Abstract
Aging results in a loss of muscle mass and strength. Myoblasts play an important role in maintaining muscle mass through regenerative processes, which are impaired during aging. Vitamin E potentially ameliorates age-related phenotypes. Hence, this study aimed to determine the effects of the tocotrienol-rich fraction (TRF) and α-tocopherol (ATF) in protecting myoblasts from replicative senescence and promoting myogenic differentiation. Primary human myoblasts were cultured into young and senescent stages and were then treated with TRF or ATF for 24 h, followed by an analysis of cell proliferation, senescence biomarkers, cellular morphology and differentiation. Our data showed that replicative senescence impaired the normal regenerative processes of myoblasts, resulting in changes in cellular morphology, cell proliferation, senescence-associated β-galactosidase (SA-β-gal) expression, myogenic differentiation and myogenic regulatory factors (MRFs) expression. Treatment with both TRF and ATF was beneficial to senescent myoblasts in reclaiming the morphology of young cells, improved cell viability and decreased SA-β-gal expression. However, only TRF treatment increased BrdU incorporation in senescent myoblasts, as well as promoted myogenic differentiation through the modulation of MRFs at the mRNA and protein levels. MYOD1 and MYOG gene expression and myogenin protein expression were modulated in the early phases of myogenic differentiation. In conclusion, the tocotrienol-rich fraction is superior to α-tocopherol in ameliorating replicative senescence-related aberration and promoting differentiation via modulation of MRFs expression, indicating vitamin E potential in modulating replicative senescence of myoblasts.
Introduction
Sarcopenia is a geriatric syndrome that is characterized by a dramatic loss of skeletal muscle mass and strength in advancing age. Although the underlying mechanism of these alterations is not clear, several risk factors have been considered, such as immobilization, chronic diseases, hormone and pro-inflammatory cytokine shift and malnutrition in the elderly [1]. Loss of muscle regenerative capacity has been suggested as one of the possible contributory factors of this age-related muscle deterioration [2].
Skeletal muscle has an established regeneration competency in restoring and maintaining muscle mass when muscle cells undergo injury [3]. Muscle regeneration essentially involves four sequential and overlapping phases: degeneration, inflammation, regeneration and remodeling. Satellite cells are the key regenerative phase and will be activated, proliferate and differentiate in response to stimuli. Proliferating satellite cells are known as myoblasts [4]. In addition to producing functional progeny via differentiation, satellite cells can replicate to maintain the satellite cell pool; thus, they are also categorized as muscle stem cells [5]. The heterogeneity of satellite cells has provoked the rationale of targeting these cells for therapeutic purposes in ameliorating age-related sarcopenia or pathological dystrophic muscle [6].
In aging, satellite cells malfunction and fail to sustain their normal quiescent state, irrevocably influencing their regenerative and self-renewal capacities [7]. A decreased number of satellite cells in old age were also observed [6,8]. However, this decrease may not be the sole reason for the gradual loss of muscle rejuvenation capacity in old age. In fact, a permissive atmosphere is imperative rather than the number of satellite cells, whereby satellite cells from old muscle can be engaged for myogenic activity when exposed to a young systemic environment [9–12].
Myogenic differentiation is regulated by a family of myogenic regulatory factors (MRFs) that includes MyoD, Myf5, Myogenin and MRF4. MRFs are transcription factors with a basic helix–loop–helix (bHLH) central domain that assist protein interactions and DNA binding to activate muscle-specific genes [4]. The deregulation of Myf5, MyoD and myogenin at an early stage of differentiation is interrelated with the differentiation capability of senescent myoblasts, resulting in the formation of smaller myotubes that resemble the condition in sarcopenia [13,14]. Thus, ongoing research in finding ways to restore the regenerative capacity in old myoblasts will presumably provide precious insight for combating muscle atrophy in aging or degenerative diseases.
Because muscle atrophy or aging itself is closely related to oxidative stress, the re-establishment of redox balance should be potentially advantageous in the amelioration of age-related muscle wasting [15,16]. Vitamin E is a lipid-soluble vitamin that is able to scavenge free radicals, boosts cellular antioxidant competency and prevents oxidative damage. There are two subgroups of vitamin E: tocopherols and tocotrienols [17]. Howard et al. reported that α-tocopherol (ATF) was able to repair the laser-induced disrupted membrane of myoblasts, which supports a therapeutic effect exerted by vitamin E [18]. A significant correlation between the ATF level and sarcopenia indicators among the elderly has been reported [19]. Vitamin E deficiency will not only affect muscle performance but also accelerate the progression of aging [20]. Therefore, it is rational to introduce antioxidants, such as vitamin E, to prevent sarcopenia, even though further studies are still required [16].
Human myoblasts can be isolated and cultured in vitro with a limited proliferation capacity, whereby at a certain stage, they will undergo growth arrest, termed replicative senescence [21]. The present study was designed to elucidate the effects of the tocotrienol-rich fraction (TRF) and α-tocopherol (ATF) in ameliorating senescent myoblasts and promoting myogenic differentiation during replicative senescence.
Methods
Cell Culture and Replicative Senescence Model
Primary human myoblasts (Human Skeletal Muscle Myoblasts; HSMM) at passage 2 from two donors, a 17-year-old Caucasian female and a 16-year-old Caucasian male were purchased from Lonza (Walkersville, MD, USA). Briefly, myoblasts were cultured in Skeletal Muscle Basal Medium (SkBM) that was supplemented with human epidermal growth factor, fetal bovine serum, dexamethasone, L-glutamine, and gentamicin sulfate/amphotericin B (Lonza, Walkersville, MD USA). Cells were cultivated at 37°C in a humid atmosphere containing 5% CO2. The myoblasts then underwent serial passaging to reach senescence. For each passage, the population doublings (PD) of cells was calculated as: In (N/n)/In2, where N is the number of cells at harvest stage, and n is the number of cells at seeding stage [14]. The starting PD in this study was 8. The cells achieved replicative senescence when they were unable to proliferate within 10 days in culture, even with consecutive replenishment.
Analysis of Cell Morphology and Myogenic Purity
Myogenic purity and myoblasts morphology were observed by the immunocytochemistry method using a mouse monoclonal anti-Desmin antibody (D33; Dako, Produktionsvej, Denmark). Myoblasts were plated in μ-Slide 8 well (ibidi, Martinsried, Germany) at a density of 1×104 cells per well. The cells were fixed in cold ethanol. Then, anti-Desmin antibody (1:50) and Alexa Fluor 488 goat anti-mouse (Life Technologies, Carlsbad, CA, USA) were used to incubate the myoblasts in sequence. Nuclei were visualized using Hoechst 33342 (Life Technologies, Carlsbad, USA). The slides were then viewed under a Confocal Laser Scanning Microscope Leica TCS SP5 II, and data were acquired using LAS AF version Lite 2.6 software (Leica Microsystems, Wetzlar, Germany). To determine the percentage of desmin-positive cells, a minimum of 50 cells were counted in three independent cultures. In addition, the morphological changes of myoblasts were observed, while the width and length of myoblasts were visualized and measured using LAS AF version Lite 2.6 software (Leica Microsystems, Wetzlar, Germany). For each group of cells, at least 30 cells were analyzed.
Determination of DNA Synthesis in Proliferating Cells
The amount of 5-bromo-2’-deoxyuridine (BrdU) incorporation indicates the total proliferating cells. Thus, the cell proliferation ELISA, BrdU (colorimetric) kit (Roche, Penzberg, Germany) was used to determine the effects of replicative senescence, TRF and ATF on cell proliferation. This immunoassay was performed according to the manufacturer’s instructions. The cells were labeled with BrdU, a pyrimidine analog that will incorporate into the DNA and was detected by a microtiter plate reader (VersaMax Molecular Devices, USA) at 450 nm with reference to 690 nm.
Determination of Senescence Biomarkers
The expression of SA-β-gal was determined as described by Dimri et al. [22] in order to confirm the presence of senescent myoblasts. This process was carried out using a Senescent Cell Histochemical Staining Kit (Sigma-Aldrich, St. Louis, Missouri, USA) according to the manufacturer’s instructions. Cells were incubated in staining solution for 8 hours at 37°C in the absence of CO2 before analysis. At least 100 cells were observed, and the percentage of blue stained cells was calculated. In addition, the morphological changes of myoblasts were also observed.
Preparation of Vitamin E Treatments
TRF Gold Tri E 70 (Sime Darby Sdn. Bhd., Selangor, Malaysia) and ATF (Malaysian Palm Oil Board, Selangor, Malaysia) were used as treatments in this study. Briefly, stock solutions of TRF were freshly prepared in 100% ethanol (1:1) and kept at −20°C for no more than one month. A similar process was applied for ATF preparation. TRF and ATF were then incubated overnight with fetal bovine serum at 37°C before use. The cell viability was assessed with a CellTiter 96® Aqueous Non-Radioactive Cell Proliferation Assay (MTS; Promega, Madison, WI USA) according to the manufacturer’s instruction. Various concentrations of TRF or ATF were used to treat the cells for 24 hours. Then, MTS was added and further incubated for 2 hours. The absorbance of MTS formazan was measured at 490 nm with a microtiter plate reader (VersaMax Molecular Devices, USA). The optimum dose of treatments was used for subsequent experiments.
Induction of Myogenic Differentiation
To induce muscle cell differentiation, the proliferating medium SkBM was replaced with DMEM:F12 (Lonza, Walkersville, MD USA) that was supplemented with 2% horse serum (ATCC, Baltimore, USA). The differentiation medium was changed every 2 days until the desired day of differentiation for parameter measurement.
Analysis of Myogenic Differentiation
To evaluate the efficiency of differentiation, a micro-insert 4 well, μ-Dish was used (ibidi, Martinsried, Germany) to culture the cells to determine myotubes formation. After 9 days of differentiation, myotubes were stained using an anti-Desmin antibody. The fusion index and the size of myotubes were calculated, indicating myotube formation. The formula below was used to calculate the fusion index, and a minimum of 50 nuclei were counted in 3 different randomly chosen optical fields.
To determine the size of the myotubes, the number of nuclei per myotube was counted in a minimum of 11 multinucleated cells in 3 different randomly chosen optical fields.
Determination of MRFs at an Early Phase of Myogenic Differentiation
At days 0, 1 and 2 of differentiation, total RNA was extracted using the TRI reagent (Molecular Research Center Inc., Ohio, USA). For gene expression determination, quantitative real-time RT-PCR (qRT-PCR) was used. The expression of MYF5, MYOD1 and MYOG mRNA was quantitatively analyzed using a one-step qRT-PCR technique. qRT-PCR was performed with 100 ng of total RNA, 400 nM each primer and KAPA SYBR FAST One-Step qPCR kit (Kapa Biosystems, Boston, Massachusetts, USA) according to the manufacturer’s instructions. The primer sequences are GAPDH forward 5’-TCCCTGAGCTGAACGGGAAG-3’, GAPDH reverse 5’-GGAGGAGTGGGTGTCGCTGT-3’, MYF5 forward 5’-TCACCTCCTCAGAGCAACCT-3’, MYF5 reverse 5’-ATTAGGCCCTCCTGGAAGAA-3’, MYOD1 forward 5’-CGCCAGGATATGGAGCTACT-3’, MYOD1 reverse 5’-GAGTGCTCTTCGGGTTTCAG-3’, MYOG forward 5’-CAGTGCCATCCAGTACATCG-3’ and MYOG reverse 5’-AGGTTGTGGGCATCTGTAGG-3’. The master mix was prepared, and PCR reactions were carried out in a Bio-Rad iQ5 Cycler (Hercules, CA, USA) with the following programmed reaction profile: cDNA synthesis for 5 min at 42°C; pre-denaturation for 4 min at 95°C; and PCR amplification for 40 cycles of 3 sec at 95°C and 20 sec at 60°C. These reactions were followed by a melt curve analysis to determine the reaction specificity and the expression of each targeted gene. The expression level of each targeted gene was normalized to that of glyceraldehyde 3-phosphate dehydrogenase (GAPDH). The relative expression value (REV) was calculated using the 2-ΔΔCt method of relative quantification and the following equation: REV = 2 Ct value of GAPDH -Ct value of the gene of interest. Then, the fold change of expression was determined.
Determination of Myogenin Expression
At day 3 of differentiation, the number of cells expressing myogenin was estimated using a mouse monoclonal anti-myogenin antibody (F5D, Dako, Produktionsvej, Denmark) at a 1:20 dilution overnight at 4°C. Alexa Fluor 488 was used as the secondary antibody. Nuclei were visualized using Hoechst 33342. The cells were observed under an EVOS FL Digital Inverted Fluorescence Microscope (Life Technologies, Carlsbad, USA).
Assessment of Intracellular Free Radical Generation
In order to measure free radicals generation by myoblasts, we used two types of dyes, i.e. dihydroethidium (DHE) and 5-(and-6)-carboxy-2′,7′-dichlorodihydrofluorescein diacetate (carboxy-H2DCFDA) (Molecular Probes, Eugene, OR, USA). The DHE-stained cells indicated oxidation by superoxide anion, while carboxy-H2DCFDA is oxidized by hydrogen peroxide (H2O2), peroxynitrite or hydroxyl radical. Superoxide anions may contribute to carboxy-H2DCFDA oxidation albeit at a lesser degree. Briefly, myoblasts were incubated in 20 μM of DHE and 40 μM of carboxy-H2DCFDA for 45 min. After that, the cells were washed with PBS and recovered in medium for 30 minutes. Then, we measured the intensity by using microplate reader (Infinite® 200, Tecan, USA) at excitation/emission wavelength (Ex/Em) 518/600 nm and 488/521 nm respectively.
Statistical Analysis
Statistical analyses were performed using SPSS 17.0 software (IBM, NY, USA). All of the data are reported as the means ± standard deviation (SD) from at least three replicates. For all of the tests, p<0.05 was considered statistically significant. To determine significance between two treatment groups, comparisons were made using an independent T-test, while ANOVA was used to analyze multiple groups, followed by a post-hoc Tukey HSD or LSD (if equal variance was assumed) and Dunnett T3 (if equal variance was not assumed) tests.
Results
Replicative Senescence Model of Myoblasts: Characteristics and Proliferation
To elucidate the effects of aging on myoblasts, we expanded the cells until replicative senescence. The lifespan curve that was plotted based on their cumulative PD showed that myoblasts have a limited proliferative capacity, and cell growth was halted at 21 divisions in culture (Fig 1a). Based on BrdU incorporation, the proliferation of myoblasts decreased with increasing total PD, whereby the percentage of BrdU incorporation at PD18 and PD21 was significantly different compared to the percentage of BrdU incorporation at PD14 (p<0.05) (Fig 1b). The percentage of SA-β-gal-stained cells increased with the serial passaging of myoblasts, which was significantly higher at PD21 compared to both PD14 and PD18 (p<0.05) (Fig 1c). Therefore, myoblasts are considered young at PD<15 and senescent at PD>20. No loss of myogenicity was observed during the replicative senescence of myoblasts, as indicated by the presence of desmin in 96% of the cell population (Table 1), allowing a reliable statistical comparison of these cells.
Fig 1. Effects of serial passaging on population doubling, cell proliferation and expression of SA-β-gal in myoblasts.
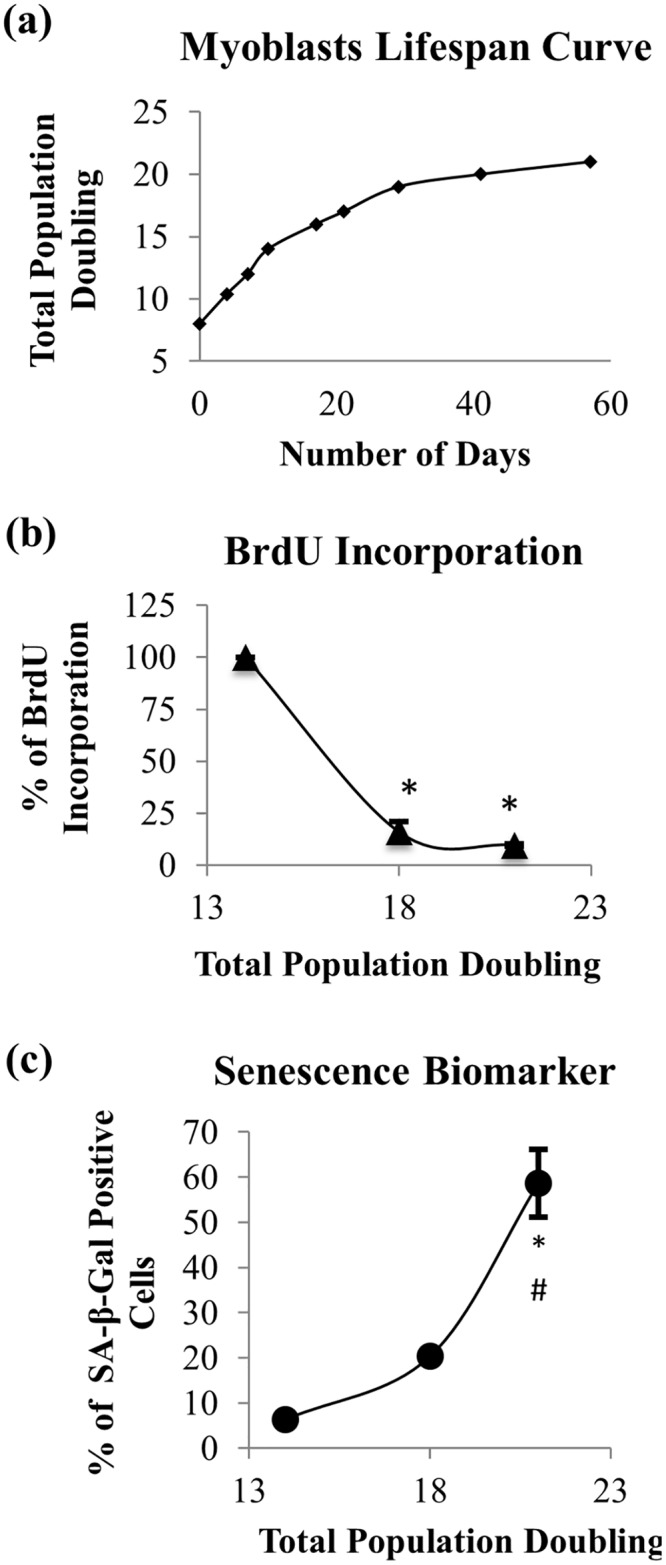
During extensive expansion, myoblasts significantly lost their proliferation capacity as represented by a hyperbolic proliferative lifespan curve (a) and decreasing percentage of BrdU incorporation (b), while the percentage of senescent cells increased, as represented by positive SA-β-gal staining (c). For (b) and (c), the data are presented as the means ± SD, n = 3. *p<0.05 compared to myoblasts at PD14 (young), #p<0.05 compared to myoblasts at PD18 (pre-senescent) with a post-hoc Dunnett T3.
Table 1. Percentage of desmin-positive cells in different cell stages.
| Myoblasts | Young(PD14) | Presenescent(PD18) | Senescent(PD21) |
|---|---|---|---|
| Desmin +ve | 96.67±2.31(n = 3) | 98.67±1.15(n = 3) | 96.00±2.00(n = 3) |
Promotion of Cell Viability and Proliferation
Incubation with various concentrations of TRF or ATF for 24 h significantly increased the viability of young and senescent myoblasts (Fig 2a and 2b). Cells that were treated with TRF and ATF at concentrations of 50 μg/ml, exhibited the greatest percentage of viability in both myoblasts. Therefore, in the subsequent experiments, 50 μg/ml TRF and 50 μg/ml ATF were used for treatment in young and senescent myoblasts. Prolonged treatment (48 hours) using the optimal dose (50 μg/ml) improved cell viability in young cells, but not in senescent myoblasts (Fig 2c). Subsequently, the optimal dose was applied on senescent myoblasts from the other donor (a 16-year-old Caucasian male). However, there was no significant different observed on the cell viability as compared to the viability of myoblast from the first donor (a 17-year-old Caucasian female) (Fig 2d). Therefore, for the following experiment, myoblasts from the first donor was used.
Fig 2. Effects of the TRF and ATF treatments on cell viability and proliferation.
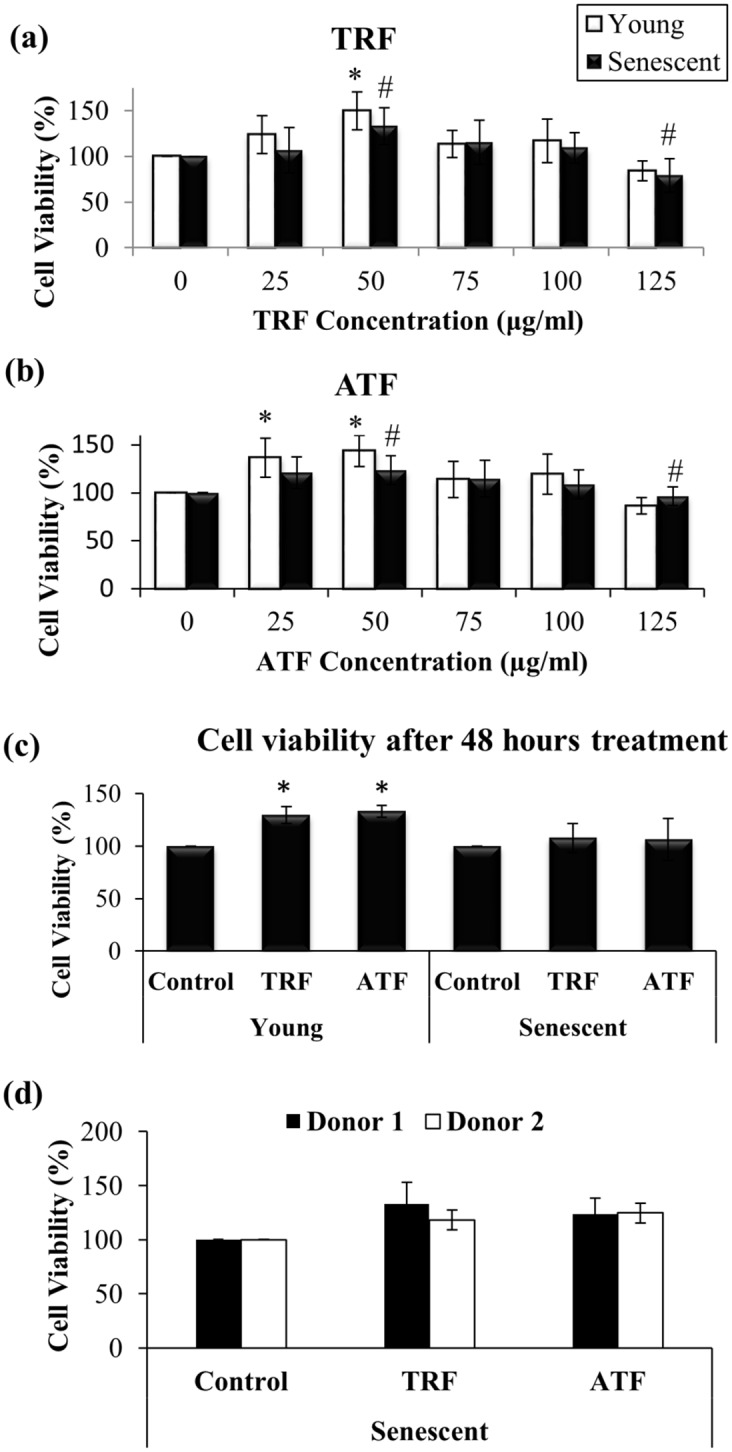
Dose-response curve of TRF (a) and ATF (b) treatments (24 h) in young and senescent myoblasts (n = 9). The prolonged treatments of TRF and ATF (48 h) at optimal dose were unlikely to further improve cell viability in senescent myoblasts (c). Therefore, 24 h treatment was used in subsequent experiment. Comparison of cell viability between myoblasts from donor 1, a 17 year-old, female Caucasian and donor 2, a 16 year-old male Caucasian (d). There were no significant different observed between the two cell lines in response to both TRF and ATF treatments based on the viability assessment. Post-hoc Dunnett T3 test for (a) and (b). *p<0.05 significantly different compared to untreated young myoblasts, #p<0.05 significantly different compared to untreated senescent myoblasts, §p<0.05 significantly different compared to TRF-treated senescent myoblasts. The data are presented as the means ± SD.
A significantly decreased percentage of BrdU incorporation was observed in senescent myoblasts compared to young cells (p<0.05). Treatment with TRF increased the percentage of BrdU incorporation in senescent myoblasts (p<0.05) (Fig 3), while no significant difference was observed in young myoblasts that were treated with TRF or ATF.
Fig 3. Effects of TRF and ATF on BrdU incorporation.
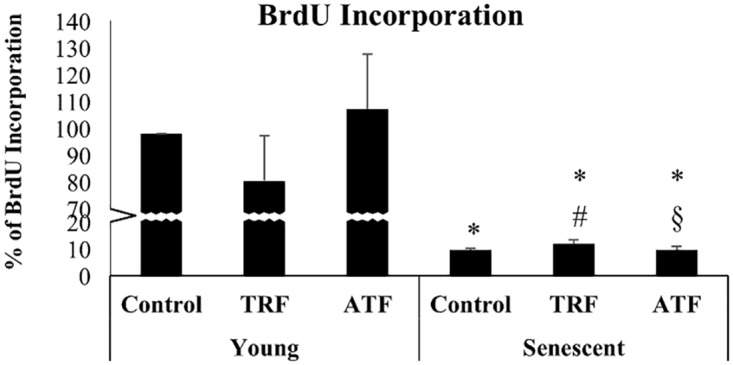
Cells were treated with optimal dose of TRF and ATF followed by cell proliferation determination based on the percentage of BrdU incorporation (n = 3). Only TRF-treated senescent myoblasts showed increased BrdU incorporation indicating promotion of cell proliferation and DNA synthesis with TRF treatment. *p<0.05 significantly different compared to untreated young myoblasts, #p<0.05 significantly different compared to untreated senescent myoblasts, §p<0.05 significantly different compared to TRF-treated senescent myoblasts, with post-hoc LSD test. The data are presented as the means ± SD.
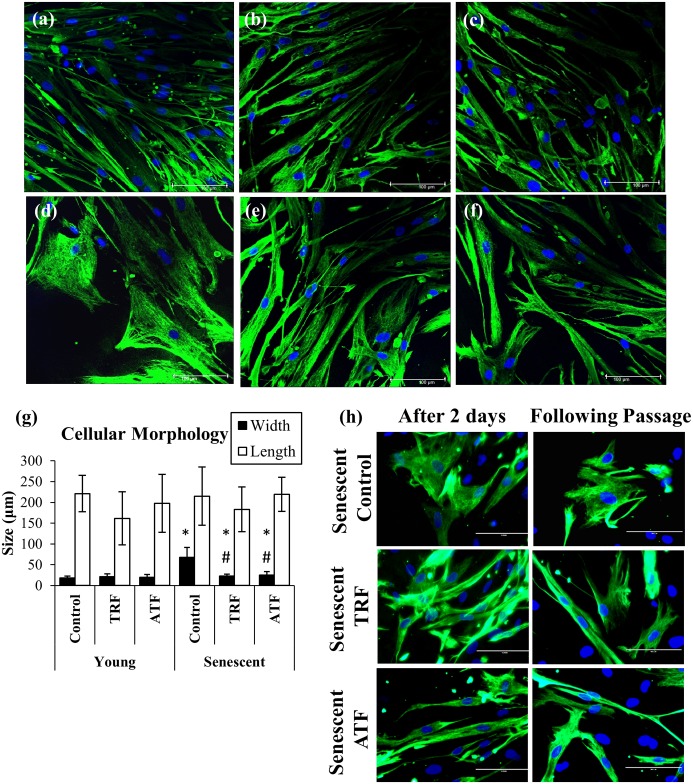
Improvement in Myoblasts Cellular Morphology with TRF and ATF Treatment
Myoblasts were spindle shaped when young but transformed into large and flat cells with a prominent intermediate filament network at the senescent stage (Fig 4a and 4d). Senescent cells exhibited a significantly higher ratio of cytoplasm to nucleus content than did young cells, as manifested by increased width during replicative senescence (p<0.05) (Fig 4g). However, both TRF- and ATF-treated senescent myoblasts retrieved the young-like morphology with the presence of more spindle-shaped cells (Fig 4e and 4f). Moreover, the average width of senescent myoblasts that were treated with both TRF and ATF significantly decreased compared to that of the untreated control (p<0.05) (Fig 4g). The spindle-shaped cells can be maintained in culture for two days after withdrawal of treatments and being observed in the following passage (Fig 4h).
Fig 4. Effects of replicative senescence and vitamin E treatment on myoblasts phenotype.
The photomicrographs of myoblasts were taken from a young control (a), TRF-treated young (b), ATF-treated young (c), senescent control (d), TRF-treated senescent (e) and ATF-treated senescent (f) cells (magnification: 400×). Myoblasts were stained for desmin (green) and Hoechst (blue). Both TRF- and ATF-treated senescent myoblasts resembled the morphology of young cells. The width and length of cells were measured (g). The width of senescent myoblasts significantly increased in the untreated control and decreased with TRF and ATF treatment. The spindle-shaped cells can be maintained in culture for two days after withdrawal of treatments and retained in the following passage (h). *p<0.05 significantly different compared to untreated young myoblasts, #p<0.05 significantly different compared to untreated senescent myoblasts, with post-hoc Dunnett T3. The data are presented as the means ± SD, n = 30.
Reversal of Replicative Senescence by TRF and ATF
Senescent myoblasts were stained positive for SA-β-gal (Fig 5d–5f). The percentage of SA-β-gal-positive cells is shown in Fig 5g. The SA-β-gal-positive cells were markedly increased in senescent myoblasts (58.67% ± 7.5) compared to young cells (6.33% ± 1.2) (p<0.05). The percentage of SA-β-gal-positive cells significantly decreased to 29.67% ± 3.8 and 32.67% ± 4.0 (p<0.05) with TRF and ATF treatment compared to the untreated groups.
Fig 5. Effects of replicative senescence and vitamin E treatment on senescence biomarker.
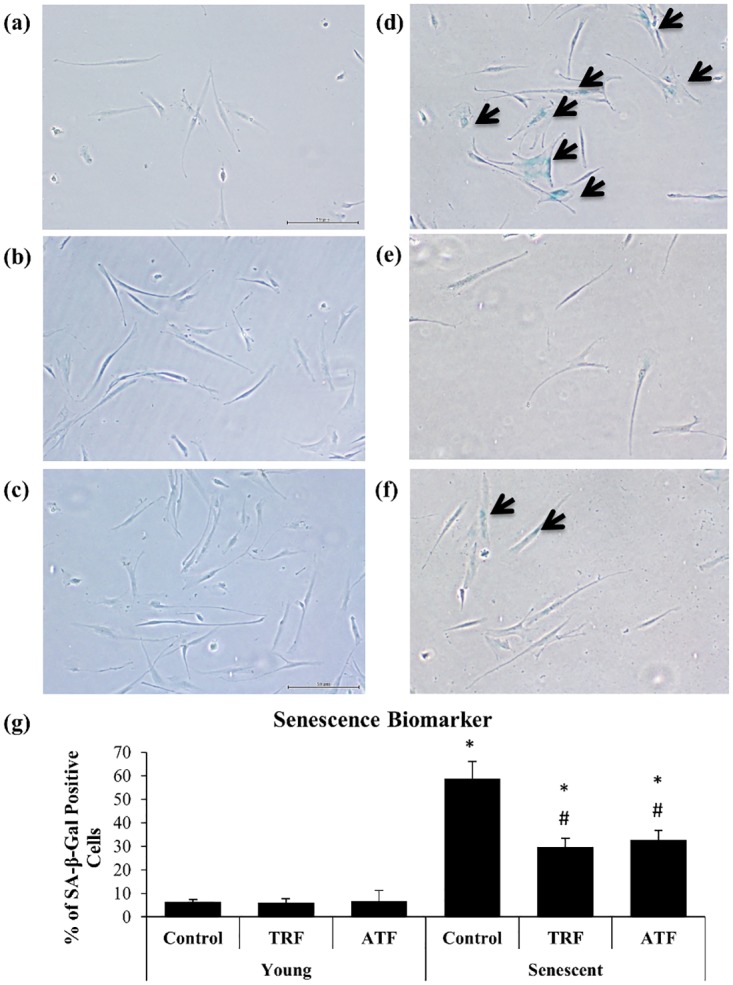
The photomicrographs of myoblasts were taken from young control (a), TRF-treated young (b), ATF-treated young (c), senescent control (d), TRF-treated senescent (e) and ATF-treated senescent (f) cells (magnification: 40×). Most of the senescent control myoblasts were stained positive for SA-β-gal (blue stained), as indicated by the arrow. The percentage of blue-stained cells was determined (g). TRF and ATF significantly reduced the number of blue-stained cells of senescent myoblasts. *p<0.05 significantly different compared to untreated young myoblasts, #p<0.05 significantly different compared to untreated senescent myoblasts, with post-hoc Tukey HSD. The data are presented as the means ± SD, n = 3.
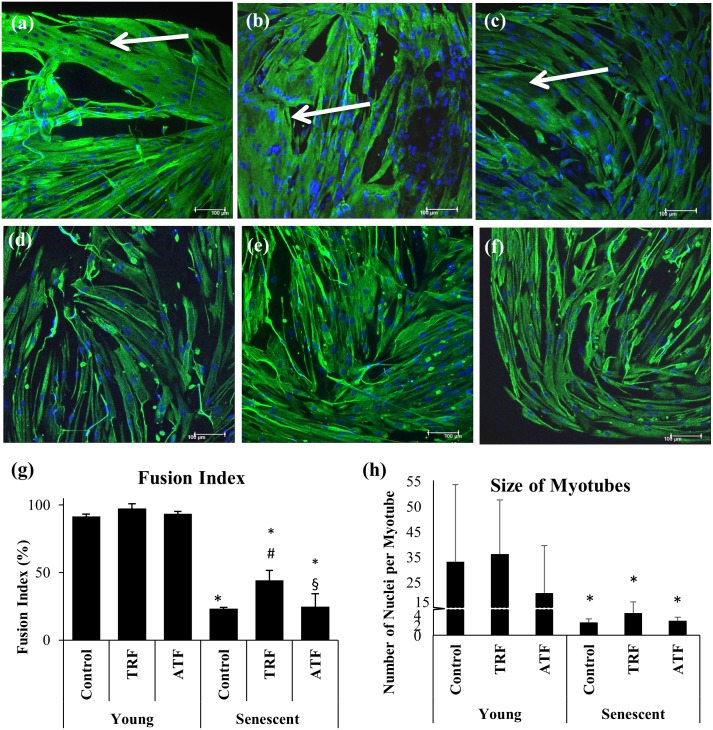
Superior Effects of TRF in Promoting Cell Differentiation in Senescent Myoblasts
Young and senescent myoblasts were allowed to differentiate for 9 days to form myotubes. Young myoblasts fused together, forming large and branched multinucleated myotubes (Fig 6a). Senescent cells, however, formed smaller myotubes with fewer branches compared to young cells (Fig 6d), indicating an inefficient differentiation process during the replicative senescence of myoblasts, which is similar to sarcopenic muscle.
Fig 6. Effects of replicative senescence and vitamin E treatment on the differentiation capacity of myoblasts.
The photomicrographs of myotubes were taken from young control (a), TRF-treated young (b), ATF-treated young (c), senescent control (d), TRF-treated senescent (e) and ATF-treated senescent (f) cells (magnification: 200×). Desmin was stained green, and the nuclei were stained blue (Hoechst). The myotubes that formed from young myoblasts were significantly bigger than the myotubes from senescent myoblasts. The fusion index (g) and the size of myotubes (h) were determined to evaluate the efficiency of muscle differentiation. TRF significantly increased the fusion index (n = 3), which was not shown with ATF treatment. No changes was observed in the size of the myotubes that formed (n = 12) with the TRF and ATF treatments. *p<0.05 significantly different compared to young control, #p<0.05 significantly different compared to senescent control, §p<0.05 significantly different compared to TRF-treated senescent myoblasts, with post-hoc Tukey HSD. The data are presented as the means ± SD.
A significant decrease in the fusion index and size of myotubes was observed in senescent myoblasts (Fig 6g and 6h). Approximately 91.66% of young myoblasts turned into myotubes, but only 23.17% of senescent myoblasts were able to fuse and form myotubes (Fig 6g). Large, branched multinucleated myotubes were formed from young myoblasts with approximately 25 nuclei per myotube, while much smaller myotubes were formed during replicative senescence with the presence of 2.5 nuclei per myotube (Fig 6h).
The multinucleated myotubes that formed from senescent myoblasts, however, were improved with TRF and ATF treatment (Fig 6e and 6f), even though they were still smaller in size and with fewer branches compared to young myoblasts. An analysis of the fusion index demonstrated that TRF treatment significantly promoted cell differentiation during cellular senescence, as indicated by a significantly increased fusion index in TRF-treated myoblasts (p<0.05) (Fig 6g). ATF, however, did not produce similar effects.
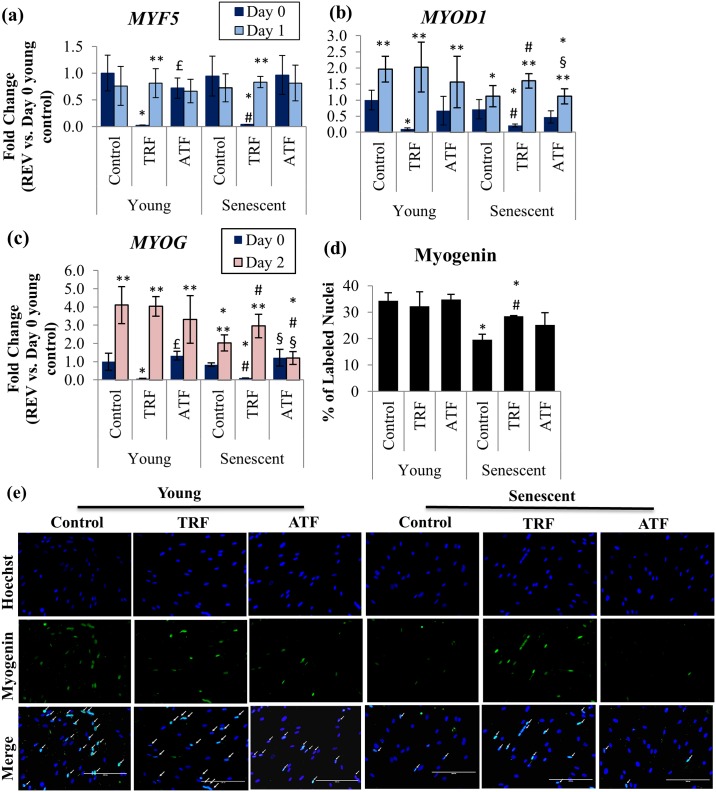
Modulation of MRFs at the Early Phase of Myogenic Differentiation
The modulation of MRFs expression by TRF and ATF during the replicative senescence of myoblasts was also investigated by determining the expression of MYF5, MYOD1 and MYOG mRNA at the early phase of differentiation in young and senescent myoblasts (Fig 7a–7c). MYOG mRNA expression was upregulated at day 2 of differentiation induction in both untreated young and untreated senescent myoblasts (p<0.05), while MYOD1 expression was upregulated at day 1 of differentiation in untreated young myoblasts only (Fig 7b and 7c). The expression of MYOD1 and MYOG mRNA, however, was lower in untreated senescent myoblasts compared to untreated young cells (p<0.05).
Fig 7. Effects of replicative senescence and vitamin E treatment at the early phase of myogenic differentiation.
The MYF5 (a) and MYOD1 (b) mRNA expression levels on day 0 and day 1 of differentiation were determined, while the MYOG (c) mRNA expression level was determined on day 0 and day 2 of differentiation. The percentage of nuclei that stained for myogenin (green) on day 3 of differentiation is shown in (d). Photomicrographs were taken from all groups using a fluorescence microscope (magnification: 200×) (e). TRF significantly increased the number of myogenin-labeled nuclei on day 3 of differentiation, as indicated by arrows. *p<0.05 significantly different compared to young control at corresponding day of differentiation, #p<0.05 significantly different compared to senescent control at corresponding day of differentiation, £p<0.05 significantly different compared to TRF-treated young myoblasts at the corresponding day of differentiation, §p<0.05 significantly different compared to TRF-treated senescent myoblasts at corresponding day of differentiation, **p<0.05 significantly different compared to the corresponding treatment at day 0 of differentiation. The data are presented as the means ± SD.
Treatment with TRF down regulated MYF5, MYOD1 and MYOG in young and senescent myoblasts at day 0 of differentiation induction compared to the untreated young control (p<0.05). At day 1 and day 2 of differentiation induction, the expression of MYF5, MYOD1 and MYOG in both young and senescent myoblasts significantly increased (p<0.05) (Fig 7a–7c).
No significant changes were observed in the expression of MYF5, MYOD1 and MYOG at day 0 of the differentiation induction of young myoblasts with ATF treatment compared to untreated control. After differentiation induction, ATF treatment caused upregulation to MYOD1 and MYOG mRNA in young myoblasts. Only MYOD1 was upregulated in senescent myoblasts at day 1 of differentiation induction (p<0.05) (Fig 7a–7c).
Myogenin protein expression significantly decreased in untreated senescent myoblasts compared to untreated young cells (p<0.05) (Fig 7d and 7e). TRF treatment significantly increased the expression of the myogenin protein in senescent myoblasts (p<0.05), while no significant changes were observed in the expression of myogenin with ATF treatment (Fig 7d and 7e).
Modulation of MRFs during Myogenic Differentiation Phase
To further validate the effect of TRF on MRFs during differentiation, expression of MRFs were determined in an extended time course and enhanced treatment protocol, in which myoblasts were treated with 50 μg/ml of TRF again during differentiation phase. Myoblasts from other donor (a 16-year-old male Caucasian) was used. The gene expression of MRFs (MYF5, MYOD1 and MYOG) at day 1 (D1), day 3 (D3) and day 5 (D5) of differentiation induction were determined (Fig 8a–8c). The expression of MYF5 mRNA was significantly decreased in senescent myoblasts at day 3 and day 5 of differentiation induction (p<0.05) (Fig 8a) while the expression of MYOD1 and MYOG mRNA was significantly lower in senescent myoblasts at day 1 till day 5 of differentiation induction as compared to untreated young myoblasts (p<0.05) (Fig 8b and 8c).
Fig 8. Effects of TRF on the MRFs mRNA expression levels during 5 days of differentiation induction.
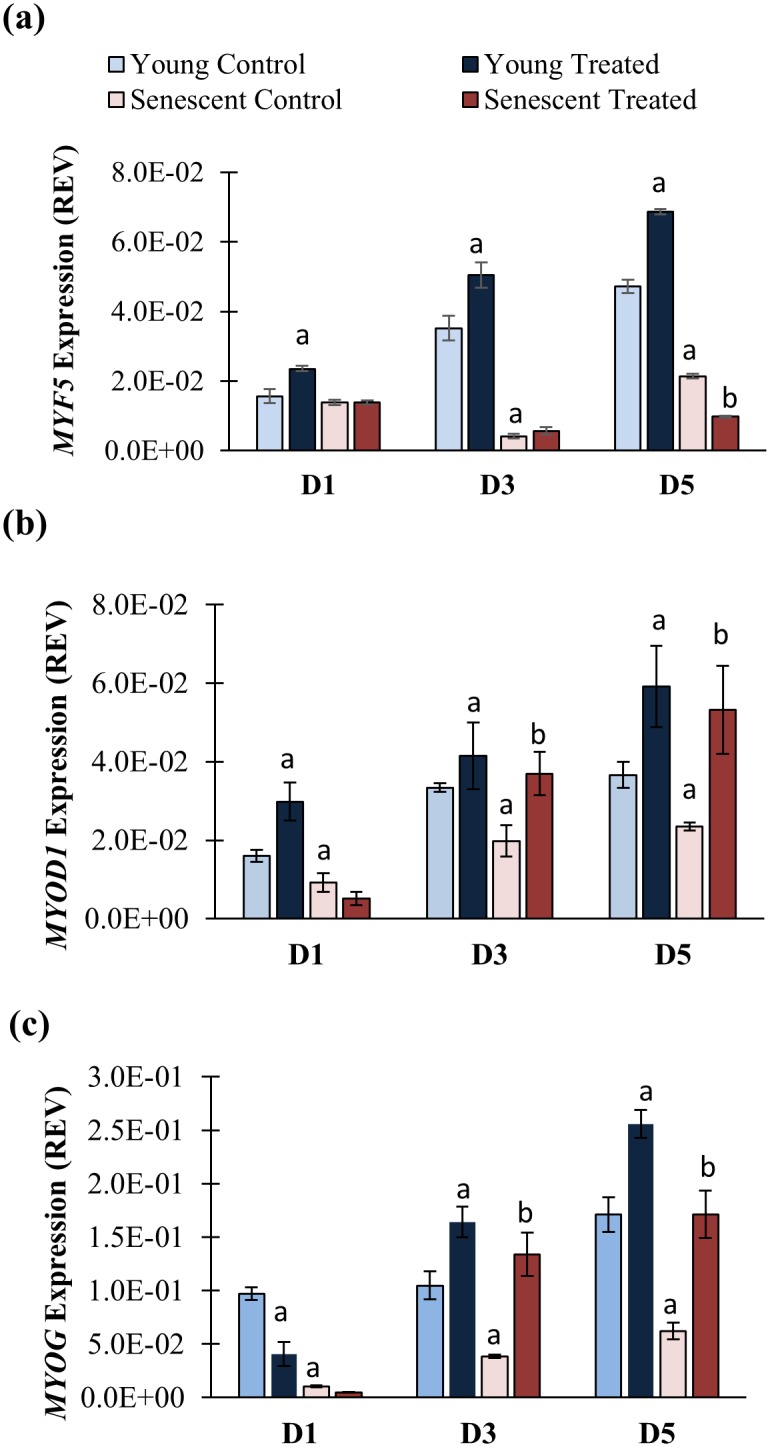
The MYF5 (a), MYOD1 (b) and MYOG (c) mRNA expression levels in senescent control were significantly lower than young control (p<0.05). TRF significantly increased the expression of both MYOD1 and MYOG mRNA at day 3 (D3) and day 5 (D5) of differentiation, resembled the expression in young control, while the expression in senescent myoblasts remained low, even after 5 days of differentiation. ap<0.05 significantly different compared to young control at corresponding day of differentiation, bp<0.05 significantly different compared to senescent control at corresponding day of differentiation, The data are presented as the means ± SD.
The expression of MRFs was modulated with TRF treatment. In TRF-treated senescent myoblasts, both MYOD1 and MYOG mRNA were significantly upregulated at day 3 and day 5 of differentiation induction as compared to untreated control (p<0.05) (Fig 8b and 8c). However, the expression of MYF5 mRNA was significantly lower in TRF-treated senescent myoblast at day 5 of differentiation as compared to senescent control (p<0.05) (Fig 8a). TRF treatment also upregulated MYF5 and MYOD1 mRNA in young myoblasts at day 1 till day 5 of differentiation induction as compared to untreated control (p<0.05) (Fig 8a and 8b) while MYOG mRNA was upregulated at day 3 and day 5 of differentiation induction (p<0.05) (Fig 8c).
Reduction of Free Radicals Generation by TRF
To further elucidate the antioxidant effect of TRF, generation of free radicals or reactive oxygen species (ROS) was determined, in which the myoblasts were labelled with DHE (in orange) and carboxy-H2DCFDA (in green). The percentage of cells which were stained positive with carboxy-H2DCFDA was gradually increased from young to senescent. Quantitative analysis showed a significantly increased in carboxy-H2DCFDA-stained senescent myoblasts (2.85 fold) as compared to young myoblasts (p<0.05) (Fig 9). TRF treatment significantly reduced the amount of intracellular ROS generation in both young and senescent myoblasts, effectively (p<0.05) (Fig 9).
Fig 9. Effects of replicative senescence and TRF treatment on intracellular ROS generation.
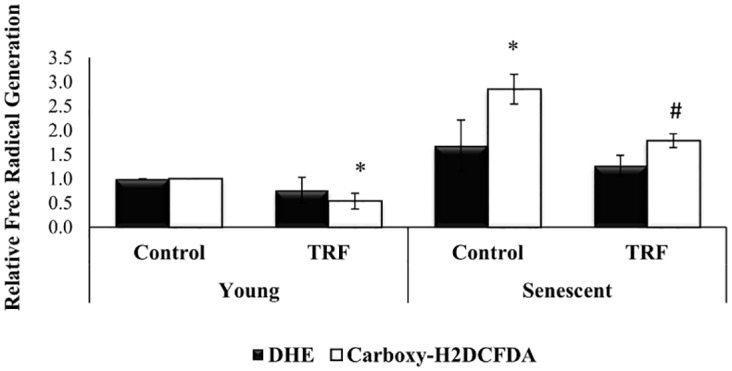
ROS was normally generated in myoblasts, however the elevated ROS level may disturb proliferation and survival of cells. The amount of intracellular ROS was significantly increased in senescent myoblasts. The fluorescence intensity of positive stained cells in senescent myoblasts was significantly reduced in TRF-treated cells, revealing free radical scavenging properties exerted in TRF. *p<0.05 significantly different compared to young control, #p<0.05 significantly different compared to senescent control, with post-hoc LSD test. Data are presented as mean ± SD, n = 3.
Discussion
The tocotrienol-rich fraction (TRF) has been shown to have not only anti-aging properties in an in vitro study [23] but also beneficial effects against aging in animal models [24] and healthy older adults [25]. Recently, positive effects of TRF were highlighted in stress-induced premature senescent (SIPS) myoblasts following a 24-hour treatment [26], acquitting its free-radical-scavenging power. The mechanism by which TRF reduces senescence phenotypes in the muscle may not only involve combating oxidative stress, but is possibly associated with its regenerative capacity. In this study, we revealed novel insight into the potential of vitamin E for improving myoblasts proliferation and differentiation for the prevention of in vitro replicative senescence.
A slight modification of skeletal muscle homeostasis may lead to unsuccessful muscle renewal as in aging and pathological dystrophic muscle. In brief, myoblasts undergo the cellular life path starts with an exponential phase that then slows and reaches a finite proliferation state, indicating the replicative senescence phase [21,27]. However, caution is required when performing the serial passaging of myoblasts, as fibroblasts may swarm in and affect the myoblasts culture [28]. Thus, in conducting the experiment, myogenic purity must be maintained, even when cells reach replicative senescence.
The number of proliferating myoblasts is represented by the total BrdU incorporated into the cells, which gradually decreased with the increased population doubling over time. Interestingly, the amount of these active cells very much depends on the donor’s age [9,21]. Moreover, old-individual-derived myoblasts had a slower response to proliferative stimuli, which may contribute to the prolonged proliferation period in senescent cells [9]. In relation to the proliferative capacity, studies have shown that aged myoblasts in culture share the same characteristics with old individual-derived myoblasts, thus indicating the perception of regenerative capacity during aging, which can be modulated by intervention. Our data show that TRF not only increases cell viability but also enhances the proliferation capacity of senescent myoblasts. The total number of proliferating cells, however, did not increase with ATF treatment in senescent myoblasts, even though the same dosage of ATF retained more viable cells than did untreated control myoblasts. These findings indicate the potential of TRF in improving the proliferation capacity of senescent myoblasts in culture.
To further verify the capability of both vitamin E treatments in rescuing senescent myoblasts, the senescence biomarker SA-β-gal was used to identify the presence of senescent cells [22]. Our results show the association between total SA-β-gal-positive cells and total population doubling, indicating the accumulation of senescent cells with increased cell expansion as previous study [29]. Both TRF and ATF dramatically decrease SA-β-gal activity in senescent cells, signifying aging reversal effects on myoblasts in culture. These findings are supported by previous reports that showed a similar decrease in SA-β-gal expression with TRF treatment in human diploid fibroblasts [23] and H2O2-induced myoblasts [26].
Apart from a significant reduction in senescence biomarkers, the eradication of typical senescence morphology further demonstrated the anti-aging effects of TRF and ATF. Senescent myoblasts normally display an enlarged and flattened morphology with the presence of more intermediate filament networks [21,29]. Elevated extracellular matrix degradation and excessive protein loss have been reported to cause failure in ultra-structural preservation, consequently presenting the unique morphology of senescent cells [30]. However, senescent myoblasts that were treated with either TRF or ATF showed a cellular morphology that resembled that of young cells, whereby more spindle-shaped cells were present, suggesting improvement in cellular physiology by both vitamin E treatments.
There is a connection between the age of the donor and the differentiation process in myoblasts [14]. Such imperfection will decelerate muscle regeneration, as observed in aging. Similar effects were shown in myoblasts that were derived from Duchenne muscular dystrophy (DMD) patients [31] and patients with myotonic dystrophy type 2 [32]. These reports supported our findings, which displayed an incomplete differentiation process in senescent myoblasts [13,14], and may explain the molecular processes that lead to muscle atrophy in aging and muscular diseases.
Our data show that the differentiation defect in senescent myoblasts is partially revived by TRF treatment. A similar finding reported that the rejuvenation of aged satellite cells can be promoted by exposure to youthful niches [10]. In addition, myoblasts that were derived from old individuals failed to differentiate when exposed to autologous serum but differentiated into myotubes in the presence of young serum, indicating that aged myoblasts were saved by circulating factors that were present in young serum [9]. In our study, senescent myoblasts regained young features with TRF treatment, which may provide a permissive environment for optimum differentiation.
Replicative senescence deregulates the expression of myogenic-differentiation-related key transcription factors, resulting in impaired myogenic differentiation as shown by differentially expressed MRFs in young and senescent myoblasts [13]. During the early phase of differentiation, the expression of Myf5, MyoD and myogenin is delayed, and their expression levels at peak are still lower than in young myoblasts, signifying the alteration of appropriate signaling for differentiation in senescent cells [13]. Previous study reported a statistically upregulation of MYOD at day 1 and returned to baseline at day 2, along with MYOG mRNA expression significantly elevated from day 2 to day 6 in culture [33]. Thus, in our study, we observed a delay in MYOD1 and MYOG mRNA expression at day 1 and day 2 respectively, as well as decreased myogenin protein expression in senescent myoblasts in the early differentiation state. Moreover, we showed that the MRFs mRNA expression remained lower in senescent myoblasts compared to young myoblasts up to 5 days of differentiation induction, which are similar to findings from a previous study [13]. In another study, a lesser induction of myogenin and MyoD in old-individual-derived muscle was observed, revealing a setback on MRFs expression during aging [34].
In our study, surprisingly, the basal MYF5 mRNA expression levels in TRF-treated senescent myoblasts were noticeably low. Previously, the MYF5 mRNA is found highly expressed in G0 myoblasts and decreased during the G0/G1 transition and persist during the rest of cell cycle progression [35]. Therefore, the expression of MYF5 may due to the dynamic of cell cycle. Besides, satellite cells in skeletal muscle are heterogeneous stem cell pool that consist of different subpopulations of cells [6] that are regenerated from the asymmetric division of satellite cells [36]. Pax7+ satellite cells with Myf5- expression can undergo asymmetric division and produce both Pax7+/Myf5- and Pax7+/Myf5+ satellite cell populations, demonstrating the role of Pax7+/Myf5- satellite cells in maintaining the muscle stem cell niche [36]. Thus, low level of MYF5 mRNA expression may be explained by this situation, even though further studies may be required to elucidate the detailed mechanism involved. However, with differentiation induction, the decreased MYF5 mRNA expression in TRF-treated senescent myoblasts returned to the normal level as in the young control. This finding agrees with a recent report that found Myf5 to be expressed in differentiating cells [37].
The results of our study also demonstrate that MYOD1 and MYOG mRNA basal expression levels in senescent myoblasts were suppressed with TRF treatment. Subsequently, these mRNA expression levels increased in response to differentiation induction. Interestingly, comparison between wild type and MYOD-/- satellite cells revealed that cell cycle progression was sustained in MYOD-/- satellite cells [38]. On the other hand, MYOG knock-down (MYOGkd) upregulates genes that are involved in cell proliferation [39]. Thus, the suppression on both MYOD1 and MYOG mRNA basal expression by TRF may favor myoblasts proliferation in the absence of differentiation stimuli.
Myogenin expression is chiefly dependent on MyoD [40]. Thus, the modulation of MyoD expression during replicative senescence as observed in this study would affect myogenin expression. Both MyoD and myogenin have a vital regulatory role in retaining myogenic differentiation [13,38,39]. Thus, a prompt increase in MYOD1 and MYOG mRNA expression which was observed immediately after differentiation induction in TRF-treated senescent cells, may indicate that myoblasts cells were rescued from senescence and more inclined to differentiation in response to stimuli. The increased expression of the myogenin protein that was observed in this study further demonstrates that TRF improves muscle differentiation. In addition, both MYOD1 and MYOG mRNA in TRF-treated senescent cells were elevated during the 5 days differentiation induction strengthening the fact that treatment with TRF during the early stage of differentiation promotes myogenic differentiation.
In this study, we attempted a nutritional approach to ameliorate senescence-associated aberration in myoblasts. To date, research findings have shown a link between vitamin E and muscle health; for instance, a population-based study and a chronic deprivation rodent model have reported that the adequate daily intake of vitamin E was correlated with muscle performance [19,20]. Sufficient vitamin E also aided in skeletal muscle survival, even in the presence of massive ROS. These findings could be attributed to the properties of vitamin E, which acts as a stabilizer for lipid membrane and scavenges ROS effectively [18]. Thus, vitamin E could be beneficial for ameliorating muscle degeneration in ageing or muscular diseases. In our study, accumulation of ROS was observed in senescent myoblasts which strengthened the fact that oxidative stress engendered age-related cell damage [41–43]. Our results demonstrated that TRF was able to reduce the accumulation of ROS in senescent myoblasts, revealing its potential in protecting myoblasts against oxidative stress during replicative senescence.
Previous studies have reported that tocotrienols have a different structure that makes it penetrate the membrane more easily than tocopherols and exerts a more potent antioxidant effect [44,45]. Compared to tocopherol, tocotrienols can efficiently be recycled and taken up by the cells [44,46]. These features may contribute to the superior effectiveness of tocotrienols in some circumstances, as has been shown in a study in which ATF required a higher dosage to produce similar effects compared to γ-tocotrienols to preserve cell viability from H2O2 insults [47]. In our study, we found that a broad mixture of TRF had better effects than did ATF alone. This result is comparable to findings from a previous study [24]. Because a discrepancy exists between isomers, TRF may be more effective than a single isomer of vitamin E.
In summary, our study highlighted the effects of vitamin E in ameliorating senescence-associated phenotypes and promoting differentiation in myoblasts during replicative senescence. We found that senescent myoblasts exhibited altered morphology and accumulated ROS with impaired proliferation and differentiation capabilities that were distinguishable from young myoblasts. Treatment with vitamin E (both TRF and ATF) was able to retrieve the young-like features in senescent myoblasts. TRF, however, exerted better effects than did ATF in promoting myoblasts proliferation, as indicated by BrdU incorporation. TRF possessed higher muscle-differentiation-promoting properties compared to ATF, as shown by the formation of myotubes and its modulation of MRFs expression. The antioxidant effect of TRF was also shown in this study. In conclusion, both the TRF and ATF have the potential to protect myoblasts from replicative senescence; however, a superior effect was shown by the TRF. The findings of this study provide the initial benefits of the TRF that may contribute to future clinical translation, in which TRF can be potentially applied to sarcopenic muscle or dystrophic muscle. Although the TRF improves senescent myoblasts by restoring their regenerative capacity, further studies are required to determine its effects in vivo, either in humans or in an animal model.
Acknowledgments
The authors would like to express gratitude to all researchers and staff of the Biochemistry Department, Faculty of Medicine, Universiti Kebangssan Malaysia Medical Centre.
Data Availability
All relevant data are within the paper.
Funding Statement
This study was funded by the Universiti Kebangsaan Malaysia grants AP-2012-012 and UKM-FF-2013-259. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Fielding RA, Vellas B, Evans WJ, Bhasin S, Morley JE, Newman AB, et al. Sarcopenia: an undiagnosed condition in older adults. Current consensus definition: prevalence, etiology, and consequences. International working group on sarcopenia. J Am Med Dir Assoc 2011; 12: 249–256. 10.1016/j.jamda.2011.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Carosio S, Berardinelli MG, Aucello M, Musaro A. Impact of ageing on muscle cell regeneration. Ageing Res Rev 2011; 10: 35–42. 10.1016/j.arr.2009.08.001 [DOI] [PubMed] [Google Scholar]
- 3.Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol Rev 2004; 84: 209–238. [DOI] [PubMed] [Google Scholar]
- 4.Yin H, Price F, Rudnicki MA. Satellite cells and the muscle stem cell niche. Physiol Rev 2013; 93: 23–67. 10.1152/physrev.00043.2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Collins CA, Olsen I, Zammit PS, Heslop L, Petrie A, Partridge TA, et al. Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 2005; 122: 289–301. [DOI] [PubMed] [Google Scholar]
- 6.Collins CA, Zammit PS, Ruiz AP, Morgan JE, Partridge TA. A population of myogenic stem cells that survives skeletal muscle aging. Stem Cells 2007; 25: 885–894. [DOI] [PubMed] [Google Scholar]
- 7.Sousa-Victor P, Gutarra S, Garcia-Prat L, Rodriguez-Ubreva J, Ortet L, Ruiz-Bonilla V, et al. Geriatric muscle stem cells switch reversible quiescence into senescence. Nature 2014; 506: 316–321. 10.1038/nature13013 [DOI] [PubMed] [Google Scholar]
- 8.Renault V, Thorne LE, Eriksson PO, Butler‐Browne G, Mouly V. Regenerative potential of human skeletal muscle during aging. Aging Cell 2002; 1: 132–139. [DOI] [PubMed] [Google Scholar]
- 9.Barberi L, Scicchitano BM, De Rossi M, Bigot A, Duguez S, Wielgosik A, et al. Age-dependent alteration in muscle regeneration: the critical role of tissue niche. Biogerontology 2013: 1–20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Conboy IM, Conboy MJ, Wagers AJ, Girma ER, Weissman IL, Rando TA. Rejuvenation of aged progenitor cells by exposure to a young systemic environment. Nature 2005; 433: 760–764. [DOI] [PubMed] [Google Scholar]
- 11.Carlson ME, Suetta C, Conboy MJ, Aagaard P, Mackey A, Kjaer M, et al. Molecular aging and rejuvenation of human muscle stem cells. EMBO Mol Med 2009; 1: 381–391. 10.1002/emmm.200900045 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bernet JD, Doles JD, Hall JK, Kelly Tanaka K, Carter TA, Olwin BB. p38 MAPK signaling underlies a cell-autonomous loss of stem cell self-renewal in skeletal muscle of aged mice. Nat Med 2014; 20: 265–271. 10.1038/nm.3465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bigot A, Jacquemin V, Debacq-Chainiaux F, Butler-Browne GS, Toussaint O, Furling D, et al. Replicative aging down-regulates the myogenic regulatory factors in human myoblasts. Biol Cell 2008; 100: 189–199. [DOI] [PubMed] [Google Scholar]
- 14.Lorenzon P, Bandi E, de Guarrini F, Pietrangelo T, Schafer R, Zweyer M, et al. Ageing affects the differentiation potential of human myoblasts. Exp Gerontol 2004; 39: 1545–1554. [DOI] [PubMed] [Google Scholar]
- 15.Arthur PG, Grounds MD, Shavlakadze T. Oxidative stress as a therapeutic target during muscle wasting: considering the complex interactions. Curr Opin Clin Nutr Metab Care 2008; 1: 408. [DOI] [PubMed] [Google Scholar]
- 16.Khor SC, Abdul Karim N, Wan Ngah WZ, Mohd Yusof YA, Makpol S. Vitamin E in Sarcopenia: Current Evidences on Its Role in Prevention and Treatment. Oxid Med Cell Longev 2014; 2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sen CK, Khanna S, Roy S. Tocotrienols: Vitamin E beyond tocopherols. Life Sciences 2006; 78: 2088–2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Howard AC, McNeil AK, McNeil PL. Promotion of plasma membrane repair by vitamin E. Nat Commun 2011; 2: 597 10.1038/ncomms1594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cesari M, Pahor M, Bartali B, Cherubini A, Penninx BW, Williams GR, et al. Antioxidants and physical performance in elderly persons: the Invecchiare in Chianti (InCHIANTI) study. Am J Clin Nutr 2004; 79: 289–294. [DOI] [PubMed] [Google Scholar]
- 20.Rafique R, Schapira AHV, Cooper JM. Mitochondrial Respiratory Chain Dysfunction in Ageing; Influence of Vitamin E Deficiency. Free Radical Res 2004; 38: 157–165. [DOI] [PubMed] [Google Scholar]
- 21.Mouly V, Aamiri A, Bigot A, Cooper R, Di Donna S, Furling D, et al. The mitotic clock in skeletal muscle regeneration, disease and cell mediated gene therapy. Acta Physiol Scand 2005; 184: 3–15. [DOI] [PubMed] [Google Scholar]
- 22.Dimri G, Lee X, Basile G, Acosta M, Scott G, Roskelley C, et al. A biomarker that identifies senescent human cells in culture and in aging skin in vivo. Proc Natl Acad Sci U S A 1995; 92: 9363–9367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Makpol S, Durani LW, Chua KH, Mohd Yusof YA, Ngah WZ. Tocotrienol-rich fraction prevents cell cycle arrest and elongates telomere length in senescent human diploid fibroblasts. J Biomed Biotechnol 2011; 2011: 506171 10.1155/2011/506171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SP, Mar GY, Ng LT. Effects of tocotrienol-rich fraction on exercise endurance capacity and oxidative stress in forced swimming rats. Eur J Appl Physiol 2009; 107: 587–595. 10.1007/s00421-009-1159-6 [DOI] [PubMed] [Google Scholar]
- 25.Chin SF, Hamid NA, Latiff AA, Zakaria Z, Mazlan M, Yusof YA, et al. Reduction of DNA damage in older healthy adults by Tri E Tocotrienol supplementation. Nutrition 2008; 24: 1–10. [DOI] [PubMed] [Google Scholar]
- 26.Lim JJ, Ngah WZ, Mouly V, Abdul Karim N. Reversal of myoblast aging by tocotrienol rich fraction posttreatment. Oxid Med Cell Longev 2013; 2013: 978101 10.1155/2013/978101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Hayflick L. The limited in vitro lifetime of human diploid cell strains. Exp Cell Res 1965; 37: 614–636. [DOI] [PubMed] [Google Scholar]
- 28.Alsharidah M, Lazarus NR, George TE, Agley CC, Velloso CP, Harridge SD. Primary human muscle precursor cells obtained from young and old donors produce similar proliferative, differentiation and senescent profiles in culture. Aging cell 2013; 12: 333–344. 10.1111/acel.12051 [DOI] [PubMed] [Google Scholar]
- 29.Nehlin JO, Just M, Rustan AC, Gaster M. Human myotubes from myoblast cultures undergoing senescence exhibit defects in glucose and lipid metabolism. Biogerontology 2011; 12: 349–365. 10.1007/s10522-011-9336-5 [DOI] [PubMed] [Google Scholar]
- 30.Cho KA, Ryu SJ, Oh YS, Park JH, Lee JW, Kim HP, et al. Morphological adjustment of senescent cells by modulating caveolin-1 status. J Biol Chem 2004; 279: 42270–42278. [DOI] [PubMed] [Google Scholar]
- 31.Decary S, Ben Hamida C, Mouly V, Barbet J, Hentati F, Butler-Browne G. Shorter telomeres in dystrophic muscle consistent with extensive regeneration in young children. Neuromuscular Disord 2000; 10: 113–120. [DOI] [PubMed] [Google Scholar]
- 32.Malatesta M, Giagnacovo M, Renna L, Cardani R, Meola G, Pellicciari C. Cultured myoblasts from patients affected by myotonic dystrophy type 2 exhibit senescence-related features: ultrastructural evidence. Eur J Histochem 2011; 55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Owens J, Moreira K, Bain G. Characterization of primary human skeletal muscle cells from multiple commercial sources. In Vitro Cell Dev Biol Anim 2013; 49: 695–705. 10.1007/s11626-013-9655-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alway SE, Lowe DA, Chen KD. The effects of age and hindlimb supension on the levels of expression of the myogenic regulatory factors MyoD and myogenin in rat fast and slow skeletal muscles. Exp Physiol 2001; 86: 509–517. [DOI] [PubMed] [Google Scholar]
- 35.Kitzmann M, Carnac G, Vandromme M, Primig M, Lamb NJ, Fernandez A. The muscle regulatory factors MyoD and myf-5 undergo distinct cell cycle–specific expression in muscle cells. J Cell Biol 1998; 142: 1447–1459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kuang S, Kuroda K, Le Grand F, Rudnicki MA. Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 2007; 129: 999–1010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Londhe P, Davie JK. Sequential association of myogenic regulatory factors and E proteins at muscle-specific genes. Skeletal Muscle 2011; 1: 14 10.1186/2044-5040-1-14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yablonka-Reuveni Z, Rudnicki MA, Rivera AJ, Primig M, Anderson JE, Natanson P. The transition from proliferation to differentiation is delayed in satellite cells from mice lacking MyoD. Dev Biol 1999; 210: 440–455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lee EJ, Malik A, Pokharel S, Ahmad S, Mir BA, Cho KH, et al. Identification of genes differentially expressed in myogenin knock-down bovine muscle satellite cells during differentiation through RNA sequencing analysis. PLoS One 2014; 9: e92447 10.1371/journal.pone.0092447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cao Y, Yao Z, Sarkar D, Lawrence M, Sanchez GJ, Parker MH, et al. Genome-wide MyoD binding in skeletal muscle cells: a potential for broad cellular reprogramming. Dev Cell 2010; 18: 662–674. 10.1016/j.devcel.2010.02.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Shigenaga MK, Hagen TM, Ames BN. Oxidative damage and mitochondrial decay in aging. Proc Natl Acad Sci U S A 1994; 91: 10771–10778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Vasilaki A, McArdle F, Iwanejko L, McArdle A. Adaptive responses of mouse skeletal muscle to contractile activity: the effect of age. Mech Ageing Dev 2006; 127: 830–839. [DOI] [PubMed] [Google Scholar]
- 43.Fano G, Mecocci P, Vecchiet J, Belia S, Fulle S, Polidori MC, et al. Age and sex influence on oxidative damage and functional status in human skeletal muscle. J Muscle Res Cell Motil 2001; 22: 345–351. [DOI] [PubMed] [Google Scholar]
- 44.Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radical Biol Med 1991; 10: 263–275. [DOI] [PubMed] [Google Scholar]
- 45.Suzuki YJ, Tsuchiya M, Wassall SR, Choo YM, Govil G, Kagan VE, et al. Structural and dynamic membrane properties of. alpha.-tocopherol and. alpha.-tocotrienol: Implication to the molecular mechanism of their antioxidant potency. Biochemistry 1993; 32: 10692–10699. [DOI] [PubMed] [Google Scholar]
- 46.Saito Y, Yoshida Y, Nishio K, Hayakawa M, Niki E. Characterization of cellular uptake and distribution of vitamin E. Ann N Y Acad Sci 2004; 1031: 368 [DOI] [PubMed] [Google Scholar]
- 47.Mazlan M, Sue Mian T, Mat Top G, Zurinah Wan Ngah W. Comparative effects of alpha-tocopherol and gamma-tocotrienol against hydrogen peroxide induced apoptosis on primary-cultured astrocytes. J Neurol Sci 2006; 243: 5–12. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All relevant data are within the paper.