Abstract
Binary fission of many prokaryotes as well as some eukaryotic organelles depends on the FtsZ protein, which self-assembles into a membrane-associated ring structure early in the division process. FtsZ is homologous to tubulin, the building block of the microtubule cytoskeleton in eukaryotes. Recent advances in genomics and cell-imaging techniques have paved the way for the remarkable progress in our understanding of fission in bacteria and organelles.
Duplication of cells occurs by the division of a mother cell into two daughter cells. This process, known as cytokinesis, provides the force to split cells and is spatially regulated to faithfully partition the genetic material. The cytoskeleton has a crucial role in cytokinesis. In animal and fungal cells, a medial ring of actin and myosin, helped by other proteins, contracts to divide the cell. Plant cells use a cell plate, and are guided by actin filaments and microtubules. Prokaryotes, which include bacteria and archaea, possess homologues of eukaryotic cytoskeletal proteins. Most prokaryotes use a tubulin homologue, a protein known as FtsZ, to divide. Eukaryotic organelles such as chloroplasts and mitochondria evolved from bacteria, and all chloroplasts and some mitochondria use FtsZ to divide.
FtsZ is thought to be the first protein to localize to the site of future division in bacteria1, and it assembles into what is known as the Z ring (FIG. 1). In Escherichia coli, the Z ring recruits at least ten other proteins, all of which are required for the progression and completion of cytokinesis2, as will be discussed below. Whereas the cytokinetic apparatus in eukaryotic cells and even organelles can be observed directly in stained thin sections by transmission electron microscopy, the Z ring and its associated factors are not detectable by these methods. This is probably because the protein machinery is largely membrane bound and resides in a densely populated cytoplasmic environment. However, the development of green fluorescent protein (GFP) fusion and improved immunofluorescence techniques over the past 10 years have been crucial in allowing the first direct visualization of many cellular components and their dynamics. For example, using GFP fusions, the dynamics of the Z ring and other components of the cell-division protein machinery can now be visualized in living bacterial cells. The combination of these new cytological tools with genomics and the already powerful genetics of several model systems have spurred rapid progress in our understanding of the molecular cell biology of bacteria and eukaryotic organelles. This review will discuss how the recent revolutions in genomics and cell biology have provided new evolutionary and mechanistic insights into how bacterial cells and organelles divide.
Figure 1. A typical cell-division cycle in Escherichia coli.
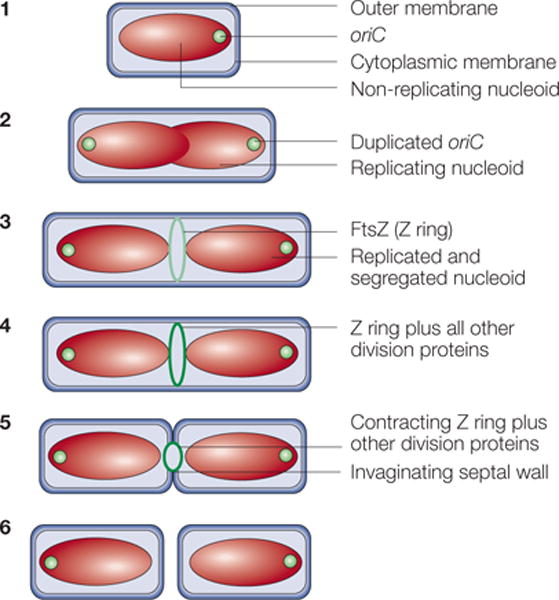
In step 1, newborn cells grown at low growth rates contain a single, non-replicating chromosome in a structure known as a nucleoid. Soon after chromosome replication initiates, the replication origins (oriC) move towards the cell poles until both daughter chromosomes are segregated (step 2). Near the end of this process, the FtsZ protein assembles into the Z ring on the inner face of the cytoplasmic membrane (light green ring) at the cell centre, marking the future division site (step 3). In step 4, the Z ring recruits at least ten membrane-associated proteins (for details, see FIG. 6) to assemble the cell-division protein machinery (dark green ring). This machinery synthesizes the division septum, which consists of cell-wall material, with the Z ring at the leading edge of membrane invagination. Contraction of the Z ring and constriction of the outer membrane follow (step 5). The result is the production of two separate newborn daughter cells (step 6).
The FtsZ family of proteins
Conservation of FtsZ
FtsZ is a highly conserved protein that is found in most of the major groups of bacteria and in the EURYARCHAEAL branch of the Archaea. However, it is absent in the CRENARCHAEA, and is also missing in a few large bacterial groups3. One of these groups, the Planctomycetes, is unusual in that some species contain a membrane-bound NUCLEOID. One representative of this group, Pirellula spp., has been completely sequenced and lacks the ftsZ gene. Several species in another diverse bacterial group that includes Chlamydiae and Verrucomicrobia also lack ftsZ. Intriguingly, Prosthecobacter dejongii, a member of the verrucomicrobial group that lacks ftsZ, contains genes that are significantly more similar to the tubulin gene than ftsZ. Although the sequences of these bacterial tubulin genes are also divergent from eukaryotic tubulins, it is likely that they were acquired from a eukaryote by horizontal gene transfer. In support of this idea, species related to P. dejongii do not have these tubulin genes. Nothing is known about the functional significance of these bacterial tubulins, although recently they have been shown to assemble into protofilaments and hydrolyse GTP4. Finally, many of the wall-less mollicutes (MYCOPLASMAS) contain ftsZ, except for Ureaplasma urealyticum, a free-living species5. The mycoplasmas have also lost many other cell division genes, but it is not clear why U. urealyticum lost ftsZ, or how it can divide without it. In fact, the mechanism by which cell division occurs in any of the species that lack ftsZ is unknown.
FtsZ is also found in eukaryotic cells. Nuclear-encoded homologues of FtsZ are imported into chloroplasts and mitochondria of primitive eukaryotes such as protists6. As a result, primitive algae have separate FtsZ homologues for their chloroplasts and mitochondria (see below). Higher plants contain two distinct families of FtsZ homologues that seem to have diverged early in plant evolution, perhaps because they have distinct, conserved functions7,8. As would be expected from their ENDOSYMBIOTIC origins, mitochondria contain FtsZ proteins that are most closely related to those of α-proteobacteria, which are the progenitors of mitochondria, whereas chloroplast FtsZs are most closely related to those of cyanobacteria, the predecessors of chloroplasts.
FtsZ domain structure
FtsZ contains four main protein domains, as determined by the crystal structure of FtsZ from the thermophilic bacterium Thermotoga maritima9 and by phylogenetic analysis3. These domains comprise a variable N-terminal segment, a highly conserved core region, a variable spacer, and a C-terminal conserved peptide (FIG. 2). The functions of the N-terminal segment and spacer have not been determined. The core region contains the tubulin signature motif and is responsible for GTP binding and hydrolysis, which is required for self-assembly of the protein (see below). Recently, this core region has been shown to consist of two independently folding N-terminal and C-terminal segments10 — the Nt core and Ct core, respectively. The Nt core contains the GTP-binding site and binds the bottom portion of the adjacent monomer in the protofilament (see below), whereas the Ct core binds the top portion of the adjacent monomer in the protofilament.
Figure 2. The domain structure of FtsZ.

The domain structure applies to most FtsZ proteins. The N terminus and variable spacer domains are highly variable in length, and their precise functions are unknown. The core region displays most similarity to tubulin and is required for GTP binding and hydrolysis as well as assembly into protofilaments. The C-terminal peptide interacts with other cell-division proteins recruited by FtsZ such as ZipA, FtsA and FtsW, and might function mainly to anchor the Z ring to the membrane using these proteins.
The C-terminal peptide is not required for assembly, but is essential for interactions with other membrane-associated cell division proteins, FtsA and ZipA. Deletion of the C-terminal peptide blocks FtsZ function, probably by preventing its interaction with both ZipA and FtsA11. The presence of either FtsA or ZipA is required for the stability of Z rings12, which implies that their functions overlap (see also below). FtsZs from different species are generally unable to substitute for E. coli FtsZ13, probably because of the specialized function of the C-terminal peptide.
Structure and assembly of FtsZ
FtsZ is a structural homologue of tubulin9. Like tubulin, purified FtsZ binds and hydrolyses GTP14–16. GTP binding induces FtsZ self-assembly into protofilaments that consist of a head-to-tail linear polymer of FtsZ10,17–19. These protofilaments and other structures, such as minirings, resemble structures formed by tubulin20. However, whereas tubulin assembles into microtubules, comprising 13 protofilaments arranged around a hollow core, FtsZ protofilaments do not assemble into microtubule-like structures. Instead, FtsZ protofilaments associate laterally to form bundles or sheets. This bundling can be induced by several factors, such as Ca2+ (REF. 21), macromolecular crowding22 and the binding of partner proteins such as ZipA23 (see FIG. 3). It is likely that FtsZ protofilament bundling is important in vivo, although without direct visualization only indirect conclusions can be drawn. Also, most biochemical studies have been done with E. coli FtsZ, but significant variations in assembly dynamics have been observed with FtsZ from Mycobacterium tuberculosis24, which emphasizes the importance of other model systems in correlating the in vivo and in vitro properties of FtsZ.
Figure 3. Assembly and disassembly of FtsZ and the Z ring.
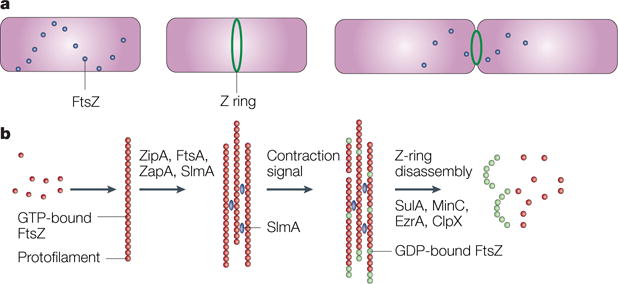
a | A time course, starting with a newborn Escherichia coli cell without a Z ring but with FtsZ in spiral patterns, followed by a cell with a Z ring, and finally, a dividing cell with a contracting Z ring. b | The putative time course of FtsZ assembly. Red dots indicate GTP-bound FtsZ dimers that assemble into protofilaments. GTP hydrolysis during assembly is probably balanced by rapid exchange of the GDP for the abundant GTP within the cell. Several protein factors, including ZipA, ZapA and probably also FtsA and the nucleoid occlusion protein SlmA (blue), bundle these protofilaments and anchor them to the cytoplasmic membrane. An unknown signal triggers ring contraction and probable disassembly; this might involve stimulation of GTP hydrolysis, which would increase the number of GDP-bound subunits at protofilament ends (shown in green), causing curved protofilaments to be formed. The free FtsZ then forms a spiral pattern throughout the cell, possibly by reassembling, and this FtsZ would then be available to form a new ring in the daughter cells. The presence of high levels of inhibitor proteins such as SulA, MinC, ClpX or EzrA (in Bacillus subtilis) might help to antagonize the ring-stabilizing proteins and tip the balance towards disassembly, although none of the inhibitor proteins is required for Z-ring contraction or disassembly.
The GTP-dependent assembly of FtsZ protofilaments in vitro is probably driven by the affinity between GTP-bound FtsZ monomers. GTP is subsequently hydrolysed by an active site formed between the two associated monomers, as in microtubule assembly25,26. However, GTP hydrolysis and subsequent nucleotide exchange seems to differ from that of microtubules. Recent data indicate that the nucleotide-binding site in FtsZ is accessible to the cytoplasm, which is rich in GTP and should therefore rapidly restore GTP binding10,27. If this conclusion is correct, then GTP hydrolysis should be the limiting step for the FtsZ assembly cycle and FtsZ protofilaments probably consist of mostly FtsZ–GTP, which is resistant to depolymerization10,28. Indeed, it has been shown in vitro that once the pool of GTP is exhausted, FtsZ protofilaments disassemble29. By contrast, microtubule protofilaments consist mostly of GDP–tubulin with a GTP cap and are susceptible to rapid depolymerization once the cap is hydrolysed30. Interestingly, GDP can allow FtsZ assembly as well, resulting in curved polymers31,32. The potential significance of such polymers in vivo is unclear, especially if nucleotide exchange from the large available pool of GTP is sufficiently rapid to saturate most FtsZ with GTP.
The actual mechanism of FtsZ assembly into protofilaments and protofilament bundles is also controversial. Most methods for monitoring FtsZ assembly indicate that the assembly of protofilaments is COOPERATIVE, with a defined critical concentration for assembly33,34. However, the presence of only one binding face for a new monomer to attach to an existing protofilament indicates an ISODESMIC ASSEMBLY mechanism35,36, as does the appearance of single protofilaments in electron micrographs of assembled FtsZ in the absence of bundling factors. How can the apparent cooperativity of assembly be rationalized with the microscopic evidence of single-stranded protofilaments? One possible answer suggested recently37 is that FtsZ initially assembles isodesmically as a curved protofilament, but after reaching a certain length the protofilament ends are able to contact each other. This process of cyclization would cause the formation of additional lateral bonds, resulting in cooperativity. In accord with this model, protofilament rings, some hundreds of nanometers in diameter, have been observed by atomic force microscopy and electron microscopy37. If cyclization could be partially suppressed in vivo, then it is conceivable that the Z ring could be a single cyclized protofilament; following longitudinal pulling, it could extend into a short spiral of cyclizing protofilaments. Spiral FtsZ structures are often observed in cells (see below). However, the 125–200 subunit circles would require 30–40 s to assemble at the diffusion-limited assembly rate of 4.6 per second34 and are therefore not compatible with the kinetics of initial assembly, nor with the 8 s half-time turnover at steady state in vitro38 and in vivo39 (H. Erickson, personal communication). As it stands, there is currently no explanation for the cooperative assembly of a single-stranded protofilament.
Regulation of Z-ring assembly
Spatial regulation
The concentration of FtsZ in the cell is approximately 10 μM, which is significantly higher than the critical concentration of 1–2 μM that is needed for protofilament assembly in vitro. This indicates that assembly inhibitors keep FtsZ from assembling except at the right time and place27. One important inhibitory system, the MIN SYSTEM, is crucial for the precise positioning of the Z ring. In E. coli, this system consists of the FtsZ assembly inhibitor MinC, and the MinD and MinE proteins, which oscillate from one cell pole to the other40,41. MinD is an ATPase that binds to the membrane in its ATP form and is released from the membrane on ATP hydrolysis. MinE drives MinD off the membrane by binding to MinD and stimulating its ATP hydrolysis. Subsequent diffusion of MinD–ADP and nucleotide exchange causes MinD–ATP to rebind to the membrane; this new binding occurs far away from the original site because of the transient presence of MinE at the original site and the kinetics of nucleotide exchange42–44. Because MinC associates with the oscillating MinD protein, FtsZ assembly is inhibited most at the cell poles and least at midcell (FIG. 4). This causes an FtsZ disassembly wave to oscillate from pole to pole45.
Figure 4. How the Z ring finds the centre of an Escherichia coli cell.
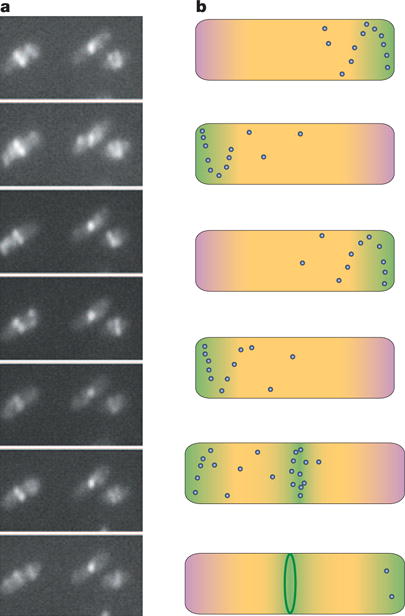
a | A time course of a group of E. coli cells expressing FtsZ–GFP, showing the Z ring at the midcell but also displaying the oscillation of FtsZ from one side of the ring to the other. b | A schematic time course of the spatial regulation of FtsZ. The top four cells span approximately 2 minutes of growth of a cell early in the cell cycle, showing how the oscillation of Min proteins (pink) and FtsZ (green), along with nucleoid occlusion (yellow), prevent assembly of the Z ring (dark green) at all locations in the cell. The fifth cell is at a later point in the cell cycle, after chromosomal replication and segregation is well underway, relieving nucleoid occlusion at the cell centre. This, along with potential positive regulators, lowers the barrier for Z-ring assembly in a zone near the middle of the cell (green), allowing Z-ring assembly (final cell). Blue dots represent higher concentrations of non-ring FtsZ that oscillate in spiral patterns. Similar mechanisms might help to centre the Z ring in chloroplasts and in other bacteria that contain the Min system, such as cyanobacteria. Nucleoid occlusion might be the main spatial regulator in species that lack Min proteins.
Whereas many bacterial species contain the MinCDE system, many others such as Caulobacter crescentus lack the Min proteins. And others, such as Bacillus subtilis, contain only MinCD. Consistent with the role of MinE in stimulating the oscillation of MinD, the MinC and MinD proteins of B. subtilis do not move but are tethered to the cell poles by another protein, DivIVA, which itself binds strongly to the cell poles46. Therefore, although it has a completely different structure and function, DivIVA is analogous to MinE in that it restricts the action of MinC to the cell poles and away from the future division site. Bacillus subtilis MinCD is important for blocking polar divisions but not for the precise placement of the Z ring at midcell47. Some spherical-shaped cells such as Neisseria gonorrhoeae and Synechocystis spp. contain all three Min proteins, which are required for normal cell division in alternating perpendicular planes48,49. It is therefore likely that the more complex problem of identifying the plane of division in such cells is determined in part by Min oscillation, which tends to follow the cell’s long axis50,51.
Another important spatial regulatory system is nucleoid occlusion52. Like the Min system, nucleoid occlusion negatively regulates Z-ring assembly. Acting independently of the Min system53, nucleoid occlusion prevents the assembly of the Z ring on top of unreplicated chromosomal DNA. As a result, Z rings do not form at the cell midpoint until after most of the chromosome has been duplicated and partitioned towards the cell poles (FIG. 4). In the absence of the Min system, multiple Z rings can assemble in DNA-free areas of the cell. This indicates that Z-ring assembly does not require a specific receptor, but instead can occur anywhere on the cytoplasmic membrane that is not blocked by nucleoid occlusion or Min54,55. Moreover, if nucleoid occlusion is inactivated, septa will form over unsegregated nucleoids, resulting in chromosome ‘guillotining’. The inactivation of nucleoid occlusion can be achieved by decondensing the chromosome56.
The recent discovery of proteins in E. coli and B. subtilis that mediate nucleoid occlusion (SlmA and Noc, respectively) has provided an important molecular framework for understanding this process57,58. Cells that lack these proteins seem normal, but when the Min system is also inactivated, FtsZ often assembles on top of nucleoids as well as between them. These multiple FtsZ assemblages sequester FtsZ subunits, thereby preventing the assembly of productive Z rings and blocking cell division. Nucleoid occlusion normally prevents Z rings from forming over unreplicated chromosomes, but artificially blocking replication in slmA− or noc− cells results in chromosome guillotining. Therefore, it seems that Noc and SlmA topologically restrict the assembly of FtsZ, enhancing the cooperativity of Z-ring assembly, and serve as a checkpoint to prevent guillotining of nucleoids. Noc and SlmA do not share sequence similarity, but both bind to multiple sites on chromosomal DNA. The normal appearance of slmA– or noc– cells indicates either that their Min systems are sufficient to restrict Z rings to the midcell, or that other, partially redundant, nucleoid occlusion systems exist. The latter is more likely, because even in the absence of Noc or SlmA and MinCD, the majority of FtsZ still localizes to spaces between segregated nucleoids, indicating that the process of nucleoid occlusion remains partially active. Curiously, purified SlmA protein from E. coli enhances the bundling of FtsZ protofilaments57, which is at odds with its role in blocking Z-ring assembly but points to the existence of other nucleoid-occlusion-mediating factors in the cell.
Although there is no evidence for the existence of proteins that localize to the midcell before FtsZ, positive factors might have a role in its localization. For example, some E. coli plasmids such as F and P1 localize at the cell quarter positions — the future cells’ midpoints — before cytokinesis. A factor that localizes to such positions might leave a mark that later helps to position the Z ring. There is some evidence that future cell poles (and therefore division sites) are recognized by proteins in filamentous cells, which is consistent with the marking of division sites59. Membrane staining has indicated that certain lipid domains might be enriched at division sites, either as a cause or an effect of Z-ring localization60. Alternative factors in ring positioning will probably be important in the many species that lack the Min system, or in species that do not exhibit obvious nucleoid occlusion61,62.
Cell-cycle timing
In E. coli, the Z ring assembles at approximately the same time as chromosome replication terminates63. Because chromosome segregation occurs continuously during replication, once replication origins are positioned near the cell poles, Z rings assemble between largely segregated bulk nucleoids. However, the basis for this temporal control is not known. No local signal from the nucleoid is required for Z-ring assembly in E. coli, as Z rings can assemble at a distance from non-replicating chromosomes in filamentous cells53,64. Moreover, although the initiation of chromosome replication is not necessary for Z-ring assembly, the proper positioning of the Z ring at the midcell is dependent on replication initiation55,56,65. In B. subtilis, the precision of Z-ring positioning at the midcell does not depend on REPLISOME positioning, because the replisome itself is highly mobile66.
Once the Z ring assembles, its contraction also becomes subject to regulation. In E. coli, Z-ring assembly is followed by a period in which the ring remains relatively stable and does not contract significantly. The reason for the delay is not clear, although during this time the process of cell-wall synthesis switches from the elongation mode to the septal mode. This switch is dependent on FtsZ but not on other cell-division proteins that are recruited by FtsZ67, which are described below. The number of Z rings that can contract in each cell cycle is also regulated. For example, cells with extra nucleoids limit the number of division events to one per cell cycle68. The factors that are involved in this cell-cycle timing of septation are not known, although DNA replication seems to be important for the proper expression of several genes, including ftsZ69. In E. coli and B. subtilis, FtsZ levels do not change significantly during the cell cycle70, which indicates that the activity of FtsZ is cell-cycle controlled. By contrast, FtsZ levels in C. crescentus vary dramatically because of proteolysis and resynthesis71; nevertheless, the artificial increase of FtsZ levels at the wrong time in the cell cycle does not trigger Z-ring assembly or change the timing of Z-ring constriction72. Finding the source of this cell-cycle control of FtsZ activity remains an important challenge for the future.
Other regulators of Z-ring assembly
The fact that Z rings can form independently of nucleoids in cells that lack Min proteins indicates that there must be regulators of Z-ring assembly other than Min and nucleoid occlusion. Indeed, several positive and negative regulators of Z-ring assembly are known (FIG. 3). ZipA, a positive regulator, bundles FtsZ protofilaments in vitro and is essential for cell division23,73. It is only present in E. coli and a few other closely related species. The topology of ZipA is unusual, with its N terminus in the cytoplasmic membrane and its C terminus, which is sufficient for the bundling activity, in the cytoplasm. A protein that has a similar topology, known as EzrA, is present in B. subtilis and other Gram-positive bacteria; EzrA, however, is a negative regulator of Z-ring assembly and inhibits FtsZ polymerization in vitro74,75. As ZipA and EzrA bind directly to FtsZ, they must therefore regulate FtsZ assembly at the membrane. Recently, the conserved chaperone ClpX was also found to inhibit Z-ring formation in B. subtilis76. Purified ClpX can inhibit FtsZ polymerization, although GTP hydrolysis is not affected, indicating that ClpX functions after the initial assembly of protofilaments.
ZapA, which was first found in B. subtilis but is present in a wide variety of species including E. coli, binds directly to FtsZ, has a strong tendency to dimerize through coiled coils and promotes the bundling of protofilaments77,78. However, as with Noc or SlmA, the loss of ZapA has no detectable consequences in vivo, except when combined with other defects78,79, which indicates that the positive regulatory function of ZapA is redundant or required only under certain circumstances. Other cell-division genes found in cyanobacteria and conserved in other species are good candidates for other FtsZ-assembly regulators61.
Finally, an inhibitor of FtsZ assembly, SulA, becomes active under conditions of DNA damage, to delay cell division until chromosomes can be properly duplicated and partitioned. Instead of being bound to DNA like Noc, the SulA protein is synthesized only following induction of the SOS RESPONSE80 and prevents Z-ring formation81, probably by directly binding FtsZ and preventing its assembly into protofilaments82. However, SulA is cleaved by the Lon protease, allowing Z rings to assemble after a delay. In B. subtilis and other Gram-positive bacteria, the YneA protein has a similar general role in disrupting Z rings after DNA damage. However, YneA has no structural similarity to SulA and its biochemical mechanism of inhibiting cell division is not yet known83.
FtsZ function in bacteria
Assembly of the cytokinesis machine
Once assembled, the Z ring recruits several membrane-associated proteins that are essential for cell division2 (FIG. 5). These proteins seem to be recruited in a linear order, with FtsA and ZipA required for all the others to arrive and the recruitment of FtsN, one of several BITOPIC membrane proteins that are essential for cell division, being dependent on all the others. FtsA and ZipA are required to anchor the Z ring to the membrane73,84. Because a point mutation in FtsA, R286W, can completely bypass the requirement for ZipA85, FtsA probably has the main role in stabilizing the Z ring at the membrane in E. coli. The recent discovery of a membrane-targeting sequence at the C terminus of FtsA, which consists of an amphipathic helix84, strongly supports this crucial role for FtsA in anchoring the Z ring to the membrane. FtsA and ZipA can bind to the same region of FtsZ because FtsZ is present at ~10,000 molecules per cell, and ZipA and FtsA are at least fivefold less abundant. In support of this idea, the presence of excess FtsA or ZipA has an inhibitory effect on cell division, which can be reversed by increasing the level of FtsZ. In M. tuberculosis, which lacks ZipA and FtsA, FtsZ might be tethered to the membrane through a unique FtsZ-binding domain that is present in another conserved cell-division protein, FtsW, which normally depends on FtsZ for its localization to the Z ring86 (FIG. 5a).
Figure 5. Fellowship of the ring.
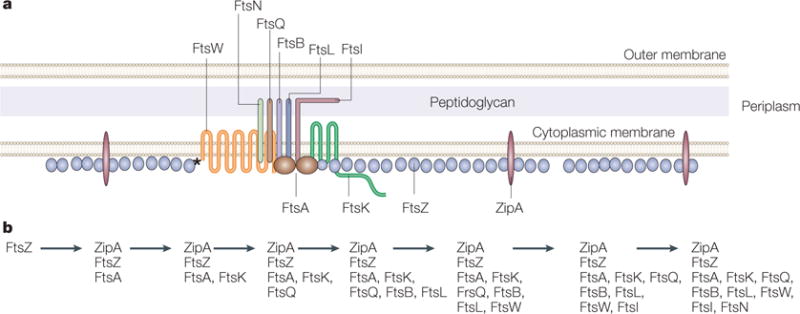
a | A model of the Escherichia coli Z ring and its essential protein partners is shown in cross section. FtsZ is shown as a series of single protofilaments at the membrane, although the actual structure of FtsZ in the Z ring is unknown. Both ZipA and FtsA contact FtsZ as well as the membrane in E. coli. However, FtsZ contacts FtsW directly in Mycobacterium tuberculosis (asterisk), which lacks ZipA and FtsA. A single transmembrane subassembly associated with an FtsA dimer is shown, based on the low relative amounts of most of the integral membrane proteins that are essential for cell division. These membrane proteins include FtsQ, FtsB, FtsL, FtsI and FtsN, which are bitopic proteins that each have a single transmembrane and periplasmic domain, and FtsW and FtsK, which are polytopic proteins with multiple transmembrane and periplasmic domains. The network of protein–protein associations is implied by the proximity of the proteins in the diagram. Proteins implicated in stabilization of the ring structure are labelled below the cytoplasmic membrane lines, whereas proteins implicated in later functions in septum formation, such as septum synthesis, are labelled above the lines. b | The dependency order of recruitment of essential cell-division proteins to the Z ring, as deduced from the requirement of a given protein for another’s localization to the Z ring.
The mechanism by which the other proteins are recruited, and how they are dependent not only on FtsZ, but also on FtsA and ZipA, is slowly being unravelled. For example, ZipA is required for the recruitment of all downstream proteins, but because it can be bypassed, it cannot be recruiting these proteins by direct, unique protein–protein contacts. Instead, ZipA probably indirectly enhances the recruitment activity of the Z ring by stabilizing the ring components. In other cases, direct protein–protein interactions are important for subassemblies of later septal proteins. This is particularly clear for FtsQ, which is needed midway in the recruitment pathway. FtsQ can co-purify with its downstream partners FtsL and FtsB87. Moreover, when targeted prematurely to the Z ring by fusion to ZapA, FtsQ can recruit FtsL, FtsB and the later protein FtsI (but not FtsN) to the Z ring in the absence of the upstream proteins FtsW or FtsA88. The failure of FtsN to be recruited in this system indicates that the proper assembly of all components at the septum, particularly FtsA, might be essential for the localization of FtsN. In support of this, FtsA or a small subdomain of FtsA, known as 1c, is able to recruit FtsI and FtsN to cell poles when fused to DivIVA, a protein that localizes to the cell poles89. Other bacterial two-hybrid assays also show interactions among many cell-division proteins, including FtsA–FtsI and FtsA–FtsN, which is consistent with a patchwork of protein–protein interactions90,91.
Assembly of the division machine and the timing of its function in other bacteria are likely to be variations on a similar theme. For example, there is more co-dependency of recruitment in B. subtilis92. In contrast to B. subtilis and E. coli, C. crescentus cell-division proteins are specifically synthesized and degraded during the cell cycle in parallel with their requirement: FtsA and FtsQ, which function after FtsZ, are synthesized and stable in predivisional cells, but are degraded post-division when they are no longer needed93. The septation process in round-shaped bacteria such as streptococci and staphylococci involves many of the same proteins, but their growth is normally dependent on the septation machinery94. As a result, there are significant differences in the timing of septal localization and function in round bacteria95,96.
Contraction and recycling of the Z ring
During the period after assembly but before visible contraction, the Z ring seems to be stable. FRAP experiments with GFP fusion proteins, however, show that individual FtsZ monomers within the ring undergo rapid exchange with FtsZ in the cytoplasm, with an average recovery time of ~8–9 s39. This indicates that subunits within the Z ring are in a constant state of flux, not unlike microtubules or actin filaments in eukaryotic cells. The GTPase-defective FtsZ84 protein, on the other hand, displays much slower turnover in the cell, providing a clear correlation between GTPase activity and ring dynamics. Nevertheless, cells with FtsZ84 divide normally at 30°C, which indicates that the high level of turnover is not essential for ring function.
How does the visible contraction of the Z ring at the leading edge of the inner membrane translate into the pinching of cell membranes and cytokinesis? Perhaps the Z ring contracts passively ahead of invaginating septal wall growth, which, in turn, is promoted by proteins such as FtsI, the septum-specific cell wall transpeptidase, that are recruited by the Z ring. Another possibility is that the rapid net loss of FtsZ monomers, which are tethered to the membrane by the other proteins of the machine, exerts a pinching force on the membrane that is analogous to the mechanical force exerted by DYNAMINS on their membrane substrates97.
Experimental evidence indicates that Z-ring contraction might be accompanied by the loss of FtsZ subunits from the Z ring. In cells that express both FtsZ and FtsZ–GFP, fluorescent polymer-like structures seem to emanate from the closing ring45,98. Moreover, this fluorescence remains in a helical pattern throughout the cell even when no Z rings are present, suggesting that non-ring FtsZ is not randomly diffuse but tethered to the membrane in a structure, possibly through ZipA or FtsA. This structure is highly mobile, as evidenced from the rapid movement of fluorescence within the helical patterns. It is not clear whether FtsZ assembly causes these helical patterns or whether FtsZ localizes to a helical track made by some other cell-envelope component.
Other cellular functions of FtsZ
The functions of FtsZ are not limited to cytokinesis. Recent evidence indicates that the machineries responsible for cell shape and cell division interact. In C. crescentus, the extended coil of the actin homologue MreB, which contributes to the rod shape of this species, contracts towards the midcell at the time of division initiation99. This contraction is dependent on FtsZ, and might be a way to spatially and temporally regulate bulk septal wall synthesis at the time of cytokinesis. In E. coli, the simultaneous inactivation of FtsZ and certain penicillin-binding proteins such as the PBP5 carboxypeptidase, neither of which induces a significant shape alteration on its own, causes cells to branch and sometimes grow helically at their poles100. It is possible that non-ring FtsZ spirals are involved in interactions with the cell wall that cause the cells to change shape. Finally, FtsZ might have a role in the duplication of certain plasmids. In several Bacillus species, in addition to the conserved chromosomal copy of ftsZ, a weak homologue of ftsZ is encoded by a plasmid3. This raises the possibility that these plasmids, one of which (pX01) is required for virulence of Bacillus anthracis, use an FtsZ variant for replication or segregation.
FtsZ and organelle division
Plastid division
Chloroplast division is probably as complex as bacterial cell division, as multiple membranes need to be coordinately invaginated and sealed. Normal chloroplast fission and distribution require FtsZ, as shown by the dramatically enlarged organelles after the inhibition of FtsZ101. The protein machine of chloroplasts, unlike that of bacteria, can be observed in electron micrographs of thin sections. One possible reason for its visibility is that this machine consists of at least three layers: an innermost ring comprising FtsZ, followed by the plastid dividing ring that is visible by negative staining but is of unknown composition102, and an outermost ring on the outside of the organelle that contains a dynamin-like protein (DRP)103,104. Dynamins are eukaryotic GTPases that assemble on membranes and mediate membrane fission97; dynamin also has a role in cytokinesis of animal and plant cells105,106. DRPs have no structural homology with FtsZ, and obvious homologues are absent from prokaryotes. Therefore, the plastid division apparatus consists of a chimaera of prokaryotic and eukaryotic fission systems (FIG. 6).
Figure 6. FtsZ and the evolution of cell and organelle fission.
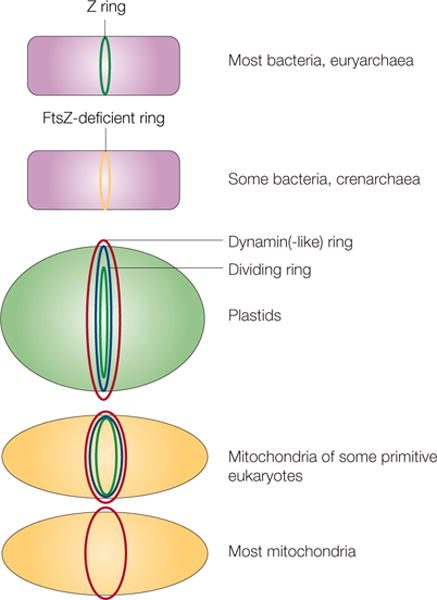
Different types of cells or organelles and their use of FtsZ or alternative proteins for fission are shown. In euryarchaea and many bacteria, FtsZ (green) localizes to the inner side of the inner membrane as the Z ring. Crenarchaea and some bacteria lack FtsZ, so some other protein must localize as a ring at the site of division (yellow). In plastids and mitochondria, dynamin or dynamin-like protein rings (red) localize to the cytoplasmic face. These organelles also contain a dividing ring (blue) and, in the case of chloroplasts and some primitive mitochondria, an innermost ring of FtsZ (green). Most mitochondria, including those of fungi and animals, lack FtsZ and a detectable dividing ring, but still rely on dynamin on the cytosolic face for fission (red).
As mentioned above, two major homologues of FtsZ are found in plants, FtsZ1 and FtsZ2. They are found in the STROMAL COMPARTMENT and co-localize to a ring structure at the midpoint of the chloroplast107. An additional homologue of FtsZ that is distinct from FtsZ1 and FtsZ2, but is still related to cyanobacterial FtsZs, is also present in plastids of primitive algae. Homologues of cyanobacterial MinD and MinE proteins are found in plants, and are required for the proper localization of plastid Z rings in Arabidopsis thaliana108,109, which indicates that the function of MinD and MinE in the spatial regulation of plastid Z rings has been conserved. Nevertheless, no homologues of MinC have been found in plants, so the factor that negatively regulates FtsZ assembly through MinD in plastids is not yet known.
Other regulators of FtsZ assembly in plastids have been identified. One protein, known as ARC6, is related to a cyanobacterial cell-division protein, Ftn2 (REF. 110). Ftn2 interacts directly with FtsZ and is important for cell division in several species of cyanobacteria49. ARC6 localizes to the membrane at the site of the plastid FtsZ ring and, like E. coli ZipA, seems to promote FtsZ assembly. Recently, a homologue of the SulA protein has been identified in A. thaliana111. However, unlike E. coli SulA, this version, which is also found in cyanobacteria, seems to have a positive role in cell and plastid division111,112. The reasons for the need for SulA in division of cyanobacteria and plastids are unknown, although it might function in the recycling of FtsZ. Other proteins might be involved in later stages of fission and in the coordination of the three membrane systems that need to be invaginated. One candidate for this function is ARTEMIS, a YidC homologue that helps to promote insertion of transmembrane proteins113. Recently, several additional cell-division genes have been discovered in cyanobacteria. Some of these, including the ylm genes, are conserved throughout most bacteria114 and are often present in plants and algae61, indicating that they might be involved in plastid division.
As in bacteria, plastid FtsZ has the potential to localize in a cytoskeletal-like array. An extensive network of filamentous structures is formed by FtsZ–GFP and a similar network is observed in electron micrographs115. This array, however, is not detected by immunostaining and might be an artefact of abnormally high expression levels107. Interestingly, in moss, one isoform of FtsZ can localize as rings in both chloroplasts and in the host-cell cytoplasm as detected by GFP fusions or by immunofluorescence116, which indicates that it might have a role in division of the host cell.
Mitochondrial fission
Like chloroplasts, mitochondria need to distribute themselves to daughter cells after each division of the eukaryotic host cell. For many types of mitochondria, this requires a dynamic balance between organelle fusion and fission. Mitochondria of animals, fungi and higher plants lack FtsZ, and it is well-established that DRPs have important functions in fission of all mitochondria6,117,118. Nevertheless, mitochondria of some primitive eukaryotes contain nuclear-encoded FtsZ119. First discovered in primitive algae120, FtsZ is also present in the mitochondria of Dictyostelium discoideum121. As with plastids, two distinct homologues of FtsZ (FszA and FszB) are found on the inside of D. discoideum mitochondria. Both are evolutionarily related to FtsZs from α-proteobacteria, which are the progenitors of mitochondria. Genetic knockouts of either FtsZ homologue in D. discoideum result in mitochondrial elongation, which is consistent with a role of each FtsZ in mitochondrial fission. However, whereas FszA localizes to bands and foci at presumptive mitochondrial fission sites, FszB localizes to foci at the ends of the organelle121. This dual localization pattern is distinct from the common medial localization seen with plastid FtsZ homologues, and indicates that the two FtsZs have different roles.
The fission apparatus of primitive mitochondria appears to be similar to that of chloroplasts, with an inner FtsZ ring, an outer ring of DRP, and a mitochondrion-dividing ring sandwiched between them117 (FIG. 6). This arrangement is consistent with the function of FtsZ in marking the initial site of division and recruiting the other two components. Once constriction by the mitochondrion-dividing ring initiates, the DRP ring functions in the late stages of fission. Apparently, this putative initial scaffolding function of FtsZ became dispensable at some point in evolution, leaving DRPs and other proteins to carry out fission. As no homologues of the Min proteins or other FtsZ regulators have been found for primitive mitochondria, it remains to be seen how the sites of fission are selected, and whether mitochondrial nucleoids have a role. It is even less clear how fission sites are selected in mitochondria that lack FtsZ.
Conclusions and future challenges
The revolutions in genomics and cell-imaging techniques have paved the way for the extraordinary progress in our understanding of fission in bacteria and organelles. Most of the players in these fundamental processes have been identified and localized to their sites of action. However, the essential nature of cytokinesis in prokaryotic cells, as well as the membrane association of most of the proteins, pose significant challenges. For example, does FtsZ function in cytokinesis by generating the constriction force itself, or is it merely a scaffold for other proteins that generate the force? How are FtsZ and other fission-associated factors such as DRPs in eukaryotes targeted to the site of fission, and what determines the timing of their action? What coordinates the invagination and fusion of multiple membranes? The present lack of biochemical assays for cytokinesis calls for novel genetic and cytological approaches that take advantage of the superb molecular genetics available for some of the bacterial and organelle model systems. In addition, the redundancy of many of the proteins, such as in E. coli cell division, means that obtaining a more minimal set of functional components is of high priority. This is where work done with diverse systems can help, as common themes will emerge that should simplify the study of the core proteins that are indispensable for cytokinesis.
Acknowledgments
Work in the Margolin laboratory is supported by grants from the National Institutes of Health and the National Science Foundation. I thank H. Erickson for helpful advice. I apologize to colleagues whose work was not cited here because of space limitations.
Glossary
- EURYARCHAEAL
Pertaining to the group of archaea that includes the methanogens and extreme halophiles
- CRENARCHAEA
The group of archaea that includes the extreme thermophiles
- NUCLEOID
The organized form of a bacterial chromosome
- MYCOPLASMAS
Wall-less bacteria
- ENDOSYMBIOTIC
Describing the engulfment of one cell by another larger cell, with the engulfed cell evolving into an organelle
- COOPERATIVE ASSEMBLY
The affinity of subunits for a polymer increases as more subunits are assembled, displaying a critical concentration below which little assembly occurs
- ISODESMIC ASSEMBLY
The opposite of cooperative assembly, in that the affinity of each new subunit for a polymer is independent of the subunit concentration
- MIN SYSTEM
A group of two or three bacterial proteins that inhibit unwanted formation of the Z ring at the cell poles
- REPLISOME
The DNA-replication protein machinery
- SOS RESPONSE
Inducible DNA repair system in bacteria invoked in response to a sudden increase in DNA damage
- BITOPIC
Describes an integral membrane protein that has one cytoplasmic, transmembrane and periplasmic domain
- FRAP
(Fluorescence recovery after photobleaching). A microscopic technique used to measure the movement (for example, diffusion rates) of fluorescently tagged molecules over time in vivo. Specific regions in a cell are irreversibly photobleached using a laser; fluorescence is restored by diffusion of fluorescently tagged unbleached molecules into the bleached area.
- DYNAMINS
A family of GTPases that are important for membrane scission
- STROMAL COMPARTMENT
The inner compartment of the chloroplast
Footnotes
Competing interests statement
The author declares no competing financial interests.
References
- 1.Bi E, Lutkenhaus J. FtsZ ring structure associated with division in Escherichia coli. Nature. 1991;354:161–164. doi: 10.1038/354161a0. [DOI] [PubMed] [Google Scholar]
- 2.Weiss DS. Bacterial cell division and the septal ring. Mol Microbiol. 2004;54:588–597. doi: 10.1111/j.1365-2958.2004.04283.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaughan S, Wickstead B, Gull K, Addinall SG. Molecular evolution of FtsZ protein sequences encoded within the genomes of archaea, bacteria, and eukaryota. J Mol Evol. 2004;58:19–29. doi: 10.1007/s00239-003-2523-5. [DOI] [PubMed] [Google Scholar]
- 4.Sontag CA, Staley JT, Erickson HP. In vitro assembly and GTP hydrolysis by bacterial tubulins BtubA and BtubB. J Cell Biol. 2005;169:233–238. doi: 10.1083/jcb.200410027. A member of the Chlamydia/Verrucomicrobia group of bacteria that lacks ftsZ contains instead two genes that are evolutionarily closer to tubulin than FtsZ. This paper investigates the assembly and nucleotide-binding properties of these two bacterial tubulins. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Glass JI, et al. The complete sequence of the mucosal pathogen Ureaplasma urealyticum. Nature. 2000;407:757–762. doi: 10.1038/35037619. [DOI] [PubMed] [Google Scholar]
- 6.Osteryoung KW, Nunnari J. The division of endosymbiotic organelles. Science. 2003;302:1698–1704. doi: 10.1126/science.1082192. This is a recent comprehensive review of organelle division. [DOI] [PubMed] [Google Scholar]
- 7.Stokes KD, Osteryoung KW. Early divergence of the FtsZ1 and FtsZ2 plastid division gene families in photosynthetic eukaryotes. Gene. 2003;320:97–108. doi: 10.1016/s0378-1119(03)00814-x. [DOI] [PubMed] [Google Scholar]
- 8.Miyagishima SY, Nozaki H, Nishida K, Matsuzaki M, Kuroiwa T. Two types of FtsZ proteins in mitochondria and red-lineage chloroplasts: the duplication of FtsZ is implicated in endosymbiosis. J Mol Evol. 2004;58:291–303. doi: 10.1007/s00239-003-2551-1. [DOI] [PubMed] [Google Scholar]
- 9.Löwe J, Amos LA. Crystal structure of the bacterial cell-division protein FtsZ. Nature. 1998;391:203–206. doi: 10.1038/34472. [DOI] [PubMed] [Google Scholar]
- 10.Oliva MA, Cordell SC, Löwe J. Structural insights into FtsZ protofilament formation. Nature Struct Mol Biol. 2004;11:1243–1250. doi: 10.1038/nsmb855. The core domain of FtsZ consists of independently folding N-terminal and C-terminal domains. In addition, this study and reference 28 suggest that nucleotide exchange occurs rapidly, with hydrolysis being the limiting step. [DOI] [PubMed] [Google Scholar]
- 11.Ma X, Margolin W. Genetic and functional analyses of the conserved C-terminal core domain of Escherichia coli FtsZ. J Bacteriol. 1999;181:7531–7544. doi: 10.1128/jb.181.24.7531-7544.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pichoff S, Lutkenhaus J. Unique and overlapping roles for ZipA and FtsA in septal ring assembly in Escherichia coli. EMBO J. 2002;21:685–693. doi: 10.1093/emboj/21.4.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Redick SD, Stricker J, Briscoe G, Erickson HP. Mutants of FtsZ targeting the protofilament interface: effects on cell division and GTPase activity. J Bacteriol. 2005;187:2727–2236. doi: 10.1128/JB.187.8.2727-2736.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Mukherjee A, Dai K, Lutkenhaus J. Escherichia coli cell division protein FtsZ is a guanine nucleotide binding protein. Proc Natl Acad Sci USA. 1993;90:1053–1057. doi: 10.1073/pnas.90.3.1053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.de Boer P, Crossley R, Rothfield L. The essential bacterial cell-division protein FtsZ is a GTPase. Nature. 1992;359:254–256. doi: 10.1038/359254a0. [DOI] [PubMed] [Google Scholar]
- 16.Raychaudhuri D, Park JT. Escherichia coli cell-division gene ftsZ encodes a novel GTP-binding protein. Nature. 1992;359:251–254. doi: 10.1038/359251a0. [DOI] [PubMed] [Google Scholar]
- 17.Mukherjee A, Lutkenhaus J. Guanine nucleotide-dependent assembly of FtsZ into filaments. J Bacteriol. 1994;176:2754–2758. doi: 10.1128/jb.176.9.2754-2758.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Erickson HP, Taylor DW, Taylor KA, Bramhill D. Bacterial cell division protein FtsZ assembles into protofilament sheets and minirings, structural homologs of tubulin polymers. Proc Natl Acad Sci USA. 1996;93:519–523. doi: 10.1073/pnas.93.1.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Löwe J, Amos LA. Tubulin-like protofilaments in Ca2+-induced FtsZ sheets. EMBO J. 1999;18:2364–2371. doi: 10.1093/emboj/18.9.2364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Erickson HP, Stoffler D. Protofilaments and rings, two conformations of the tubulin family conserved from bacterial FtsZ to α, β, and γ tubulin. J Cell Biol. 1996;135:5–8. doi: 10.1083/jcb.135.1.5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yu XC, Margolin W. Ca2+-mediated GTP-dependent dynamic assembly of bacterial cell division protein FtsZ into asters and polymer networks in vitro. EMBO J. 1997;16:5455–5463. doi: 10.1093/emboj/16.17.5455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez JM, et al. Essential cell division protein FtsZ assembles into one monomer-thick ribbons under conditions resembling the crowded intracellular environment. J Biol Chem. 2003;278:37664–37671. doi: 10.1074/jbc.M305230200. [DOI] [PubMed] [Google Scholar]
- 23.Raychaudhuri D. ZipA is a MAP-Tau homolog and is essential for structural integrity of the cytokinetic FtsZ ring during bacterial cell division. EMBO J. 1999;18:2372–2383. doi: 10.1093/emboj/18.9.2372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.White EL, et al. Slow polymerization of Mycobacterium tuberculosis FtsZ. J Bacteriol. 2000;182:4028–4034. doi: 10.1128/jb.182.14.4028-4034.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sossong TM, Jr, Brigham-Burke MR, Hensley P, Pearce KH., Jr Self-activation of guanosine triphosphatase activity by oligomerization of the bacterial cell division protein FtsZ. Biochemistry. 1999;38:14843–14850. doi: 10.1021/bi990917e. [DOI] [PubMed] [Google Scholar]
- 26.Scheffers DJ, de Wit JG, den Blaauwen T, Driessen AJ. GTP hydrolysis of cell division protein FtsZ: evidence that the active site is formed by the association of monomers. Biochemistry. 2002;41:521–529. doi: 10.1021/bi011370i. [DOI] [PubMed] [Google Scholar]
- 27.Romberg L, Levin PA. Assembly dynamics of the bacterial cell division protein FtsZ: poised at the edge of stability. Annu Rev Microbiol. 2003;57:125–154. doi: 10.1146/annurev.micro.57.012903.074300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Romberg L, Mitchison TJ. Rate-limiting guanosine 5′-triphosphate hydrolysis during nucleotide turnover by FtsZ, a prokaryotic tubulin homologue involved in bacterial cell division. Biochemistry. 2004;43:282–288. doi: 10.1021/bi035465r. [DOI] [PubMed] [Google Scholar]
- 29.Mukherjee A, Lutkenhaus J. Dynamic assembly of FtsZ regulated by GTP hydrolysis. EMBO J. 1998;17:462–469. doi: 10.1093/emboj/17.2.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Desai A, Mitchison TJ. Microtubule polymerization dynamics. Annu Rev Cell Dev Biol. 1997;13:83–117. doi: 10.1146/annurev.cellbio.13.1.83. [DOI] [PubMed] [Google Scholar]
- 31.Huecas S, Andreu JM. Energetics of the cooperative assembly of cell division protein FtsZ and the nucleotide hydrolysis switch. J Biol Chem. 2003;278:46146–46154. doi: 10.1074/jbc.M307128200. [DOI] [PubMed] [Google Scholar]
- 32.Lu C, Reedy M, Erickson HP. Straight and curved conformations of FtsZ are regulated by GTP hydrolysis. J Bacteriol. 2000;182:164–170. doi: 10.1128/jb.182.1.164-170.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Caplan M, Erickson HP. Apparent cooperative assembly of the bacterial cell-division protein FtsZ demonstrated by isothermal titration calorimetry. J Biol Chem. 2003;278:13784–13788. doi: 10.1074/jbc.M300860200. [DOI] [PubMed] [Google Scholar]
- 34.Chen Y, Bjornson K, Redick SD, Erickson HP. A rapid fluorescence assay for FtsZ assembly indicates cooperative assembly with a dimer nucleus. Biophys J. 2005;88:505–514. doi: 10.1529/biophysj.104.044149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Rivas G, et al. Magnesium-induced linear self-association of the FtsZ bacterial cell division protein monomer. The primary steps for FtsZ assembly. J Biol Chem. 2000;275:11740–11749. doi: 10.1074/jbc.275.16.11740. [DOI] [PubMed] [Google Scholar]
- 36.Romberg L, Simon M, Erickson HP. Polymerization of FtsZ, a bacterial homolog of tubulin: Is assembly cooperative? J Biol Chem. 2001;276:11743–11753. doi: 10.1074/jbc.M009033200. [DOI] [PubMed] [Google Scholar]
- 37.Gonzalez JM, et al. Cooperative behavior of Escherichia coli cell-division protein FtsZ assembly involves the preferential cyclization of long single-stranded fibrils. Proc Natl Acad Sci USA. 2005;102:1895–1900. doi: 10.1073/pnas.0409517102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chen Y, Erickson HP. Rapid in vitro assembly dynamics and subunit turnover of FtsZ demonstrated by fluorescence resonance energy transfer. J Biol Chem. 2005;280:22549–22554. doi: 10.1074/jbc.M500895200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Anderson DE, Gueiros-Filho FJ, Erickson HP. Assembly dynamics of FtsZ rings in Bacillus subtilis and Escherichia coli and effects of FtsZ-regulating proteins. J Bacteriol. 2004;186:5775–5781. doi: 10.1128/JB.186.17.5775-5781.2004. Although the Z ring seems to be stable, FtsZ subunits turn over rapidly within the ring, with a half-time of 8–9 s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Raskin DM, de Boer PA. Rapid pole-to-pole oscillation of a protein required for directing division to the middle of Escherichia coli. Proc Natl Acad Sci USA. 1999;96:4971–4976. doi: 10.1073/pnas.96.9.4971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hu Z, Mukherjee A, Pichoff S, Lutkenhaus J. The MinC component of the division site selection system in Escherichia coli interacts with FtsZ to prevent polymerization. Proc Natl Acad Sci USA. 1999;96:14819–14824. doi: 10.1073/pnas.96.26.14819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Suefuji K, Valluzzi R, Raychaudhuri D. Dynamic assembly of MinD into filament bundles modulated by ATP, phospholipids, and MinE. Proc Natl Acad Sci USA. 2002;99:16776–16781. doi: 10.1073/pnas.262671699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Hu Z, Lutkenhaus J. Topological regulation of cell division in E. coli.: spatiotemporal scillation of MinD requires stimulation of its ATPase by MinE and phospholipid. Mol Cell. 2001;7:1337–1343. doi: 10.1016/s1097-2765(01)00273-8. [DOI] [PubMed] [Google Scholar]
- 44.Huang KC, Meir Y, Wingreen NS. Dynamic structures in Escherichia coli: spontaneous formation of MinE rings and MinD polar zones. Proc Natl Acad Sci USA. 2003;100:12724–12728. doi: 10.1073/pnas.2135445100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Thanedar S, Margolin W. FtsZ exhibits rapid movement and oscillation waves in helix-like patterns in Escherichia coli. Curr Biol. 2004;14:1167–1173. doi: 10.1016/j.cub.2004.06.048. Non-ring FtsZ is not randomly dispersed within the cell but instead oscillates from pole to pole in a Min-dependent manner, in a spiral pattern. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Marston AL, Thomaides HB, Edwards DH, Sharpe ME, Errington J. Polar localization of the MinD protein of Bacillus subtilis and its role in selection of the mid-cell division site. Genes Dev. 1998;12:3419–3430. doi: 10.1101/gad.12.21.3419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Migocki MD, Freeman MK, Wake RG, Harry EJ. The Min system is not required for precise placement of the midcell Z ring in Bacillus subtilis. EMBO Rep. 2002;3:1163–1167. doi: 10.1093/embo-reports/kvf233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Szeto J, et al. Gonococcal MinD affects cell division in Neisseria gonorrhoeae and Escherichia coli and exhibits a novel self-interaction. J Bacteriol. 2001;183:6253–6264. doi: 10.1128/JB.183.21.6253-6264.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Mazouni K, Domain F, Cassier-Chauvat C, Chauvat F. Molecular analysis of the key cytokinetic components of cyanobacteria: FtsZ, ZipN and MinCDE. Mol Microbiol. 2004;52:1145–1158. doi: 10.1111/j.1365-2958.2004.04042.x. [DOI] [PubMed] [Google Scholar]
- 50.Corbin BD, Yu XC, Margolin W. Exploring intracellular space: function of the Min system in round-shaped Escherichia coli. EMBO J. 2002;21:1988–2008. doi: 10.1093/emboj/21.8.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ramirez-Arcos S, Szeto J, Dillon JA, Margolin W. Conservation of dynamic localization among MinD and MinE orthologues: oscillation of Neisseria gonorrhoeae proteins in Escherichia coli. Mol Microbiol. 2002;46:493–504. doi: 10.1046/j.1365-2958.2002.03168.x. [DOI] [PubMed] [Google Scholar]
- 52.Woldringh C, et al. Role of the nucleoid in the toporegulation of division. Res Microbiol. 1990;141:39–49. doi: 10.1016/0923-2508(90)90096-9. [DOI] [PubMed] [Google Scholar]
- 53.Sun Q, Margolin W. Influence of the nucleoid on placement of FtsZ and MinE rings in Escherichia coli. J Bacteriol. 2001;183:1413–1422. doi: 10.1128/JB.183.4.1413-1422.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yu XC, Margolin W. FtsZ ring clusters in min and partition mutants: role of both the Min system and the nucleoid in regulating FtsZ ring localization. Mol Microbiol. 1999;32:315–326. doi: 10.1046/j.1365-2958.1999.01351.x. [DOI] [PubMed] [Google Scholar]
- 55.Quardokus EM, Brun YV. DNA replication initiation is required for mid-cell positioning of FtsZ rings in Caulobacter crescentus. Mol Microbiol. 2002;45:605–616. doi: 10.1046/j.1365-2958.2002.03040.x. [DOI] [PubMed] [Google Scholar]
- 56.Sun Q, Margolin W. Effects of perturbing nucleoid structure on nucleoid occlusion-mediated toporegulation of FtsZ ring assembly. J Bacteriol. 2004;186:3951–3959. doi: 10.1128/JB.186.12.3951-3959.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bernhardt TG, de Boer PA. SlmA, a nucleoid-associated, FtsZ-binding protein required for blocking septal ring assembly over chromosomes in E. coli. Mol Cell. 2005;18:555–564. doi: 10.1016/j.molcel.2005.04.012. This paper, along with reference 58, demonstrates that nucleoid occlusion is mediated by DNA-binding proteins that interact with FtsZ. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Wu LJ, Errington J. Coordination of cell division and chromosome segregation by a nucleoid occlusion protein in Bacillus subtilis. Cell. 2004;117:915–925. doi: 10.1016/j.cell.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 59.Janakiraman A, Goldberg MB. Evidence for polar positional information independent of cell division and nucleoid occlusion. Proc Natl Acad Sci USA. 2004;101:835–840. doi: 10.1073/pnas.0305747101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Mileykovskaya E, Dowhan W. Visualization of phospholipid domains in Escherichia coli by using the cardiolipin-specific fluorescent dye 10-N-nonyl acridine orange. J Bacteriol. 2000;182:1172–1175. doi: 10.1128/jb.182.4.1172-1175.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Miyagishima SY, Wolk CP, Osteryoung KW. Identification of cyanobacterial cell division genes by comparative and mutational analyses. Mol Microbiol. 2005;56:126–143. doi: 10.1111/j.1365-2958.2005.04548.x. [DOI] [PubMed] [Google Scholar]
- 62.Ramos A, et al. Altered morphology produced by ftsZ expression in Corynebacterium glutamicum ATCC 13869. Microbiology. 2005;151:2563–2572. doi: 10.1099/mic.0.28036-0. [DOI] [PubMed] [Google Scholar]
- 63.Den Blaauwen T, Buddelmeijer N, Aarsman ME, Hameete CM, Nanninga N. Timing of FtsZ assembly in Escherichia coli. J Bacteriol. 1999;181:5167–5175. doi: 10.1128/jb.181.17.5167-5175.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gullbrand B, Nordström K. FtsZ ring formation without subsequent cell division after replication runout in Escherichia coli. Mol Microbiol. 2000;36:1349–1359. doi: 10.1046/j.1365-2958.2000.01949.x. [DOI] [PubMed] [Google Scholar]
- 65.Harry EJ, Rodwell J, Wake RG. Co-ordinating DNA replication with cell division in bacteria: a link between the early stages of a round of replication and mid-cell Z ring assembly. Mol Microbiol. 1999;33:33–40. doi: 10.1046/j.1365-2958.1999.01439.x. [DOI] [PubMed] [Google Scholar]
- 66.Migocki MD, Lewis PJ, Wake RG, Harry EJ. The midcell replication factory in Bacillus subtilis is highly mobile: implications for coordinating chromosome replication with other cell cycle events. Mol Microbiol. 2004;54:452–463. doi: 10.1111/j.1365-2958.2004.04267.x. [DOI] [PubMed] [Google Scholar]
- 67.de Pedro MA, Quintela JC, Holtje JV, Schwarz H. Murein segregation in Escherichia coli. J Bacteriol. 1997;179:2823–2834. doi: 10.1128/jb.179.9.2823-2834.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bach T, Skarstad K. Re-replication from non-sequesterable origins generates three-nucleoid cells which divide asymmetrically. Mol Microbiol. 2004;51:1589–1600. doi: 10.1111/j.1365-2958.2003.03943.x. [DOI] [PubMed] [Google Scholar]
- 69.Liu G, Begg K, Geddes A, Donachie WD. Transcription of essential cell division genes is linked to chromosome replication in Escherichia coli. Mol Microbiol. 2001;40:909–916. doi: 10.1046/j.1365-2958.2001.02434.x. [DOI] [PubMed] [Google Scholar]
- 70.Weart RB, Levin PA. Growth rate-dependent regulation of medial FtsZ ring formation. J Bacteriol. 2003;185:2826–2834. doi: 10.1128/JB.185.9.2826-2834.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kelly AJ, Sackett M, Din N, Quardokus E, Brun YV. Cell cycle dependent transcriptional and proteolytic regulation of FtsZ in Caulobacter. Genes Dev. 1998;12:880–893. doi: 10.1101/gad.12.6.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Quardokus EM, Din N, Brun YV. Cell cycle and positional constraints on FtsZ localization and the initiation of cell division in Caulobacter crescentus. Mol Microbiol. 2001;39:949–959. doi: 10.1046/j.1365-2958.2001.02287.x. [DOI] [PubMed] [Google Scholar]
- 73.Hale CA, de Boer PA. Direct binding of FtsZ to ZipA, an essential component of the septal ring structure that mediates cell division in E. coli Cell. 1997;88:175–185. doi: 10.1016/s0092-8674(00)81838-3. [DOI] [PubMed] [Google Scholar]
- 74.Levin PA, Kurtser IG, Grossman AD. Identification and characterization of a negative regulator of FtsZ ring formation in Bacillus subtilis. Proc Natl Acad Sci USA. 1999;96:9642–9647. doi: 10.1073/pnas.96.17.9642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Haeusser DP, Schwartz RL, Smith AM, Oates ME, Levin PA. EzrA prevents aberrant cell division by modulating assembly of the cytoskeletal protein FtsZ. Mol Microbiol. 2004;52:801–814. doi: 10.1111/j.1365-2958.2004.04016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Weart RB, Nakano S, Lane BE, Zuber P, Levin PA. The ClpX chaperone modulates assembly of the tubulin-like protein FtsZ. Mol Microbiol. 2005;57:238–249. doi: 10.1111/j.1365-2958.2005.04673.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Low HH, Moncrieffe MC, Lowe J. The crystal structure of ZapA and its modulation of FtsZ polymerisation. J Mol Biol. 2004;341:839–852. doi: 10.1016/j.jmb.2004.05.031. [DOI] [PubMed] [Google Scholar]
- 78.Gueiros-Filho FJ, Losick R. A widely conserved bacterial cell division protein that promotes assembly of the tubulin-like protein FtsZ. Genes Dev. 2002;16:2544–2556. doi: 10.1101/gad.1014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Johnson JE, Lackner LL, Hale CA, de Boer PA. ZipA is required for targeting of DMinC/DicB, but not DMinC/MinD, complexes to septal ring assemblies in Escherichia coli. J Bacteriol. 2004;186:2418–2429. doi: 10.1128/JB.186.8.2418-2429.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Huisman O, D’Ari R. An inducible DNA replication-cell division coupling mechanism in E. coli. Nature. 1981;290:797–799. doi: 10.1038/290797a0. [DOI] [PubMed] [Google Scholar]
- 81.Bi E, Lutkenhaus J. Cell division inhibitors SulA and MinCD prevent formation of the FtsZ ring. J Bacteriol. 1993;175:1118–1125. doi: 10.1128/jb.175.4.1118-1125.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Cordell SC, Robinson EJ, Lowe J. Crystal structure of the SOS cell division inhibitor SulA and in complex with FtsZ. Proc Natl Acad Sci USA. 2003;100:7889–7894. doi: 10.1073/pnas.1330742100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Kawai Y, Moriya S, Ogasawara N. Identification of a protein, YneA, responsible for cell division suppression during the SOS response in Bacillus subtilis. Mol Microbiol. 2003;47:1113–1122. doi: 10.1046/j.1365-2958.2003.03360.x. [DOI] [PubMed] [Google Scholar]
- 84.Pichoff S, Lutkenhaus J. Tethering the Z ring to the membrane through a conserved membrane targeting sequence in FtsA. Mol Microbiol. 2005;55:1722–1734. doi: 10.1111/j.1365-2958.2005.04522.x. An independent membrane-targeting sequence is required for FtsA to interact with the membrane, which in turn helps to keep the Z ring anchored to the membrane. [DOI] [PubMed] [Google Scholar]
- 85.Geissler B, Elraheb D, Margolin W. A gain of function mutation in ftsA bypasses the requirement for the essential cell division gene zipA in Escherichia coli. Proc Natl Acad Sci USA. 2003;100:4197–4202. doi: 10.1073/pnas.0635003100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Datta P, Dasgupta A, Bhakta S, Basu J. Interaction between FtsZ and FtsW of Mycobacterium tuberculosis. J Biol Chem. 2002;277:24983–24987. doi: 10.1074/jbc.M203847200. [DOI] [PubMed] [Google Scholar]
- 87.Buddelmeijer N, Beckwith J. A complex of the Escherichia coli cell division proteins FtsL, FtsB and FtsQ forms independently of its localization to the septal region. Mol Microbiol. 2004;52:1315–1327. doi: 10.1111/j.1365-2958.2004.04044.x. [DOI] [PubMed] [Google Scholar]
- 88.Goehring NW, Gueiros-Filho F, Beckwith J. Premature targeting of a cell division protein to midcell allows dissection of divisome assembly in Escherichia coli. Genes Dev. 2005;19:127–137. doi: 10.1101/gad.1253805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Corbin BD, Geissler B, Sadasivam M, Margolin W. A Z-ring-independent interaction between a subdomain of FtsA and late septation proteins as revealed by a polar recruitment assay. J Bacteriol. 2004;186:7736–7744. doi: 10.1128/JB.186.22.7736-7744.2004. This paper, along with reference 88, uses novel cytological methods for dissecting the complex interactions among cell-division proteins and provides additional evidence for the existence of membrane-protein subassemblies. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Di Lallo G, Fagioli M, Barionovi D, Ghelardini P, Paolozzi L. Use of a two-hybrid assay to study the assembly of a complex multicomponent protein machinery: bacterial septosome differentiation. Microbiology. 2003;149:3353–3359. doi: 10.1099/mic.0.26580-0. [DOI] [PubMed] [Google Scholar]
- 91.Karimova G, Dautin N, Ladant D. Interaction network among Escherichia coli membrane proteins involved in cell division as revealed by bacterial two-hybrid analysis. J Bacteriol. 2005;187:2233–2243. doi: 10.1128/JB.187.7.2233-2243.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Robson SA, Michie KA, Mackay JP, Harry EJ, King GF. The Bacillus subtilis cell division proteins FtsL and DivIC are intrinsically unstable and do not interact with one another in the absence of other septasomal components. Mol Microbiol. 2002;44:663–674. doi: 10.1046/j.1365-2958.2002.02920.x. [DOI] [PubMed] [Google Scholar]
- 93.Martin ME, Trimble MJ, Brun YV. Cell cycle-dependent abundance, stability and localization of FtsA and FtsQ in Caulobacter crescentus. Mol Microbiol. 2004;54:60–74. doi: 10.1111/j.1365-2958.2004.04251.x. [DOI] [PubMed] [Google Scholar]
- 94.Pinho MG, Errington J. Dispersed mode of Staphylococcus aureus cell wall synthesis in the absence of the division machinery. Mol Microbiol. 2003;50:871–881. doi: 10.1046/j.1365-2958.2003.03719.x. [DOI] [PubMed] [Google Scholar]
- 95.Morlot C, Noirclerc-Savoye M, Zapun A, Dideberg O, Vernet T. The D, D-carboxypeptidase PBP3 organizes the division process of Streptococcus pneumoniae. Mol Microbiol. 2004;51:1641–1648. doi: 10.1046/j.1365-2958.2003.03953.x. [DOI] [PubMed] [Google Scholar]
- 96.Pinho MG, Errington J. Recruitment of penicillin-binding protein PBP2 to the division site of Staphylococcus aureus is dependent on its transpeptidation substrates. Mol Microbiol. 2005;55:799–807. doi: 10.1111/j.1365-2958.2004.04420.x. [DOI] [PubMed] [Google Scholar]
- 97.Praefcke GJ, McMahon HT. The dynamin superfamily: universal membrane tubulation and fission molecules? Nature Rev Mol Cell Biol. 2004;5:133–147. doi: 10.1038/nrm1313. [DOI] [PubMed] [Google Scholar]
- 98.Sun Q, Margolin W. FtsZ dynamics during the cell division cycle of live Escherichia coli. J Bacteriol. 1998;180:2050–2056. doi: 10.1128/jb.180.8.2050-2056.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Figge RM, Divakaruni AV, Gober JW. MreB, the cell shape-determining bacterial actin homologue, co-ordinates cell wall morphogenesis in Caulobacter crescentus. Mol Microbiol. 2004;51:1321–1332. doi: 10.1111/j.1365-2958.2003.03936.x. [DOI] [PubMed] [Google Scholar]
- 100.Varma A, Young KD. FtsZ collaborates with penicillin binding proteins to generate bacterial cell shape in. Escherichia coli J Bacteriol. 2004;186:6768–6774. doi: 10.1128/JB.186.20.6768-6774.2004. FtsZ is involved in the maintenance of proper cell shape in addition to its established role in cytokinesis. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Osteryoung KW, Stokes KD, Rutherford SM, Percival AL, Lee WY. Chloroplast division in higher plants requires members of two functionally divergent gene families with homology to bacterial FtsZ. Plant Cell. 1998;10:1991–2004. doi: 10.1105/tpc.10.12.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Miyagishima S, Takahara M, Kuroiwa T. Novel filaments 5 nm in diameter constitute the cytosolic ring of the plastid division apparatus. Plant Cell. 2001;13:707–721. doi: 10.1105/tpc.13.3.707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Miyagishima SY, et al. A plant-specific dynamin-related protein forms a ring at the chloroplast division site. Plant Cell. 2003;15:655–665. doi: 10.1105/tpc.009373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Gao H, Kadirjan-Kalbach D, Froehlich JE, Osteryoung KW. ARC5, a cytosolic dynamin-like protein from plants, is part of the chloroplast division machinery. Proc Natl Acad Sci USA. 2003;100:4328–4333. doi: 10.1073/pnas.0530206100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Thompson HM, Skop AR, Euteneuer U, Meyer BJ, McNiven MA. The large GTPase dynamin associates with the spindle midzone and is required for cytokinesis. Curr Biol. 2002;12:2111–2117. doi: 10.1016/s0960-9822(02)01390-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Kang BH, Busse JS, Bednarek SY. Members of the Arabidopsis dynamin-like gene family, ADL1, are essential for plant cytokinesis and polarized cell growth. Plant Cell. 2003;15:899–913. doi: 10.1105/tpc.009670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Vitha S, McAndrew RS, Osteryoung KW. FtsZ ring formation at the chloroplast division site in plants. J Cell Biol. 2001;153:111–120. doi: 10.1083/jcb.153.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Colletti KS, et al. A homologue of the bacterial cell division site-determining factor MinD mediates placement of the chloroplast division apparatus. Curr Biol. 2000;10:507–516. doi: 10.1016/s0960-9822(00)00466-8. [DOI] [PubMed] [Google Scholar]
- 109.Itoh R, Fujiwara M, Nagata N, Yoshida S. A chloroplast protein homologous to the eubacterial topological specificity factor MinE plays a role in chloroplast division. Plant Physiol. 2001;127:1644–1655. [PMC free article] [PubMed] [Google Scholar]
- 110.Vitha S, et al. ARC6 is a J-domain plastid division protein and an evolutionary descendant of the cyanobacterial cell division protein Ftn2. Plant Cell. 2003;15:1918–1933. doi: 10.1105/tpc.013292. Evidence is mounting that several cyanobacterial cell-division proteins might have been retained by plants for chloroplast division. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Raynaud C, Cassier-Chauvat C, Perennes C, Bergounioux C. An Arabidopsis homolog of the bacterial cell division inhibitor SulA is involved in plastid division. Plant Cell. 2004;16:1801–1811. doi: 10.1105/tpc.022335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Maple J, et al. GIANT CHLOROPLAST 1 is essential for correct plastid division in Arabidopsis. Curr Biol. 2004;14:776–781. doi: 10.1016/j.cub.2004.04.031. [DOI] [PubMed] [Google Scholar]
- 113.Fulgosi H, Gerdes L, Westphal S, Glockmann C, Soll J. Cell and chloroplast division requires ARTEMIS. Proc Natl Acad Sci USA. 2002;99:11501–11506. doi: 10.1073/pnas.172032599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Fadda D, et al. Characterization of divIVA and other genes located in the chromosomal region downstream of the dcw cluster in Streptococcus pneumoniae. J Bacteriol. 2003;185:6209–6214. doi: 10.1128/JB.185.20.6209-6214.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Reski R. Rings and networks: the amazing complexity of FtsZ in chloroplasts. Trends Plant Sci. 2002;7:103–105. doi: 10.1016/s1360-1385(02)02232-x. [DOI] [PubMed] [Google Scholar]
- 116.Kiessling J, et al. Dual targeting of plastid division protein FtsZ to chloroplasts and the cytoplasm. EMBO Rep. 2004;5:889–894. doi: 10.1038/sj.embor.7400238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Nishida K, et al. Dynamic recruitment of dynamin for final mitochondrial severance in a primitive red alga. Proc Natl Acad Sci USA. 2003;100:2146–2151. doi: 10.1073/pnas.0436886100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Bleazard W, et al. The dynamin-related GTPase Dnm1 regulates mitochondrial fission in yeast. Nature Cell Biol. 1999;1:298–304. doi: 10.1038/13014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Kiefel BR, Gilson PR, Beech PL. Diverse eukaryotes have retained mitochondrial homologues of the bacterial division protein FtsZ. Protist. 2004;155:105–115. doi: 10.1078/1434461000168. [DOI] [PubMed] [Google Scholar]
- 120.Beech PL, et al. Mitochondrial FtsZ in a chromophyte alga. Science. 2000;287:1276–1279. doi: 10.1126/science.287.5456.1276. [DOI] [PubMed] [Google Scholar]
- 121.Gilson PR, et al. Two Dictyostelium orthologs of the prokaryotic cell division protein FtsZ localize to mitochondria and are required for the maintenance of normal mitochondrial morphology. Eukaryot Cell. 2003;2:1315–1326. doi: 10.1128/EC.2.6.1315-1326.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]


