, BMI, and eGFR. DR, diabetic retinopathy; OR, odds ratio; UACR, urine albumin to creatinine ratio.
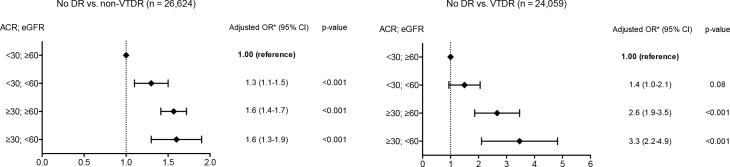
The prevalence of DR using the KDIGO combination of UACR and eGFR categories is shown in S1 Table. Moreover, Fig 2 shows the association between CKD and DR severity. Patients with eGFR <60 mL/min/1.73m2 and UACR <30 mg/g had an increased non-VTDR rate (OR 1.3; 95% CI 1.1–1.5) and a non-significant increase in VTDR (OR 1.4; 95% CI 1.0–2.1; p = 0.082). In individuals with UACR ≥30 mg/g and eGFR ≥60 mL/min/1.73m2 the rate of VTDR (OR 2.6; 95% CI 1.9–3.5) was higher than non-VTDR (OR 1.6; 95% CI 1.4–1.7).
Fig 2. Association of chronic kidney disease and diabetic retinopathy.
*Adjusted by sex, age at digital photography, duration of type 2 diabetes, systolic blood pressure <140 mmHg, and A1C ≤7%; DR, diabetic retinopathy; eGFR, estimated glomerular filtration rate; UACR, urine albumin to creatinine ratio; VTDR, vision threatening diabetic retinopathy (severe non proliferative DR, proliferative DR, and diabetic macular edema); non-VTDR, mild non-proliferative DR and moderate non-proliferative DR.
Discussion
Our study showed that high UACR level significantly increases the prevalence of diabetic retinopathy in type 2 diabetic patients, even after adjustment for variables, and this association was significant from UACR values ≥10 mg/g. These findings concur with the RIACE study cohort [21]. We observed that individuals with elevated UACR had a higher DR prevalence than those with decreased eGFR. A number of factors could have contributed to the renal and retinal tissue injury observed; including A1C levels, hypertension, dyslipidemia, diabetes duration, age at onset of DM2, smoking, and UACR levels [6, 10, 22, 23]. Patients with CKD are more likely to present with DR VTDR [24]. High UACR doubles the possibility of developing DR with respect to normal UACR levels, a risk that increases considerably with macroalbuminuria, even after adjusting for other factors [7, 14, 25]. In addition, an association was observed between low eGFR and greater prevalence of DR. Lu et al. [26] showed that a decrease in eGFR was significantly correlated with DR, after controlling for sex, age, and albuminuria staging. Wu J et al. [27] observed that, after the adjustment of the variables affecting the relationship between eGFR and DR, results of a univariate analysis suggested that eGFR remained significantly associated with DR. In our study we observed that eGFR levels <45 ml/min/1.73m2 were associated with higher DR prevalence, but we did not find this association with eGFR levels ≥45/ml/min/1.73 m2. This association was not significant with eGFR levels <30 mL/min/1.73m2, probably because there were few patients in this group.
Penno et al. [28] reported an association between UACR ≥300 mg/g and DR (OR 2.9; 95% CI, 2.1–4.0) higher than that observed in our work. Such a variation could be due to the different grouping of DR types. Nevertheless, in both studies elevated UACR had a greater association with DR than decreased eGFR.
CKD in DM2 is more heterogeneous than in type 1 diabetes, retinopathy and albuminuria were both absent in 30% of adults with DM2 and chronic renal insufficiency according to the Third National Health and Nutrition Survey [29]. Patients with diabetes are also susceptible to non diabetic renal disease [30]. A decrease in eGFR with normoalbuminuria in such patients sometimes may be linked to the presence of arteriosclerosis.
Recently, Lee et al. [31] showed a direct association between DR and CKD. The presence of CKD identified a group of DM2 patients of being at a greater risk of presenting macro and microvascular complications. Moreover, the presence of CKD and DR was associated with a more rapid reduction in renal function and greater mortality in this group of patients who might benefit from more aggressive treatment.
Our study should be interpreted with consideration of the following limitations. First, this study was a cross-sectional analysis; therefore, the data were recorded from an electronic database and diabetic patients with DR-VTDR, who were attended by an ophthalmologist or endocrinologist, could have been poorly represented. Second, because of the small number of cases in some categories, we could not carry out the final analysis using all KDIGO categories. Despite these limitations, this study used a nationally representative sample of DM2 patients. Moreover, to the best of our knowledge, this is the first large population based study to examine the association between CKD, decreased eGFR and high UACR, and DR among a representative Catalonian, Spain, DM2 population.
Conclusions
CKD appears to be associated with DR in this DM2 population. Elevated UACR had a greater association with DR prevalence than decreased eGFR, but both were associated with DR in DM2 patients.
Supporting Information
(DOCX)
Acknowledgments
This study was supported by a grant from the IDIAP J. Gol and the Education and Research in Family Practice Chair (UAB-Novartis). We acknowledge Mònica Gratacòs and Amanda Prowse for providing support in the manuscript preparation and editing. CIBER of Diabetes and Associated Metabolic Diseases (CIBERDEM) is an initiative from Instituto de Salud Carlos III.
Data Availability
Due to ethical restrictions, data are available upon request. The data contain identifying human information and are unsuitable for public deposition. Requests may be made to the corresponding author.
Funding Statement
This study was supported by a grant from the IDIAP J. Gol (SIDIAP-2013 from Fundacio J Gol i Gurina, www.idiapjordigol.com) and the Education and Research in Family Practice Chair (UAB-Novartis). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Mathiesen ER, Ronn B, Storm B, Foght H, Deckert T. The natural course of microalbuminuria in insulin-dependent diabetes: a 10-year prospective study. Diabet Med. 1995;12(6): 482–487. [DOI] [PubMed] [Google Scholar]
- 2.The Diabetes Control and Complications (DCCT) Research Group. Effect of intensive therapy on the development and progression of diabetic nephropathy in the Diabetes Control and Complications Trial. Kidney Int. 1995;47(6): 1703–1720. [DOI] [PubMed] [Google Scholar]
- 3.UK Prospective Diabetes Study (UKPDS) Group. Effect of intensive blood-glucose control with metformin on complications in overweight patients with type 2 diabetes (UKPDS 34). Lancet. 1998;352(9131): 854–865. [PubMed] [Google Scholar]
- 4.Krolewski AS, Warram JH, Rand LI, Christlieb AR, Busick EJ, Kahn CR. Risk of proliferative diabetic retinopathy in juvenile-onset type I diabetes: a 40-yr follow-up study. Diabetes Care. 1986;9(5): 443–452. [DOI] [PubMed] [Google Scholar]
- 5.Parving HH, Hommel E, Mathiesen E, Skott P, Edsberg B, Bahnsen M, et al. Prevalence of microalbuminuria, arterial hypertension, retinopathy and neuropathy in patients with insulin dependent diabetes. Br Med J (Clin Res Ed). 1988;296(6616): 156–160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stratton IM, Kohner EM, Aldington SJ, Turner RC, Holman RR, Manley SE, et al. UKPDS 50: risk factors for incidence and progression of retinopathy in Type II diabetes over 6 years from diagnosis. Diabetologia. 2001;44(2): 156–163. [DOI] [PubMed] [Google Scholar]
- 7.Manaviat MR, Afkhami M, Shoja MR. Retinopathy and microalbuminuria in type II diabetic patients. BMC Ophthalmol. 2004;4: 9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Estacio RO, McFarling E, Biggerstaff S, Jeffers BW, Johnson D, Schrier RW. Overt albuminuria predicts diabetic retinopathy in Hispanics with NIDDM. Am J Kidney Dis. 1998;31(6): 947–953. [DOI] [PubMed] [Google Scholar]
- 9.Wolf G, Müller N, Mandecka A, Müller UA. Association of diabetic retinopathy and renal function in patients with types 1 and 2 diabetes mellitus. Clin Nephrol. 2007;68: 81–6. [DOI] [PubMed] [Google Scholar]
- 10.Pedro RA, Ramon SA, Marc BB, Juan FB, Isabel MM. Prevalence and relationship between diabetic retinopathy and nephropathy, and its risk factors in the North-East of Spain, a population-based study. Ophthalmic Epidemiol. 2010;17(4): 251–265. 10.3109/09286586.2010.498661 [DOI] [PubMed] [Google Scholar]
- 11.Sabanayagam C, Foo VH, Ikram MK, Huang H, Lim SC, Lamoureux EL, et al. Is chronic kidney disease associated with diabetic retinopathy in Asian adults?. J Diabetes. 2014;6: 556–63. 10.1111/1753-0407.12148 [DOI] [PubMed] [Google Scholar]
- 12.Satchell SC, Tooke JE. What is the mechanism of microalbuminuria in diabetes: a role for the glomerular endothelium?. Diabetologia. 2008;51: 714–25. 10.1007/s00125-008-0961-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Man RE, Sasongko MB, Wang JJ, MacIsaac R, Wong TY, Sabanayagam C, et al. The Association of Estimated Glomerular Filtration Rate With Diabetic Retinopathy and Macular Edema. Invest Ophthalmol Vis Sci. 2015;56: 4810–6. 10.1167/iovs.15-16987 [DOI] [PubMed] [Google Scholar]
- 14.Chen YH, Chen HS, Tarng DC. More impact of microalbuminuria on retinopathy than moderately reduced GFR among type 2 diabetic patients. Diabetes Care. 2012;35(4): 803–808. 10.2337/dc11-1955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care. 2010;33 Suppl 1: S62–S69. 10.2337/dc10-S062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Vinagre I, Mata-Cases M, Hermosilla E, Morros R, Fina F, Rosell M, et al. Control of glycemia and cardiovascular risk factors in patients with type 2 diabetes in primary care in Catalonia (Spain). Diabetes Care. 2012;35(4): 774–779. 10.2337/dc11-1679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bolibar B, Fina Aviles F, Morros R, Garcia-Gil Mdel M, Hermosilla E, Ramos R, et al. SIDIAP database: electronic clinical records in primary care as a source of information for epidemiologic research. Med Clin (Barc). 2012;138(14): 617–621. [DOI] [PubMed] [Google Scholar]
- 18.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, et al. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150(9): 604–612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lopez-Bastida J, Cabrera-Lopez F, Serrano-Aguilar P. Sensitivity and specificity of digital retinal imaging for screening diabetic retinopathy. Diabet Med. 2007;24(4): 403–407. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson CP, Ferris FL 3rd, Klein RE, Lee PP, Agardh CD, Davis M, et al. Proposed international clinical diabetic retinopathy and diabetic macular edema disease severity scales. Ophthalmology. 2003;110(9): 1677–1682. [DOI] [PubMed] [Google Scholar]
- 21.Pugliese G, Solini A, Bonora E, Orsi E, Zerbini G, Fondelli C, et al. Distribution of cardiovascular disease and retinopathy in patients with type 2 diabetes according to different classification systems for chronic kidney disease: a cross-sectional analysis of the renal insufficiency and cardiovascular events (RIACE) Italian multicenter study. Cardiovasc Diabetol. 2014;13: 59 10.1186/1475-2840-13-59 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Adler AI, Stratton IM, Neil HA, Yudkin JS, Matthews DR, Cull CA, et al. Association of systolic blood pressure with macrovascular and microvascular complications of type 2 diabetes (UKPDS 36): prospective observational study. BMJ. 2000;321(7258): 412–419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong TY, Cheung N, Tay WT, Wang JJ, Aung T, Saw SM, et al. Prevalence and risk factors for diabetic retinopathy: the Singapore Malay Eye Study. Ophthalmology. 2008;115(11): 1869–1875. 10.1016/j.ophtha.2008.05.014 [DOI] [PubMed] [Google Scholar]
- 24.Harris Nwanyanwu K, Talwar N, Gardner TW, Wrobel JS, Herman WH, Stein JD. Predicting development of proliferative diabetic retinopathy. Diabetes Care. 2013;36(6): 1562–1568. 10.2337/dc12-0790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ra H, Yoo JH, Ban WH, Song HC, Lee SS, Kim SR, et al. Predictors for diabetic retinopathy in normoalbuminuric people with type 2 diabetes mellitus. Diabetol Metab Syndr. 2012;4(1): 29 10.1186/1758-5996-4-29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lu B, Song X, Dong X, Yang Y, Zhang Z, Wen J, et al. High prevalence of chronic kidney disease in population-based patients diagnosed with type 2 diabetes in downtown Shanghai. J Diabetes Complications. 2008;22(2): 96–103. 10.1016/j.jdiacomp.2007.08.001 [DOI] [PubMed] [Google Scholar]
- 27.Wu J, Geng J, Liu L, Teng W, Liu L, Chen L. The Relationship between Estimated Glomerular Filtration Rate and Diabetic Retinopathy. J Ophthalmol. 2015;2015: 326209 10.1155/2015/326209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Penno G, Solini A, Zoppini G, Orsi E, Zerbini G, Trevisan R, et al. Rate and determinants of association between advanced retinopathy and chronic kidney disease in patients with type 2 diabetes: the Renal Insufficiency And Cardiovascular Events (RIACE) Italian multicenter study. Diabetes Care. 2012;35(11): 2317–2323. 10.2337/dc12-0628 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer HJ, Nguyen QD, Curhan G, Hsu CY. Renal insufficiency in the absence of albuminuria and retinopathy among adults with type 2 diabetes mellitus. JAMA. 2003;289(24): 3273–3277. [DOI] [PubMed] [Google Scholar]
- 30.Tone A, Shikata K, Matsuda M, Usui H, Okada S, Ogawa D, et al. Clinical features of non-diabetic renal diseases in patients with type 2 diabetes. Diabetes Res Clin Pract. 2005;69(3): 237–242. [DOI] [PubMed] [Google Scholar]
- 31.Lee WJ, Sobrin L, Kang MH, Seong M, Kim YJ, Yi JH, et al. Ischemic diabetic retinopathy as a possible prognostic factor for chronic kidney disease progression. Eye (Lond). 2014;28(9): 1119–1125. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
Data Availability Statement
Due to ethical restrictions, data are available upon request. The data contain identifying human information and are unsuitable for public deposition. Requests may be made to the corresponding author.