Abstract
Under environmental stress, microbes are known to alter their translation patterns using sequence-specific endoribonucleases that we call RNA interferases. However, there has been limited insight regarding which RNAs are specifically cleaved by these RNA interferases, hence their physiological functions remain unknown. In the current study, we developed a novel method to effectively identify cleavage specificities with massive parallel sequencing. This approach uses artificially designed RNAs composed of diverse sequences, which do not form extensive secondary structures, and it correctly identified the cleavage sequence of a well-characterized Escherichia coli RNA interferase, MazF, as ACA. In addition, we also determined that an uncharacterized MazF homologue isolated from Pseudomonas putida specifically recognizes the unique triplet, UAC. Using a real-time fluorescence resonance energy transfer assay, the UAC triplet was further proved to be essential for cleavage in P. putida MazF. These results highlight an effective method to determine cleavage specificity of RNA interferases.
Introduction
Toxin-antitoxin (TA) systems are genetic modules composed of a stable toxin and a volatile antitoxin. They are widely distributed among archaeal and bacterial lineages and allow microbes to withstand environmental stresses [1–3]. When microbes face these environmental stresses, antitoxin, which prevents toxin activity, is rapidly degraded. Then, toxin molecules inhibit requisite cellular functions, causing microbial growth arrest and eventually cell death [4]. Although the molecular functions of toxins are diverse, a very large number of toxins across the archaeal and bacterial lineages are known to function as endoribonucleases [5,6]. In Escherichia coli, several types of toxin ribonucleases have been shown to catalyze intracellular RNA cleavage in a ribosome dependent (RelE, HigB, YafO, YafQ, and YoeB) [7–11] or independent (MazF, MqsR, ChpBK, HicA, and RnlA) [12–16] fashion.
MazF, which is one of the most investigated RNA interferases, is a part of a TA system along with its cognate antitoxin MazE [4]. As with other TA systems, the lethality of MazF is triggered by various stresses such as antibiotic addition, heat shock, and amino acid starvation [17,18]. In such situations, MazE is degraded by ClpAP protease [4], releasing the MazF toxin endoribonuclease. This liberated MazF subsequently cleaves intracellular RNA at ACA triplets [12] and permits cells to change their translation pattern [19,20]. To date, many MazF homologues were predicted in the genomes and plasmids of various prokaryotes [21], and some of them have been characterized. Intriguingly, their cleavage patterns differed between microbes in terms of recognition-site length (in most cases three, five, or seven bases) and RNA sequences [12,22–27]. These reports indicate that RNA interferases are diverse, even within one MazF family, and that these proteins play broad roles in degrading the bulk of intracellular RNA and/or silencing specific genes to cope with the environmental fluctuations [28,29].
The cleavage patterns produced by most RNA interferases are yet to be identified because of limitations from using classical methods. In most studies, primer extension analysis with MS2 bacteriophage RNA and chaperone protein CspA has been used [22,26,27]. Although it enables direct detection of RNA cleavage sites, the experimental method is cumbersome and impractical to adapt for high-throughput studies. Mass spectrometry was also developed as a rapid, sensitive, and accurate method for detection of cleavage sites [30], however, one major drawback of this approach is that mass resolution decreases as RNA oligonucleotide length increases [31]. Gel electrophoresis is one of the simplest methods to observe the cleaved products, but it is difficult to identify the sequence of the cleavage sites without prior information. To overcome these limitations, a novel method termed MORE (mapping by overexpression of an RNase in E. coli) RNA-seq was developed [32]. In this method, RNA interferase was ectopically overexpressed in E. coli and the 5′-end sequences of the extracted RNAs that correspond to the cleavage sequence were detected with SOLiD platform [32]. This method is useful to accurately identify the cleavage sequence of RNA interferases, particularly for cleavage sequences longer than five nucleotide bases.
In our study, we also determined the cleavage sequence of RNA interferases using RNA-seq. The RNAs used in this study were designed with a computer algorithm that generates diverse sequences [33], and thus, the RNAs can be studied for many potential cleavage sequences in a high-throughput manner with the Illumina MiSeq platform. When we tested our approach with E. coli MazF, ACA was identified as the cleavage sequence. Thus, to verify this approach in another microbe, an uncharacterized MazF homologue that is predicted in the Pseudomonas putida chromosome was also analyzed. The RNA-seq showed that UAC was the RNA cleavage sequence. This observation was further confirmed by a fluorometric assay [34], demonstrating the efficacy of this approach for identifying RNA interferase-specific cleavage sequences.
Materials and Methods
Bacterial Strains and Plasmids
The following E. coli strains were used in this study: DH5α (Nippon Gene, Tokyo, Japan) for the constructed plasmid propagation and BL21 (DE3) pLysS (Novagen, Madison, WI, USA) for recombinant protein expression. Two plasmids were purchased from Takara Bio Service, pMD19 encoding the graA gene and empty vector pET21c (Takara, Shiga, Japan).
Oligonucleotides
PCR primers were purchased from Tsukuba Oligo Service (Ibaraki, Japan). All fluorescent-labeled oligonucleotides were purchased from Japan Bio Services (Saitama, Japan). Barcode RNA was also purchased from Japan Bio Services (Saitama, Japan). The sequence of PCR primers and a barcode RNA are listed in S1 Table.
Plasmid Construction
PCR was carried out to amplify the antitoxin (mazEpp) and the toxin (mazFpp) genes with Taq HS DNA polymerase (Takara) using genomic DNA of Pseudomonas putida as a template. These PCR products were purified by a PCR purification kit (Qiagen, Venlo, Netherlands). pET21c and PCR products were digested with BamHI and EcoRI (Toyobo, Osaka, Japan) and cleaned using phenol/chloroform extraction and ethanol precipitation. Digested mazEpp and mazFpp fragments were inserted into the corresponding pET21c multiple cloning sites with a DNA ligation kit (Takara). This generated the plasmids pET21c-mazEpp and pET21c-mazFpp, respectively. Each plasmid was then introduced into Escherichia coli DH5α and the colonies were grown overnight at 37°C on LB plate containing ampicillin (100 μg/ml). Plasmids were extracted using QIAprep Spin Miniprep Kit (Qiagen) and their sequences were determined using AB 3500 Genetic Analyzer (Applied Biosystems, Foster City, CA, USA) according to the manufacturer’s protocol.
Protein Expression
The pET21c-mazEpp and pET21c-mazFpp plasmids were introduced into E. coli BL21 (DE3) pLysS via heatshock. The E. coli harboring pET21c-mazEpp or pET21c-mazFpp were pre-cultivated in LB medium containing 100 μg/ml ampicillin at 37°C for overnight and then inoculated into the 0.5 L or 1 L of LB medium supplemented with 100 μg/ml ampicillin, respectively. MazEpp and MazFpp were induced by the addition of 1 mM isopropyl β-D-1-thiogalactopyranoside when OD600 reached 2.0 and 4.0, respectively. After 3.5 hours of incubation, cells were harvested by centrifugation at 7,000 g and then stored at -80°C for future use.
MazEpp Purification
E. coli cells containing MazEpp were thawed on ice and resuspended in lysis buffer (100 mM sodium phosphate buffer, 300 mM NaCl, 2.5% glycerol, 3.2 mM 2-mercaptoethanol, and 10 mM imidazole). Cells were lysed by sonication for 15 minutes with a Handy Sonic UR-20P (Tomy Seiko, Tokyo, Japan). The supernatant containing MazEpp was collected by centrifugation at 7,000 g for 15 minutes. Ni-NTA agarose (Qiagen) was added to the supernatant and rotated for 2 hours at 4°C to allow MazEpp to adsorb onto the Ni-NTA column. Non-specific proteins were eliminated with washing buffer (90 mM sodium phosphate buffer, 770 mM NaCl, 2.3% glycerol, 2.9 mM 2-mercaptoethanol, and 20 mM imidazole) and 100 mM imidazole buffer (97.5 mM sodium phosphate buffer, 290 mM NaCl, 2.4% glycerol, and 100 mM imidazole). MazEpp was eluted with elution buffer (92.5 mM sodium phosphate buffer, 280 mM NaCl, 2.3% glycerol, and 300 mM imidazole). The molecular weight and purity were confirmed by SDS-PAGE. Protein concentration was determined using Qubit Protein Assay Kit (Life Technologies).
MazFpp Purification
E. coli cells containing MazFpp were thawed on ice and resuspended in binding buffer (20 mM sodium phosphate buffer, 50 mM imidazole, 300 mM NaCl, and 5 mM 2-mercaptoethanol). Suspended cells were sonicated for 15 minutes with a Handy Sonic UR-20P and then centrifuged at 7,000 g for 15 minutes. The supernatant was then filtered with a 0.45 μm membrane (Millex, Darmstadt, Germany). After the 1 mL His-Trap HP column (GE Healthcare, Waukesha, WI, USA) was equilibrated with 10 column volumes (cv) of binding buffer, the filtered supernatant was applied to this column and washed with 32 cv of binding buffer. Residual non-specific proteins and hexa-histidine tagged MazFpp were separated by gradually increasing the imidazole concentration using AKTA pure plus (GE Healthcare). The molecular weight and purity were confirmed by SDS-PAGE. Protein concentration was determined using Qubit Protein Assay Kit.
RNA Preparation
The transcript of graA and six artificial synthetic RNAs named 500–2, 1000–1, 1000–2, 1000–3, 1000–4, and 1000–5 [33] were used in this study. All synthetic RNAs had 500-nucleotide (500–2) or 1000-nucleotide (1000–1 to 1000–5) diverse sequences [33], and each had between three-guanine bases at the 5′ end and 30-base polyadenylated tail at the 3′ end. The pMD19 plasmid encoding the graA gene was digested with HindIII (New England Biolabs, Ipswich, MA). pUC19 plasmids encoding synthetic RNA (500–2, 1000–1, 1000–2, 1000–3, and 1000–4) and the pUC19 plasmid encoding 1000–5 synthetic RNA were digested with BbsI and BtgZI, respectively (New England Biolabs). Digested fragments were purified with a PCR purification kit and then in vitro transcription was carried out with MEGAscript T7 Kit (Life Technologies) according to the manufacturer’s instruction. Transcribed RNAs were purified with RNA Clean & Concentrator™-5 (Zymo Research, Orange, CA, USA) and their concentration was determined using Qubit RNA Assay Kit (Life Technologies).
Massive Parallel Sequencing
Substrate RNA was prepared by mixing 300 ng of RNA 1000–1, 1000–2, 1000–3, 1000–4, and 1000–5. This substrate was incubated at 37°C for 15 minutes with two distinct RNA interferases: 2 U of MazF (Takara) in MazF buffer (Takara) containing 4 U of recombinant RNase inhibitor (Takara) or 300 ng of MazFpp in MazFpp buffer (20 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol, 0.01% tritonX-100, and 4 U of recombinant RNase inhibitor). As the control experiment, the substrate RNA was also treated with 2.5 U of RNase III (New England Biolabs) in NEBNext RNase III Reaction Buffer (New England Biolabs) at 37°C for 15 minutes. These samples were cleaned with RNA Clean & Concentrator™-5 and then incubated with 20 U of T4 polynucleotide kinase (Takara) in T4 Polynucleotide Kinase Buffer (Takara) containing 1 mM ATP (Ambion, Austin, TX, USA) at 37°C for 1 hour. They were purified using RNA Clean & Concentrator™-5. Subsequently, 125 pmol of barcode RNA and purified RNA fragments were incubated with 50 U of T4 RNA ligase (Takara) in the RNA ligation buffer (Takara) for 18 hours at 15°C. Samples were purified with RNA Clean & Concentrator™-5 and then the RNA concentration was determined using the Qubit RNA Assay Kit. Sequencing library was constructed according to the NEB Ultra RNA Library Prep Kit for Illumina protocols (New England Biolabs). For this study, the protocol for longer size RNA inserts was used. The constructed library quality was validated using the Agilent High Sensitivity DNA Kit (Agilent Technologies, Santa Clara, CA, USA). Sequencing was performed using the MiSeq platform with the MiSeq 500 cycles reagent kit v2 (Illumina, San Diego, CA, USA) according to the manufacturer’s protocol.
Cleavage Sequence Identification
The output files configured in fastaq format for R1 and R2 reads were separately analyzed with CLC Genomics Workbench version 7.5.1 (CLC bio, Aarhus, Denmark). Nucleotides with low quality or ambiguity were eliminated with the parameters limit of 0.05 and a maximum number of ambiguities equals to zero. Reads that included the 15-base sequence (CTGGCTTTGATGAAA) corresponding to the 3′-end sequence of the barcode were extracted from both strands via the following parameters: mismatch cost and gap cost equal five, while minimum scores for internal match and end match both equal 15. All the 5′ nucleotides upstream of this 15-base sequence were removed and the reads shorter than 15 bases were discarded. The resulting reads were mapped against five references (1000–1, 1000–2, 1000–3, 1000–4, and 1000–5). The parameters used for mapping were as follows: mismatch cost, insertion cost, and deletion cost equal three; length and similarity fraction both equal one. The fastaq files were deposited into the DNA Data Bank of Japan Sequence Read Archive (DRA004282).
Enzymatic Activity of MazFpp and MazEpp
Purified MazFpp was incubated with RNA 500–2. Next, 3 pmol of MazFpp was pre-incubated with 0.3, 3, or 30 pmol of MazEpp at room temperature for 10 minutes. After the pre-incubation, 100 ng of RNA 500–2 was added and the mix was incubated at 37°C for 30 minutes in MazFpp buffer (20 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol, 0.01% tritonX-100, and 4 U of recombinant RNase inhibitor). Samples were purified by RNA Clean & Concentrator™-5 and the gel loading buffer II (Ambion) was added to each sample. Samples were incubated at 95°C for 5 minutes and separated on a 10% polyacrylamide gel containing 7 M urea. RNA was stained using SYBR Gold (Life Technologies) and then detected with a Typhoon 9210 imager (GE Healthcare).
Fluorometric Detection of MazFpp Activity
A fluorometric assay [34] was used to validate the cleavage sequences. One hundred nanograms of MazFpp or RNase A (Novagen) and 20 pmol of fluorescent-labeled oligonucleotides were incubated in MazFpp buffer (20 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol, 0.01% tritonX-100, and 4 U of recombinant RNase inhibitor). All reactions were conducted at 37°C in triplicate and fluorescent intensity was recorded every 60 seconds using a Light Cycler 480 system (Roche, Basel, Switzerland) with 483 nm excitation and 533 nm detection filters.
RNA Cleavage Specificity of MazFpp
One hundred nanograms of graA transcript and RNA 500–2 were incubated with 50 ng of MazFpp at 37°C in MazFpp buffer (20 mM Tris-HCl (pH 8.0), 1 mM dithiothreitol, 0.01% tritonX-100, and 4 U of recombinant RNase inhibitor). After incubation for 1, 5, 15, or 30 minutes, gel loading buffer II was added to each sample. Samples were incubated at 95°C for 5 minutes before electrophoresis on a 10% polyacrylamide gel containing 7 M urea. RNA was then stained with SYBR Gold and detected with a Typhoon 9210 imager.
Accession Numbers and GI Numbers
The GenBank accession numbers and GI numbers were as follows: mazEpp gene (NC_002947.3, 26986745), mazFpp gene (NC_002947.3, 26986745), graA gene (AE015451.1, 24987239), 500–2 (AB610940.1, 321117288), 1000–1 (AB610944.1, 321117292), 1000–2 (AB610945.1, 321117293), 1000–3 (AB610946.1, 321117294), 1000–4 (AB610947.1, 321117295), and 1000–5 (AB610948.1, 321117296).
Results
Massive Parallel Sequencing Identified the MazF Cleavage Sequence
In massive parallel sequencing, nucleotides are sequenced with significant depth, which generates reliable data about the frequency of the detected molecules [35]. Indeed, this sequence depth was critical for identifying the RNA cleavage sites using a high-throughput approach. We used five 1000-nt synthetic RNAs designed by a computer algorithm as the substrates for RNA interferases. These RNAs are well suited to this approach because they are long enough and they are composed of nearly equal numbers of each RNA base (A, U, G, and C) [33]. This increases the possibility that the cleavage sequence will be included in the RNAs. Another advantage of these RNAs is that they were designed in a way that they do not form complex secondary structures [33]. Because of this smart design, RNA unwinding proteins like CspA, which prevent secondary-structure formation of substrate RNA and enhances the accessibility of RNA interferases to their cleavage sites [22,26,27], were not required for the experiment.
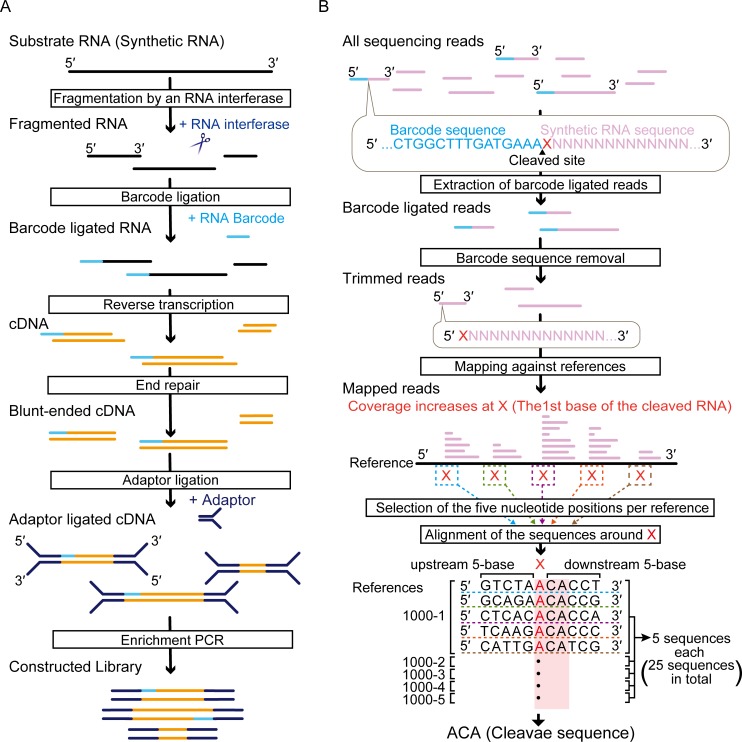
MazF is one of the best-characterized RNA interferases, and it was originally identified in an Escherichia coli chromosome [4]. Since the ACA triplet in single-stranded RNA was already elucidated as its cleavage sequence, we chose E. coli MazF as a model endoribonuclease and then evaluated if our approach was consistent with previous results [12,36]. To this end, using the Illumina platform we examined the 5′-end sequences of the MazF fragmented RNAs. A challenge of this approach was that the sequence information for the cleaved RNA sites was eliminated during the procedure in order to construct the sequence library: single-stranded DNA overhangs were polished in the end repair step. To overcome this challenge and to maintain the 5′-end nucleotides derived from the original RNA strand, which would retain the MazF cleaved sequences, we ligated a 45-nt RNA barcode oligonucleotide whose sequence was identical to the 5′ RACE Adapter of the FirstChoice RLM-RACE Kit (S1 Table, Fig 1A).
Fig 1. Schematic representation of the experimental methods.
(A) Overview of the sequence library construction method. (B) Workflow for identification of RNA cleavage site (see text).
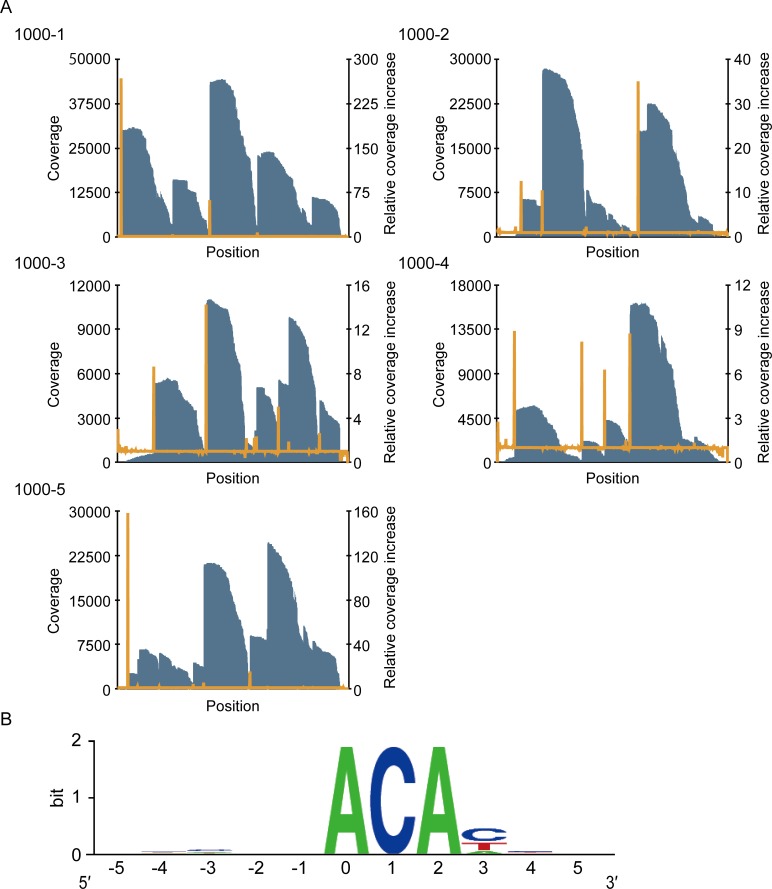
In our approach, the 5′-end sequence of the fragmented RNAs immediately downstream of the 3′-end sequence of the barcode is considered to be identical with the MazF cleavage sequence (Fig 1B). We extracted the reads containing the 15-base sequence (CTGGCTTTGATGAAA) corresponding to the 3′-end sequence of the barcode and then removed all the 5′-end nucleotides including the 15 bases from the extracted reads. These trimmed reads were mapped against the five reference sequences (1000–1, 1000–2, 1000–3, 1000–4, and 1000–5) (Fig 1B), resulting in the recovery of 9.21% of total reads. The reason for this low recovery was considered to be the low efficiency of the barcode ligation, as most of the reads discarded in the analysis procedures were derived from non-ligated RNA molecules. In all reference sequences, we found increases in coverage at specific nucleotides (Fig 2A). The sequence adjacent to these nucleotides includes the cleavage sequences. To identify the cleavage sequence, we searched for the nucleotides that met the following two criteria (X in Fig 1B): first, the position showed the coverage over 1000; and second, the positions had a large relative coverage increase, which is the value defined as the coverage at the n+1th position divided by the coverage at the nth position. The relative coverage increase at each nucleotide position was arranged in decreasing order in each reference, and five nucleotides that showed the five largest relative coverage increases were selected from all references (S2 Table). We extracted the sequences including five bases upstream and five bases downstream (Fig 1B). When next aligned 25 sequences (five sequences from five references) (Fig 1B, S2 Table), we found ACA was correctly highlighted as the MazF cleavage sequence (Fig 2B). Importantly, no specific sequence was detected in a control experiment that used RNase III, a structure-specific endoribonuclease (S1 Fig, S3 Table). These results prove that our approach is useful for detection of RNA interferase-specific cleavage sequences.
Fig 2. Identification of the MazF cleavage sequence using massive parallel sequencing.
(A) Graph of the coverage (blue bar) and the relative coverage increase (orange line). (B) Graphical representation of the conserved sequences. The nucleotide position with significant increases in coverage was numbered as zero. Twenty-five sequences were analyzed (S2 Table) and the frequency at each position was visualized with the WebLogo program [37].
MazFpp Is a Bona-Fide RNA Interferase and Comprises a TA System with MazEpp
Having determined the MazF cleavage sequence as ACA, we next tried to characterize the RNA interferase with unknown cleavage specificity. For this purpose, we chose a putative MazF homologue encoded by the locus PP0771 in Pseudomonas putida chromosome (hereafter MazFpp). Since this bacterium is widely used for production of chemical compounds and it is also a xenobiotic decomposer in environmental engineering [38], characterization of the toxin enzyme that regulates its growth would be beneficial to understand its industrial utility.
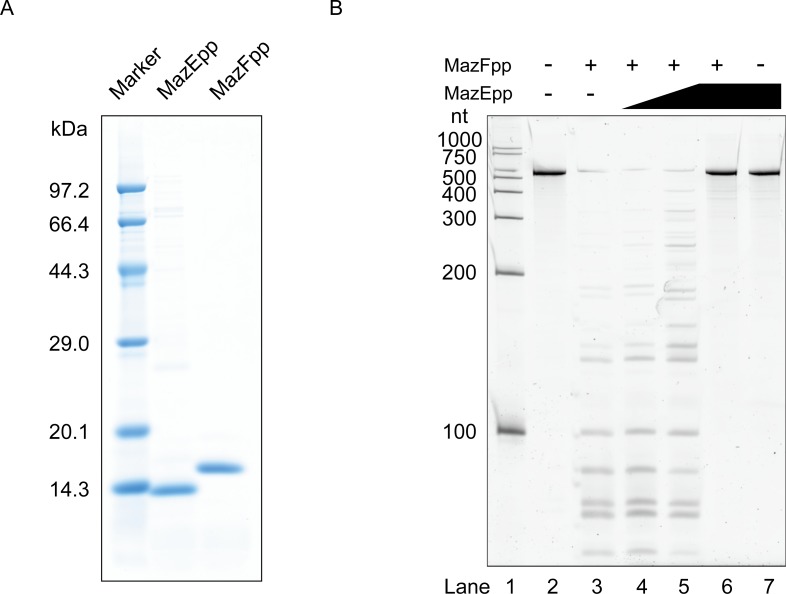
Though MazFpp shows 34.8% identity to E. coli MazF, there is no evidence of its function. Hence, we isolated histidine-tagged MazFpp (Fig 3A) and then incubated it with an RNA substrate to examine if MazFpp possesses endoribonuclease activity. Several short fragments were observed when substrate RNA was treated with MazFpp, indicating that MazFpp indeed functions as an RNA interferase and recognizes relatively short sequences (Fig 3B, Lane 3). In order to rule out the possibility that this cleavage was due to the contaminated RNases, we next purified a cognate antitoxin MazEpp (PP_0770) (Fig 3A). Following the pre-incubation with MazEpp, we observed that the ribonuclease activity of MazFpp was inhibited in a dose-dependent manner (Fig 3B, Lanes 4–6). On the basis of these findings, we concluded that MazFpp is a bona-fide RNA interferase and constitutes a TA system together with MazEpp.
Fig 3. MazEF pairs in Pseudomonas putida.
(A) Molecular weight and the purity of the hexa-histidine tagged MazEpp and MazFpp. (B) Enzymatic activity of MazEpp and MazFpp. A 533-base synthetic RNA (RNA 500–2) was incubated with these enzymes. Lane 2, a control reaction without any enzymes; Lanes 3–6, 3 pmol of MazFpp was added. For lanes 4–6, MazE-pp was also added to each lane: 0.3, 3, and 30 pmol respectively. Lane 7, 30 pmol of MazEpp was added.
MazFpp Specifically Recognizes UAC Triplet
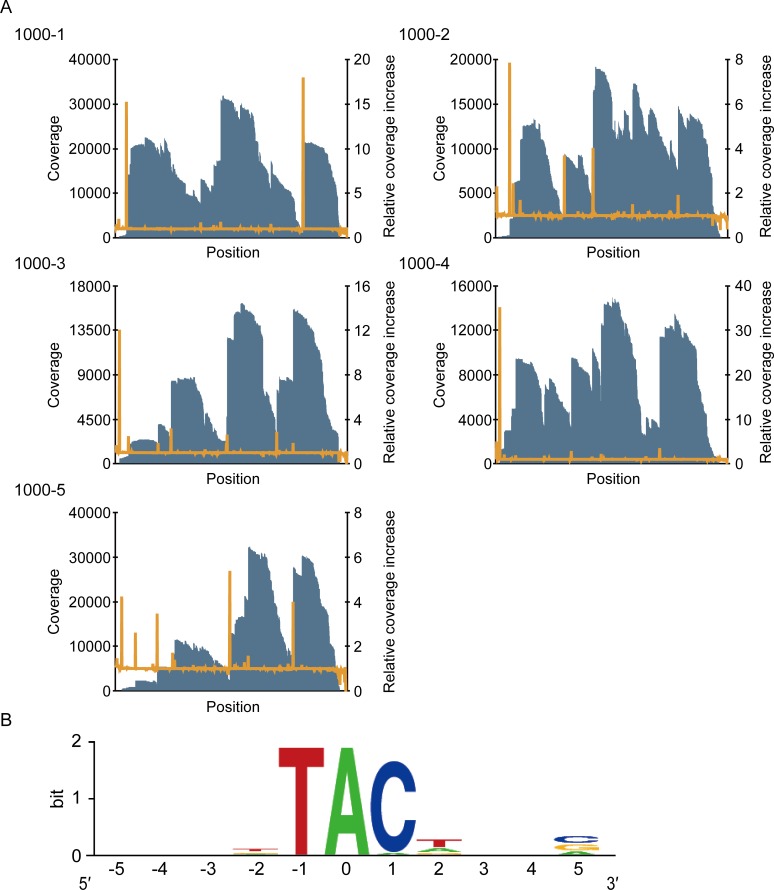
To determine the cleavage specificity of MazFpp, the MazFpp cleaved sites within the five synthetic RNAs were analyzed using massive parallel sequencing in the same manner as for E.coli MazF (Fig 4A). When the 25 sequences were aligned, the UAC triplet was highlighted as a potential cleavage sequence. Since the coverage increased at the adenine, we speculated that MazFpp specifically recognizes a UAC triplet and cuts the RNA between U and A (S4 Table, Fig 4B).
Fig 4. Analysis of the cleavage sequence of MazFpp.
(A) Graph of the coverage (blue bar) and the relative coverage increase (orange line). (B) The conserved sequence around nucleotide positions with increased coverage. The nucleotide position with significant increases in coverage was set to zero.
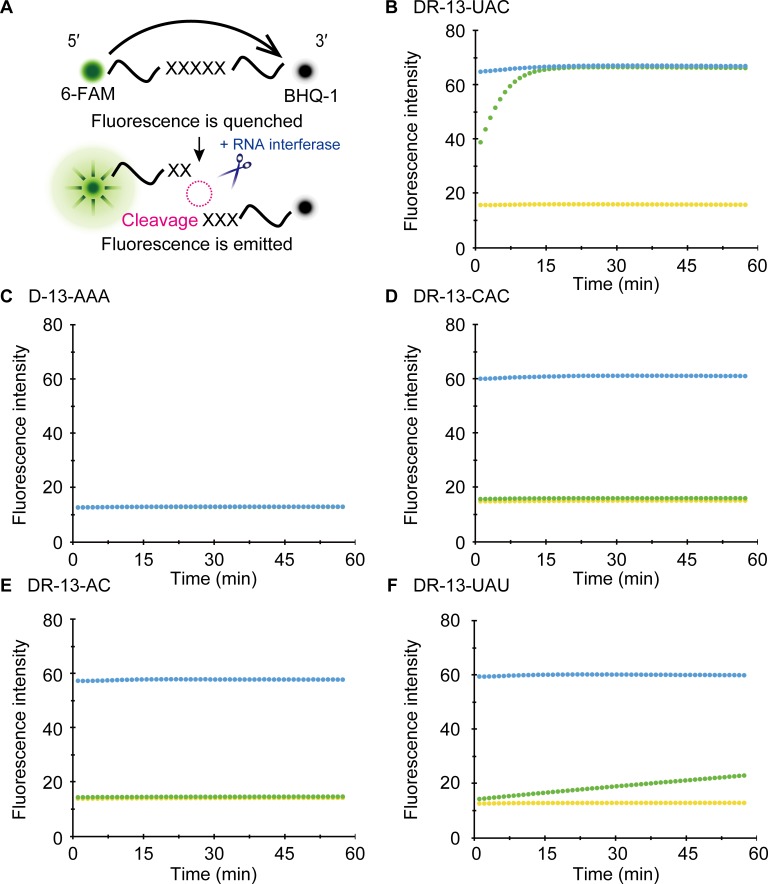
We next used a fluorometric assay [34] to further validate our results. In this assay, oligonucleotides were tagged with two fluorescent dyes, 6-carboxyfluorescein (6-FAM) as a reporter and black hole quencher-1 (BHQ-1) as a quencher, and then they were incubated with MazFpp. Fluorescence intensity was continuously measured. Since 6-FAM and BHQ-1 are in close proximity, 6-FAM is normally quenched. When MazFpp cleaves the oligonucleotides, however, these two dyes are spatially separated, resulting in a gradual increase of the fluorescence (Fig 5A). We designed various 13-base DNA, RNA, and DNA/RNA chimeric oligonucleotides (Table 1) and sought to verify the cleavage specificity of MazFpp.
Fig 5. Fluorometric detection.
(A) Principle of the fluorometric detection based on the fluorescence resonance energy transfer [34]. The fluorescent of 6-FAM is typically quenched by BHQ-1, but it emits a bright signal when the oligonucleotide is cleaved. (B-F) Investigation on the MazFpp cleavage site. One hundred nanograms of RNase A (blue) or MazFpp (green) was incubated with each oligonucleotide. The yellow plot represents a control reaction in which no enzyme was added.
Table 1. Fluorescent-labeled oligonucleotides used in fluorometric assay.
| Name | Sequence (5′ to 3′) a |
|---|---|
| DR-13-UAC | AAAAAUACAAAAA |
| D-13-AAA | AAAAAAAAAAAAA |
| R-13-UCUCG | UCUCGGUGCGUUG |
| R-13-GUUGU | GUUGUCAUGCCGG |
| R-13-UGACA | UGACACGAACCGC |
| DR-13-CAC | AAAAACACAAAAA |
| DR-13-AC | AAAAATACAAAAA |
| DR-13-UAU | AAAAAUAUAAAAA |
a Underlined letters represent RNA nucleotides and the other letters represent DNA nucleotides.
In accordance with the RNA-seq results, when a DNA/RNA chimeric oligonucleotide, DR-13-UAC, was treated with MazFpp, fluorescence intensity rapidly increased to values similar to the RNase A treated oligonucleotides (Fig 5B). In contrast, D-13-AAA, which is composed of a 13-base DNA adenine repeat, was not digested (Fig 5C). No cleavages were observed with three other RNA oligonucleotides (R-13-UCUCG, R-13-GUUGU, and R-13-UGACA) whose sequences are present in one of the synthetic RNA constructs (RNA 1000–4) used as a substrate for massive parallel sequencing (S2 Fig). As they did not include any UAC sequences, these results reinforced the notion that MazFpp shows endoribonuclease activity specifically against the UAC triplet.
We prepared three additional fluorogenic oligonucleotides with single base replacement (DR-13-CAC, DR-13-AC, DR-13-UAU). As we anticipated, substitution of the first U to the other pyrimidine base C or T (DR-13-CAC or DR-13-AC) completely impaired the MazFpp-mediated cleavage (Fig 5D and 5E). However, with DR-13-UAU, slight increase in fluorescent intensity was observed (Fig 5F). Taken together, MazFpp cleaves the UAC triplet between U and A, but also weakly recognizes other specific sequences. In addition, since the replacement of U to T completely blocked activity (DR-13-AC) (Fig 5E), the 2′ OH of the pentose [36,39] and/or the presence of a methyl group are important for the RNA cleavage. In contrast to the RNA 500–2, the transcript lacking UAC triplets (graA transcript in P. putida genome) was indeed stable even in the presence of MazFpp (S3 Fig). Taken together, these results demonstrated that the UAC sequence is the main target of this RNA interferase.
Discussion
RNA interferases, which compose TA systems, are the toxin endoribonucleases that disrupt the stability of intracellular RNAs by cleaving them in a ribosome independent [12–16] or dependent [7–11] manner. Intriguingly, some RNA interferase families such as VapC, MqsR, and MazF were reported to cleave the RNA molecules (tRNA, mRNA, and/or rRNA) in a sequence-specific manner [12,13,20,22–27,32,40–42] and to be implicated in microbial stress adaptation by regulating the translation patterns [28,29,43–46]. However, despite their widespread representation in microbial genomes, often with multiple loci [2,3,21], their sequence-specificities are poorly understood due to limitations of conventional methods for cleavage sequence detection [22,26,27,30].
In this study, we developed an easy-to-use method to define the cleavage pattern of RNA interferases with the Illumina platform (Fig 1). This method correctly identified the requisite sequence for the E. coli MazF cleavage as ACA (Fig 2). One unanticipated finding was that of the two different, previously identified positions of the cleavage site of E. coli MazF (^ACA or A^CA, where ^ is the position of the cleavage sites) [36], only ^ACA was detected in our analysis, suggesting that this endoribonuclease preferably cleaves the designed RNAs at the 5′ end of ACA. Furthermore, it successively revealed that P. putida MazF could cleave RNA at UAC triplets (Fig 4). Notably, the sequence identity between MazFpp and MazF-mt1 (Rv2801c), a MazF endoribonuclease conserved in Mycobacterium tuberculosis [47], is low (28.8%), despite the fact that both RNA interferases specifically recognize the UAC triplet. VapCRv0065 and VapCRv0617 only share 22% identity, but recognize the same 4-nt motif [30]. Similarly, MazFpp and MazF-mt1 recognize the same sequence, but the reasons for this remain unclear.
The five 1033 nucleotide synthetic RNAs employed in this study also facilitated this detection. When compared to the MORE RNA-seq, the sequence diversity of these RNAs was not rich, and thus, our method is not suitable for characterizing long base cutters such as MazF-hw, which recognizes seven bases in a strict manner [26]; indeed, these RNAs cover only 25.6% of the possible heptads (S5 Table). However, they still cover a variety of triplets, quartets, and pentads (S6 Table): 100%, 98.4%, and 95.5%, respectively. Moreover, it is feasible to design several other RNAs, which would further improve the accuracy of the cleavage-specificity determination. Based on previous reports stating that most of the RNA interferases specifically recognize less than or equal to five bases [12–14,22–24,27,41,48], these sequence combinations would be helpful for identifying which RNAs are targets in microbial cells in many cases. The notion that pathogenic bacteria and autotrophic microorganisms encode multiple RNA interferases is now widely accepted [2]. Therefore, an effective strategy to study their physiological functions would be to induce RNA interferases that are indispensable for stress adaptation, and then characterize them first with whole transcriptome and/or proteome analysis followed by our approach. A possible roadblock of our approach is that some bases might be counted as a potential cleavage sequence. For example, some might speculate that C or T at position 3 in Fig 2B and T at position 2 in Fig 4B are also involved in cleavage. The possibilities are negated with fluorometric analysis from fluorescence resonance energy transfer experiments [34], because 6-FAM-mediated intense signal was only detected when the oligonucleotide is cleaved by the RNA interferase (Fig 5 and S2 Fig).
It is known that transcripts lacking recognition sequences are not cleaved in cells [22,24,26,32]. Therefore, we attempted to identify transcripts that are translated under the environments where MazFpp is liberated. To this end, we searched for all 5350 coding sequences predicted in the P. putida genome and we found that 97 coding sequences lack UAC triplets. Interestingly, UAC triplets were also absent in the transcript of the antitoxin gene, encoded by locus PP1585. Recently, Tamman et al. demonstrated that this antitoxin composes a genuine TA pair that belongs to the HigBA family, and they named this TA pair GraTA (Growth rate-affecting toxin-antitoxin), consisting of GraT toxin and GraA antitoxin [49]. In this report, the graTA operon was efficiently repressed by GraA antitoxin, probably via its DNA binding domain. Since the graA transcript is tolerant of MazFpp (S3 Fig), the expression of GraT, which inhibits microbial growth at low temperatures but improves tolerance against some antibiotics [49], might also be negatively regulated by the preferentially translated GraA when MazFpp is released in cells. Therefore, P. putida might use these toxins selectively, depending on the environmental stresses. Moreover, since P. putida GraT has 37% sequence similarity to Vibrio chrolerae HigB, an RNA interferase that also mediates mRNA cleavage [49,50], GraT might also destabilize some intracellular RNAs. In that case, P. putida presumably improves the resistance against specific stresses by utilizing RNAs that elude the MazFpp or GraT-mediated cleavage.
In this report, we described a novel method to analyze RNA interferases with massive parallel sequencing. This approach is not specific to the MazF family of endoribonucleases but all RNA interferases that cleave intracellular RNAs in a sequence-specific manner. Furthermore, it is compatible with the fluorometric assays, enabling an easy and accurate determination of cleavage sequences in a high-throughput manner. Our approach would be useful to investigate the majority of uncharacterized RNA interferases that are conserved in the prokaryotic domain. This would further expand our understanding about physiological functions of RNA interferases.
Supporting Information
(A) Graph of the coverage (blue bar) and relative coverage increase (orange line). (B) Graphical representation of the sequences around the base with increases in coverage. The nucleotide position with significant increases in coverage was numbered as zero. Twenty-five sequences were analyzed (S3 Table) and the frequency at each position was visualized with the WebLogo program.
(TIF)
One hundred nanograms of RNase A (blue) or MazFpp (green) was incubated with RNA oligonucleotides. The yellow plot represents a control reaction where no enzyme was added.
(TIF)
Synthetic RNA 500–2 or graA transcript, which do or do not include UAC triplets, respectively, were incubated with MazFpp. Lanes 2 and 8, control reactions in which no enzyme was added; Lanes 3–6, 100 ng of MazFpp was incubated with RNA 500–2; Lanes 9–12, 100 ng of MazFpp was incubated with graA transcript.
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability
The fastaq files were deposited into the DNA Data Bank of Japan Sequence Read Archive (DRA004282). The GenBank accession numbers and GI numbers were as follows: mazEpp gene (NC_002947.3, 26986745), mazFpp gene (NC_002947.3, 26986745), graA gene (AE015451.1, 24987239), 500-2 (AB610940.1, 321117288), 1000-1 (AB610944.1, 321117292), 1000-2 (AB610945.1, 321117293), 1000-3 (AB610946.1, 321117294), 1000-4 (AB610947.1, 321117295), and 1000-5 (AB610948.1, 321117296).
Funding Statement
The authors have no support or funding to report.
References
- 1.Shao Y, Harrison EM, Bi D, Tai C, He X, Ou HY, et al. TADB: A web-based resource for Type 2 toxin-antitoxin loci in bacteria and archaea. Nucleic Acids Res. 2011;39: 606–611. 10.1093/nar/gkq908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Pandey DP, Gerdes K. Toxin-antitoxin loci are highly abundant in free-living but lost from host-associated prokaryotes. Nucleic Acids Res. 2005;33: 966–976. 10.1093/nar/gki201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Makarova KS, Wolf YI, Koonin E V. Comprehensive comparative-genomic analysis of type 2 toxin-antitoxin systems and related mobile stress response systems in prokaryotes. Biol Direct. 2009;4: 19 10.1186/1745-6150-4-19 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aizenman E, Engelberg-Kulka H, Glaser G. An Escherichia coli chromosomal “addiction module” regulated by guanosine 3′, 5′-bispyrophosphate: a model for programmed bacterial cell death. Proc Natl Acad Sci U S A. 1996;93: 6059–6063. 10.1073/pnas.93.12.6059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yamaguchi Y, Inouye M. Regulation of growth and death in Escherichia coli by toxin–antitoxin systems. Nat Rev Microbiol. 2011;9: 779–790. 10.1038/nrmicro2651 [DOI] [PubMed] [Google Scholar]
- 6.Schuster CF, Bertram R. Toxin-antitoxin systems are ubiquitous and versatile modulators of prokaryotic cell fate. FEMS Microbiol Lett. 2013;340: 73–85. 10.1111/1574-6968.12074 [DOI] [PubMed] [Google Scholar]
- 7.Christensen SK, Gerdes K. RelE toxins from Bacteria and Archaea cleave mRNAs on translating ribosomes, which are rescued by tmRNA. Mol Microbiol. 2003;48: 1389–1400. 10.1046/j.1365-2958.2003.03512.x [DOI] [PubMed] [Google Scholar]
- 8.Christensen-Dalsgaard M, Jørgensen MG, Gerdes K. Three new RelE-homologous mRNA interferases of Escherichia coli differentially induced by environmental stresses. Mol Microbiol. 2010;75: 333–348. 10.1111/j.1365-2958.2009.06969.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang Y, Yamaguchi Y, Inouye M. Characterization of YafO, an Escherichia coli toxin. J Biol Chem. 2009;284: 25522–25531. 10.1074/jbc.M109.036624 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Prysak MH, Mozdzierz CJ, Cook AM, Zhu L, Zhang Y, Inouye M, et al. Bacterial toxin YafQ is an endoribonuclease that associates with the ribosome and blocks translation elongation through sequence-specific and frame-dependent mRNA cleavage. Mol Microbiol. 2009;71: 1071–1087. 10.1111/j.1365-2958.2008.06572.x [DOI] [PubMed] [Google Scholar]
- 11.Zhang Y, Inouye M. The inhibitory mechanism of protein synthesis by YoeB, an Escherichia coli toxin. J Biol Chem. 2009;284: 6627–6638. 10.1074/jbc.M808779200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang Y, Zhang J, Hoeflich KP, Ikura M, Qing G, Inouye M. MazF cleaves cellular mRNAs specifically at ACA to block protein synthesis in Escherichia coli. Mol Cell. 2003;12: 913–923. 10.1016/S1097-2765(03)00402-7 [DOI] [PubMed] [Google Scholar]
- 13.Yamaguchi Y, Park JH, Inouye M. MqsR, a crucial regulator for quorum sensing and biofilm formation, is a GCU-specific mRNA interferase in Escherichia coli. J Biol Chem. 2009;284: 28746–28753. 10.1074/jbc.M109.032904 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhang Y, Zhu L, Zhang J, Inouye M. Characterization of ChpBK, an mRNA interferase from Escherichia coli. J Biol Chem. 2005;280: 26080–26088. 10.1074/jbc.M502050200 [DOI] [PubMed] [Google Scholar]
- 15.Jørgensen MG, Pandey DP, Jaskolska M, Gerdes K. HicA of Escherichia coli defines a novel family of translation-independent mRNA interferases in bacteria and archaea. J Bacteriol. 2009;191: 1191–1199. 10.1128/JB.01013-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Koga M, Otsuka Y, Lemire S, Yonesaki T. Escherichia coli rnlA and rnlB compose a novel toxin-antitoxin system. Genetics. 2011;187: 123–130. 10.1534/genetics.110.121798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sat B, Hazan R, Fisher T, Khaner H. Programmed Cell Death in Escherichia coli: Some Antibiotics Can Trigger mazEF Lethality. J Bacteriol. 2001;183: 2041–2045. 10.1128/JB.183.6.2041 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hazan R, Sat B, Engelberg-kulka H. Escherichia coli mazEF-Mediated Cell Death Is Triggered by Various Stressful Conditions. J Bacteriol. 2004;186: 3663–3669. 10.1128/JB.186.11.3663 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Amitai S, Kolodkin-Gal I, Hananya-Meltabashi M, Sacher A, Engelberg-kulka H. Escherichia coli MazF leads to the simultaneous selective synthesis of both “death proteins” and “survival proteins”. PLoS Genet. 2009;5 10.1371/journal.pgen.1000390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Vesper O, Amitai S, Belitsky M, Byrgazov K, Kaberdina AC, Engelberg-Kulka H, et al. Selective translation of leaderless mRNAs by specialized ribosomes generated by MazF in Escherichia coli. Cell. 2011;147: 147–157. 10.1016/j.cell.2011.07.047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chopra N, Saumitra, Pathak A, Bhatnagar R, Bhatnagar S. Linkage, mobility, and selfishness in the MazF family of bacterial toxins: A snapshot of bacterial evolution. Genome Biol Evol. 2013;5: 2268–2284. 10.1093/gbe/evt175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhu L, Inoue K, Yoshizumi S, Kobayashi H, Zhang Y, Ouyang M, et al. Staphylococcus aureus MazF specifically cleaves a pentad sequence, UACAU, which is unusually abundant in the mRNA for pathogenic adhesive factor SraP. J Bacteriol. 2009;191: 3248–3255. 10.1128/JB.01815-08 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Park JH, Yamaguchi Y, Inouye M. Bacillus subtilis MazF-bs (EndoA) is a UACAU-specific mRNA interferase. FEBS Lett. 2011;585: 2526–2532. 10.1016/j.febslet.2011.07.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rothenbacher FP, Suzuki M, Hurley JM, Montville TJ, Kirn TJ, Ouyang M, et al. Clostridium difficile MazF toxin exhibits selective, not global, mRNA cleavage. J Bacteriol. 2012;194: 3464–3474. 10.1128/JB.00217-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schuster CF, Park JH, Prax M, Herbig A, Nieselt K, Rosenstein R, et al. Characterization of a MazEF toxin-antitoxin homologue from Staphylococcus equorum. J Bacteriol. 2013;195: 115–125. 10.1128/JB.00400-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yamaguchi Y, Nariya H, Park J- H, Inouye M. Inhibition of specific gene expressions by protein-mediated mRNA interference. Nat Commun. 2012;3: 607 10.1038/ncomms1621 [DOI] [PubMed] [Google Scholar]
- 27.Zhu L, Phadtare S, Nariya H, Ouyang M, Husson RN, Inouye M. The mRNA interferases, MazF-mt3 and MazF-mt7 from Mycobacterium tuberculosis target unique pentad sequences in single-stranded RNA. Mol Microbiol. 2008;69: 559–569. 10.1111/j.1365-2958.2008.06284.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tiwari P, Arora G, Singh M, Kidwai S, Narayan OP, Singh R. MazF ribonucleases promote Mycobacterium tuberculosis drug tolerance and virulence in guinea pigs. Nat Commun. 2015;6: 6059 10.1038/ncomms7059 [DOI] [PubMed] [Google Scholar]
- 29.Nariya H, Inouye M. MazF, an mRNA Interferase, Mediates Programmed Cell Death during Multicellular Myxococcus Development. Cell. 2008;132: 55–66. 10.1016/j.cell.2007.11.044 [DOI] [PubMed] [Google Scholar]
- 30.McKenzie JL, Duyvestyn JM, Smith T, Bendak K, MacKay J, Cursons R, et al. Determination of ribonuclease sequence-specificity using Pentaprobes and mass spectrometry. RNA. 2012;18: 1267–1278. 10.1261/rna.031229.111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kirpekar F, Nordhoff E, Kristiansen K, Roepstorff P, Lezius A, Hahner S, et al. Matrix assisted laser desorption/ionization mass spectrometry of enzymatically synthesized RNA up to 150 kDa. Nucleic Acids Res. 1994;22: 3866–3870. 10.1093/nar/22.19.3866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schifano JM, Vvedenskaya IO, Knoblauch JG, Ouyang M, Nickels BE, Woychik NA. An RNA-seq method for defining endoribonuclease cleavage specificity identifies dual rRNA substrates for toxin MazF-mt3. Nat Commun. 2014;5: 3538 10.1038/ncomms4538 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Akiyama H, Ueda Y, Nobumasa H, Ooshima H, Ishizawa Y, Kitahiro K, et al. A set of external reference controls/probes that enable quality assurance between different microarray platforms. Anal Biochem. 2015;472: 75–83. 10.1016/j.ab.2014.11.012 [DOI] [PubMed] [Google Scholar]
- 34.Wang NR, Hergenrother PJ. A continuous fluorometric assay for the assessment of MazF ribonuclease activity. Anal Biochem. 2007;371: 173–183. 10.1016/j.ab.2007.07.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Buermans HPJ, den Dunnen JT. Next generation sequencing technology: Advances and applications. Biochim Biophys Acta. 2014;1842: In Press. 10.1016/j.bbadis.2014.06.015 [DOI] [PubMed] [Google Scholar]
- 36.Zhang Y, Zhang J, Hara H, Kato I, Inouye M. Insights into the mRNA cleavage mechanism by MazF, an mRNA interferase. J Biol Chem. 2005;280: 3143–3150. 10.1074/jbc.M411811200 [DOI] [PubMed] [Google Scholar]
- 37.Crooks G, Hon G, Chandonia J, Brenner SE. WebLogo: a sequence logo generator. Genome Res. 2004;14: 1188–1190. 10.1101/gr.849004.1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Poblete-Castro I, Becker J, Dohnt K, Santos VM Dos, Wittmann C. Industrial biotechnology of Pseudomonas putida and related species. Appl Microbiol Biotechnol. 2012;93: 2279–2290. 10.1007/s00253-012-3928-0 [DOI] [PubMed] [Google Scholar]
- 39.Verma S, Bhatnagar R. MoxT toxin of Bacillus anthracis exhibits sequence specific ribonuclease activity. Biochem Biophys Res Commun. 2014;450: 998–1004. 10.1016/j.bbrc.2014.06.092 [DOI] [PubMed] [Google Scholar]
- 40.Sharp JD, Cruz JW, Raman S, Inouye M, Husson RN, Woychik NA. Growth and translation inhibition through sequence-specific RNA binding by Mycobacterium tuberculosis VapC toxin. J Biol Chem. 2012;287: 12835–12847. 10.1074/jbc.M112.340109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cruz JW, Sharp JD, Hoffer ED, Maehigashi T, Vvedenskaya IO, Konkimalla A, et al. Growth-regulating Mycobacterium tuberculosis VapC-mt4 toxin is an isoacceptor-specific tRNase. Nat Commun. 2015;6: 7480 10.1038/ncomms8480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Schifano JM, Edifor R, Sharp JD, Ouyang M, Konkimalla A, Husson RN, et al. Mycobacterial toxin MazF-mt6 inhibits translation through cleavage of 23S rRNA at the ribosomal A site. Proc Natl Acad Sci U S A. 2013;110: 8501–6. 10.1073/pnas.1222031110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Maisonneuve E, Shakespeare LJ, Jørgensen MG, Gerdes K. Bacterial persistence by RNA endonucleases. Proc Natl Acad Sci U S A. 2011;108: 13206–13211. 10.1073/pnas.1100186108 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 44.Maisonneuve E, Castro-Camargo M, Gerdes K. (p)ppGpp controls bacterial persistence by stochastic induction of toxin-antitoxin activity. Cell. 2013;154: 1140–1150. 10.1016/j.cell.2013.07.048 [DOI] [PubMed] [Google Scholar]
- 45.Germain E, Roghanian M, Gerdes K, Maisonneuve E. Stochastic induction of persister cells by HipA through (p)ppGpp-mediated activation of mRNA endonucleases. Proc Natl Acad Sci. 2015; 201423536 10.1073/pnas.1423536112 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 46.Otsuka Y, Koga M, Iwamoto A, Yonesaki T. A role of RnlA in the RNase LS activity from Escherichia coli. Genes Genet Syst. 2007;82: 291–299. 10.1266/ggs.82.291 [DOI] [PubMed] [Google Scholar]
- 47.Zhu L, Zhang Y, Teh JS, Zhang J, Connell N, Rubin H, et al. Characterization of mRNA interferases from Mycobacterium tuberculosis. J Biol Chem. 2006;281: 18638–18643. 10.1074/jbc.M512693200 [DOI] [PubMed] [Google Scholar]
- 48.Zhang J, Zhang Y, Zhu L, Suzuki M, Inouye M. Interference of mRNA function by sequence-specific endoribonuclease PemK. J Biol Chem. 2004;279: 20678–20684. 10.1074/jbc.M314284200 [DOI] [PubMed] [Google Scholar]
- 49.Tamman H, Ainelo A, Ainsaar K, Hõrak R. A moderate toxin, GraT, modulates growth rate and stress tolerance of Pseudomonas putida. J Bacteriol. 2014;196: 157–169. 10.1128/JB.00851-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Christensen-Dalsgaard M, Gerdes K. Two higBA loci in the Vibrio cholerae superintegron encode mRNA cleaving enzymes and can stabilize plasmids. Mol Microbiol. 2006;62: 397–411. 10.1111/j.1365-2958.2006.05385.x [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(A) Graph of the coverage (blue bar) and relative coverage increase (orange line). (B) Graphical representation of the sequences around the base with increases in coverage. The nucleotide position with significant increases in coverage was numbered as zero. Twenty-five sequences were analyzed (S3 Table) and the frequency at each position was visualized with the WebLogo program.
(TIF)
One hundred nanograms of RNase A (blue) or MazFpp (green) was incubated with RNA oligonucleotides. The yellow plot represents a control reaction where no enzyme was added.
(TIF)
Synthetic RNA 500–2 or graA transcript, which do or do not include UAC triplets, respectively, were incubated with MazFpp. Lanes 2 and 8, control reactions in which no enzyme was added; Lanes 3–6, 100 ng of MazFpp was incubated with RNA 500–2; Lanes 9–12, 100 ng of MazFpp was incubated with graA transcript.
(TIF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
(PDF)
Data Availability Statement
The fastaq files were deposited into the DNA Data Bank of Japan Sequence Read Archive (DRA004282). The GenBank accession numbers and GI numbers were as follows: mazEpp gene (NC_002947.3, 26986745), mazFpp gene (NC_002947.3, 26986745), graA gene (AE015451.1, 24987239), 500-2 (AB610940.1, 321117288), 1000-1 (AB610944.1, 321117292), 1000-2 (AB610945.1, 321117293), 1000-3 (AB610946.1, 321117294), 1000-4 (AB610947.1, 321117295), and 1000-5 (AB610948.1, 321117296).