Abstract
Background
Upper extremity deficits are prevalent in individuals with Parkinson disease (PD). In the early stages of PD, such deficits can be subtle and challenging to document on clinical examination.
Objective
The purpose of this study was to use a novel force sensor system to characterize grip force modulation, including force, temporal, and movement quality parameters, during a fine motor control task in individuals with early stage PD.
Design
A case-control study was conducted.
Methods
Fourteen individuals with early stage PD were compared with a control group of 14 healthy older adults. The relationship of force modulation parameters with motor symptom severity and disease chronicity also was assessed in people with PD. Force was measured during both precision and power grasp tasks using an instrumented twist-cap device capable of rotating in either direction.
Results
Compared with the control group, the PD group demonstrated more movement arrests during both precision and power grasp and longer total movement times during the power grasp. These deficits persisted when a concurrent cognitive task was added, with some evidence of force control deficits in the PD group, including lower rates of force production during the precision grasp task and higher peak forces during the power grasp task. For precision grasp, a higher number of movement arrests in single- and dual-task conditions as well as longer total movement times in the dual-task condition were associated with more severe motor symptoms.
Limitations
The sample was small and consisted of individuals in the early stages of PD with mild motor deficits. The group with PD was predominantly male, whereas the control group was predominantly female.
Conclusion
The results suggest that assessing grip force modulation deficits during fine motor tasks is possible with instrumented devices, and such sensitive measures may be important for detecting and tracking change early in the progression of PD.
Parkinson disease (PD) affects about 0.3% of the total population, including approximately 1% of people aged 60 years or older and up to 4% of the population in higher age groups.1 Upper extremity involvement is common among people with PD, with the prevalence of action tremor as high as 92%.2 Although individuals with PD often report upper extremity clumsiness, functional deficits related to hand function are not well documented, possibly because these deficits may not be evident on clinical examination.
The basal ganglia have been shown to play a role in grip force control; consequently, several grip force abnormalities have been documented in individuals with PD. Movements requiring internal regulation of the rate of change of force pose increased metabolic demands on the basal ganglia, specifically the internal component of the globus pallidus and the subthalamic nucleus.3 Specific contributions to force control during precision tasks also have been related to the basal ganglia. The caudate nucleus is responsible for selection of force amplitude, whereas the subthalamic nucleus and posterior putamen are active during force production and not during force amplitude selection.4 Behavioral studies have demonstrated several aspects of deficient fine motor control in individuals with PD. Abnormalities in grip force have been identified as an intrinsic feature of the pathophysiology of PD, rather than an effect or side effect of the medications.5 Hejdukova et al6 showed grip force abnormalities specifically during a precision grip task requiring self-initiated components. People with PD also demonstrate greater impairments of individual finger movements or grips involving 2 digits compared with multidigit grasping7 and reduced coordination between the fingers and the wrist.8 The role of the cortex in the control of fractionated movements and precision activities is well known,9–11 so difficulties with precision grip activities in PD might reflect the failure of the basal ganglia to reinforce the cortical mechanisms responsible for executing fractionated movements during fine motor coordination. This notion is supported, in part, by the fact that there is increased corticocortical activation during performance of sequential finger movements to compensate for the striatal dysfunction.12 If this compensation is rendered less effective with the addition of a cognitive task that is reliant on cortical resources, deficits in motor control may become more obvious.
Although motor impairments in the upper extremity are common presenting symptoms in PD,13 these deficits are subtle and may be missed on clinical examination. The importance of upper extremity deficits is reflected in the inclusion of several items related to hand function and impairment in standardized outcome measures such as the Movement Disorder Society Unified Parkinson's Disease Rating Scale (MDS-UPDRS) and self-reports of clumsiness by individuals with PD during activities of daily living. The addition of a secondary cognitive task may unmask early deficits in upper extremity motor control. Although the effects on gait impairment of adding a simultaneous cognitive task have been widely studied,14–17 the effects of cognitive tasks on fine motor control are less well studied.18 The use of an instrumented device to assess movements both with and without a concurrent cognitive task may aid in identifying subtle deficits in upper extremity motor control that may not be evident on clinical examination, thus providing an objective quantification of movement changes with disease progression.
The goals of this study were: (1) to describe characteristics of force modulation during a fine motor control task performed both with and without a simultaneous cognitive task using multiaxial force sensors and (2) to examine the relationship between force control parameters and disease severity status of individuals with PD and age-matched controls. We developed a fine motor control task that was continuous and required rapid switching of movement direction. This task was designed based on the finding that individuals with PD have significant difficulty performing multiple motor acts in sequence,19,20 which may be attributed, in part, to difficulty switching from one motor program to another during the transition from one movement component to the next.21 We examined 14 individuals with PD and 14 age-matched healthy older adults (control group) while this task was performed in isolation or in the presence of a secondary cognitive task. We hypothesized that individuals with PD would be significantly slower and would produce higher forces when performing the task compared with the control group. We also hypothesized that there would be a significant association between force control parameters and motor symptom severity and disease chronicity based on number of years from initial diagnosis.
Method
Participants with PD were tested in the off-medication condition, at least 8 hours since their last dose of medication. Participants were recruited between July 2010 and September 2010. Eligibility criteria were a clinical diagnosis of PD as established by a movement disorders neurologist, no history of deep brain stimulator implanted, and no history of other neurological conditions or severe arthritis in the hands that could affect the ability to perform the protocol. Age-matched control participants were older adults without PD who were matched by age (within 5 years of PD group participant) and handedness with individuals with PD. Eligibility criteria for the control group were no history of neurologic condition, arthritis, pain, or any other impairment affecting their ability to complete the protocol. Eligibility criteria for all participants included no sensory or motor deficits in the hand and no significant cognitive dysfunction, as indicated by a score of 26 or greater on the Montreal Cognitive Assessment (MoCA). The MoCA is a brief screening test for mild cognitive impairment that assesses various cognitive domains, including visuospatial function, executive function, memory, attention, language, conceptual thinking, and orientation.22 The MoCA provides excellent discrimination for mild cognitive impairment and dementia in PD.23 All participants provided informed consent prior to the study.
Participants with PD also were tested on the MDS-UPDRS24 by an investigator who completed the Movement Disorder Society online training and was trained in the administration of the test by a movement disorders neurologist. The MDS-UPDRS is an instrument used for rating symptom severity in PD based on history (2 sections), physical examination (1 section), and motor complications (1 section). Symptom severity is rated on a scale of 0 to 4 for each item, with higher scores indicating greater severity of disease. The MDS-UPDRS has been widely used in clinical studies of PD as a reliable composite scale of physical function in this population.
Fine Motor Control Task
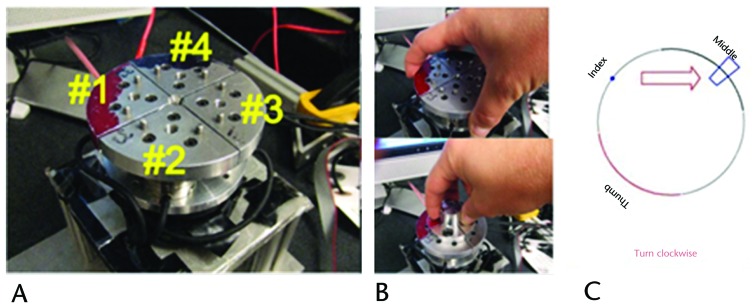
In order to assess force control during precision and power grip, we developed an instrumented twist-cap device (Fig. 1A). The twist-cap device consisted of four 6-axis force/torque transducers (Nano25, ATI Industrial Automation, Apex, North Carolina) patterned radially about a central axis on a metal platform capable of rotating in either direction. One side of the transducers was rigidly fixed to the rotor of a magnetic particle brake (B15, Placid Industries Inc, Lake Placid, New York), which was driven by a linear amplifier (TA105, Trust Automation Inc, San Luis Obispo, California). The twist cap itself was made up of 4 metal quadrants that were interchangeably attached to the other side of the transducers, one quadrant per transducer. Small gaps between quadrants allow the transducer to detect an individual finger's motion exclusive of the force production of other digits. Data were collected at a sampling rate of 1,000 Hz.
Figure 1.
Twist-cap device: (A) circular platform with power grasp cap, (B) power (top) and precision (bottom) caps, and (C) computer screen display showing position of fingers and direction/magnitude of movement.
Two sizes of twist caps were used. The small cap had a radius of 12.5 mm (the size of a soda bottle cap), and the large cap had a radius of 42.5 mm (the size of an average peanut butter jar) (Figs. 1A and 1B). Participants were asked to twist each cap in clockwise and counterclockwise directions. They were given a visual diagram on a computer screen to show the placement of fingers, direction of rotation, and range of rotation (Fig. 1C). They were instructed to rotate the platform in order to bring a target into a box depicted on this diagram. As soon as the target moved into the box, the direction of the arrow changed, and they were asked to change direction of rotation as quickly as they could. Each testing condition consisted of 20 rotations clockwise and 20 rotations counterclockwise in alternating order. Each participant completed 20 trials in each direction for the precision grasp and for the power grasp under single-task conditions, where only the fine motor control task was performed.
This protocol was repeated under dual-task conditions, during which participants were asked to perform a concurrent cognitive task. The cognitive task used was an auditory analog of the Stroop test, in which participants listened to a randomized series of the words “high” and “low” that were said in either high or low pitches. Participants were instructed to identify the pitch of the sound irrespective of the word by saying it aloud as quickly and as accurately as possible. Participants performed the cognitive task under single-task conditions (cognitive task only while seated) as well as dual-task conditions (while performing the fine motor control task). During dual-task conditions, participants were instructed to focus on the fine motor control task, performing it as quickly and as accurately as possible. The order of testing was randomized for precision and power grasp and for single- and dual-task conditions. The dominant extremity was always tested first.
Data Processing
Each sensor output consisted of forces along the 3 axes. Forces exerted by the platform and other components were subtracted to generate true baseline forces exerted by the participant on each sensor. Data were filtered using a third-order, dual-pass, 8-Hz, low-pass Butterworth filter with a cutoff frequency of 5 Hz. MATLAB version R2012 (The MathWorks Inc, Natick, Massachusetts) was used for all data processing.
Outcome Variables
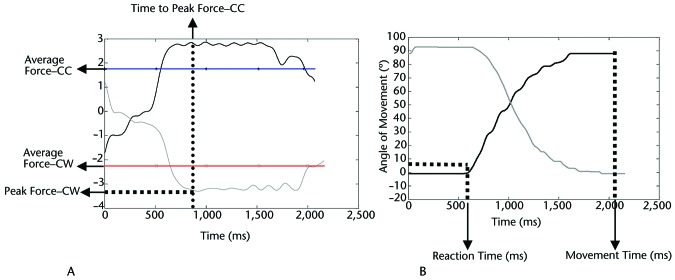
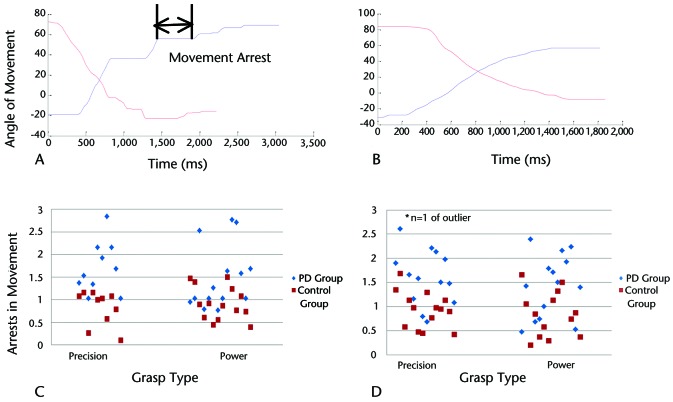
Performance on both precision and power grasp tasks was quantified using force and temporal parameters, as well as measures of movement quality. For all parameters, average values were calculated for the 20 clockwise and 20 counterclockwise trials. Force parameters included average force, peak force, and rate of force production for the tangential forces, measured in newtons (Fig. 2A). Average force levels were calculated as the mean force exerted in a direction perpendicular to the radius of the twist cap and reflect the average force required to rotate the twist cap. Peak force levels were calculated for the tangential forces as the maximum force exerted in a direction perpendicular to the radius of the twist cap. Rate of force production was averaged across the ramp period after calculating the first derivative of forces between the onset of movement and the target acquisition. Temporal parameters included time to peak force, reaction time, and movement time, measured in milliseconds (Fig. 2B). Time to peak force was defined as the time period between the onset of movement and the peak force. Reaction time was defined as the time interval between the “go” signal (ie, appearance of the target on the visual display) and the start of actual movement of the twist-cap device. Movement time was defined as the total time required to twist the cap through one complete trial. Quality of movement was assessed using the total number of arrests in ongoing movement per trial (Fig. 3A). A movement arrest was defined as a time period of ≥50 milliseconds where there was no change in the position of the twist cap, signifying that no movement had occurred. As an arrest in movement was an abrupt cessation of movement for a brief period of time, it contributed to the lack of smoothness in movement (or a decline in quality of movement).
Figure 2.
Outcome variables for force task. (A) Representative force trace for tangential force (averaged over 3 fingers) from an individual with Parkinson disease showing method for calculating peak force (for clockwise [CW] movement), time to peak force (for counterclockwise [CC] movement), and average force (for CW and CC movements). (B) Representative movement angle and time.
Figure 3.
Movement arrest analysis showing a representative trial (with clockwise and counterclockwise rotation angle traces) in an individual with Parkinson disease (PD) (panel A) and a healthy older adult (panel B) during the power grasp task. Panels C and D show total number of submovements (arrests in movement) in PD and control groups during single-task (panel C) and dual-task (panel D) conditions for both grasps.
Outcome variables calculated for the cognitive task were response latency, which was defined as the time from stimulus onset to response onset, and response accuracy, which was defined as the number of correct responses divided by the total number of responses, expressed as a percentage.
Data Analysis
Data were analyzed using IBM SPSS version 19 (IBM Corp, Armonk, New York). Descriptive statistics included means and estimates of variability computed for all outcome variables. Independent-samples t tests with alpha set at .05 were used to examine differences in the motor and cognitive outcome measures between groups. We also examined the association between motor and cognitive outcome measures and disease status based on the MDS-UPDRS motor subscale scores and disease chronicity based on number of years from initial diagnosis.
Results
Participant demographics for the PD and control groups are shown in Table 1. We compared results from the dominant and nondominant extremities and found no differences, so only results from the dominant extremity are reported here. Test-retest reliability for the primary variable of interest (average force) was established for individuals with PD as well as controls across the 20 trials during precision and power grasp and was found to be excellent (r=.990–.998). Within each group (PD and control), there were no significant differences between the single- and dual-task conditions for any of the force, temporal, or movement quality variables, suggesting that individuals with PD were able to appropriately prioritize motor performance during the dual-task condition compared with the single-task condition as instructed. Tables 2 and 3 provide outcomes for both groups during the single- and dual-task conditions for the precision grasp (Tab. 2) and the power grasp (Tab. 3).
Table 1.
Descriptive Characteristics of Samplea
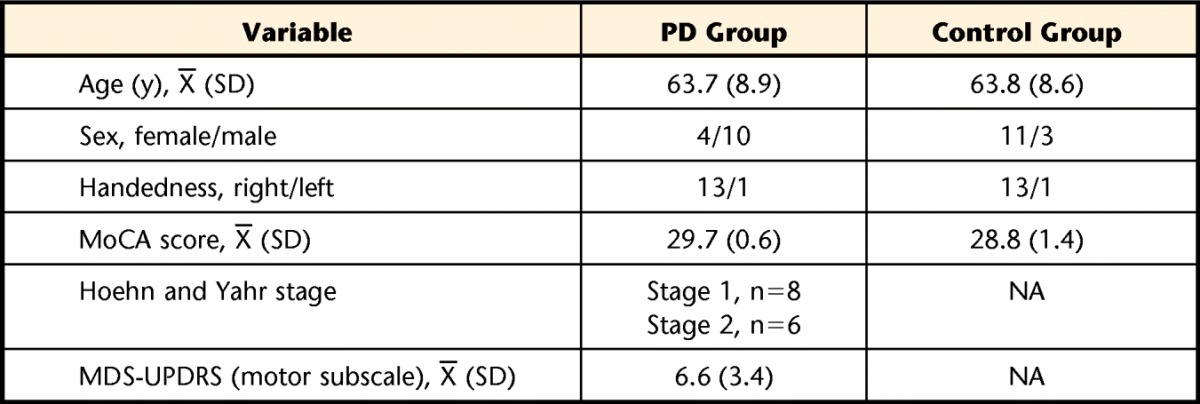
Demographics and clinical test results from individuals with Parkinson disease (PD) and healthy older adults (control group). MoCA=Montreal Cognitive Assessment, MDS-UPDRS=Movement Disorders Society Unified Parkinson's Disease Rating Scale, NA=not applicable.
Table 2.
Precision Grasp Task: Force Control and Cognitive Task Outcomes for People With Parkinson Disease (PD Group) and Healthy Older Adults (Control Group)a
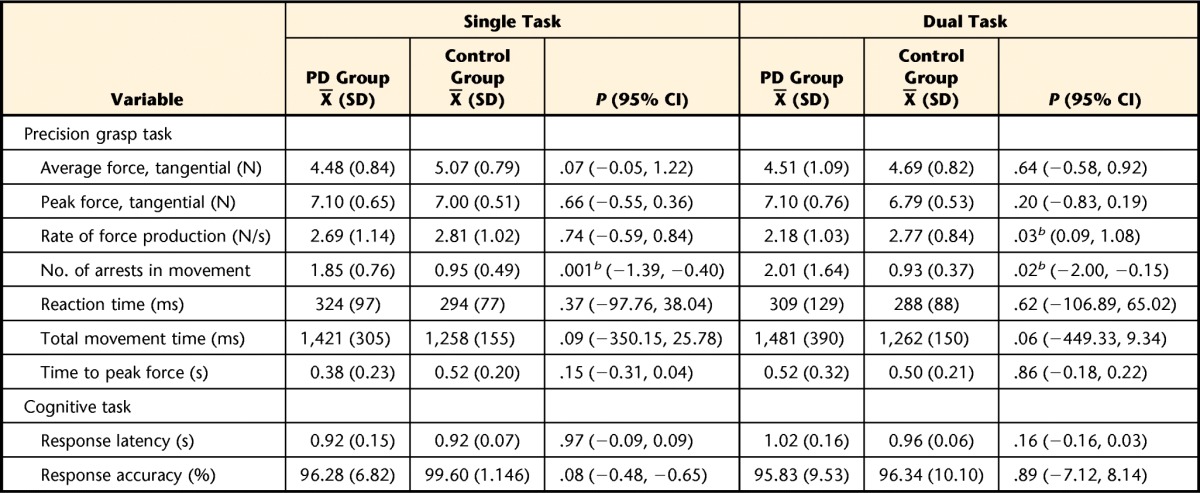
Outcomes for PD and control groups during the single-task and dual-task conditions for the precision grip task; 95% confidence intervals (95% CI) represent intervals around the difference between the group means.
b Statistically significant difference between groups at α=.05.
Table 3.
Power Grasp Task: Force Control and Cognitive Task Outcomes for People With Parkinson Disease (PD Group) and Healthy Older Adults (Control Group)a
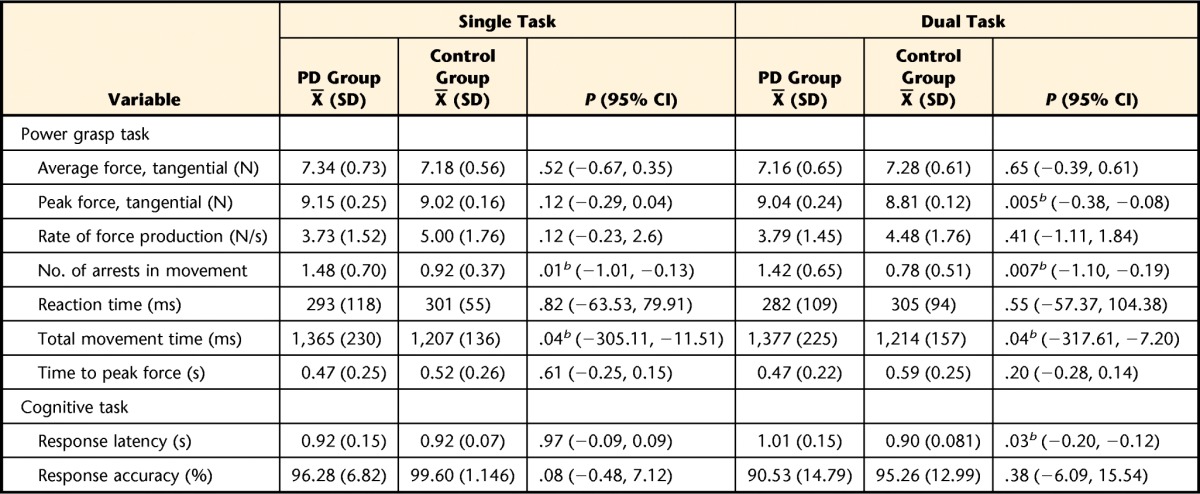
Outcomes for PD and control groups during the single-task and dual-task conditions for the power grip task; 95% confidence intervals (95% CI) represent intervals around the difference between the group means.
b Statistically significant difference between groups at α=.05.
Precision Grasp
During single-task performance of the precision grasp task, the PD and control groups did not differ on any force or temporal parameters. However, people with PD had more movement arrests in the single-task condition compared with the control group (PD group: X̅=1.85, SD=0.76; control group: X̅=0.95, SD=0.49; P=.001). During dual-task performance, individuals with PD demonstrated a slower rate of force production compared with the control group (PD group: X̅=2.18 N/s, SD=1.03; control group: X̅=2.77 N/s, SD=0.84; P=.03) and more movement arrests (PD group: X̅=2.01, SD=1.64; control group: X̅=0.93, SD=0.37; P=.024). Cognitive task response latency and accuracy were similar between groups under both single- and dual-task conditions.
Power Grasp
During single-task performance, the PD group demonstrated longer movement times compared with the control group (PD group: X̅=1,365 milliseconds, SD=230; control group: X̅=1,207 milliseconds, SD=136; P=.036) and more movement arrests (PD group: X̅=1.48, SD=0.70; control group: X̅=0.92, SD=0.37; P=.014). During dual-task performance, peak force was higher in the PD group compared with the control group (PD group: X̅=9.04 N, SD=0.24; control group: X̅=8.81 N, SD=0.12; P=.005). People with PD continued to demonstrate longer movement times compared with the control group (PD group: X̅=1,377 milliseconds, SD=225; control group: X̅=1,214 milliseconds, SD=157; P=.041) and more movement arrests (PD group: X̅=1.42, SD=0.65; control group: X̅=0.78, SD=0.51; P=.007) during the dual-task condition. Figure 3 shows a representative trace demonstrating movement arrests for an individual with PD and a matched control group participant as well as group differences in the number of movement arrests. In the dual-task condition, cognitive task response latency was longer among individuals with PD compared with the control group (PD group: X̅=1.01 seconds, SD=0.15; control group: X̅=0.90 seconds, SD=0.08; P=.03).
Association of Grip Force Task Performance With Motor Symptom Severity and Chronicity
For the precision grasp task, the total number of movement arrests was significantly associated with motor symptom severity, as measured by the MDS-UPDRS motor subscale scores, during the single- (r=.72, P=.005) and dual-task (r=.67, P=.013) conditions. Additionally, movement time in the dual-task condition was correlated with the MDS-UPDRS motor subscale scores (r=.61, P=.027). For the power grasp task, there were no associations between force, temporal, or quality parameters and MDS-UPDRS motor subscale scores. No aspect of performance during the precision and power grasp tasks was correlated with chronicity of the disease.
Discussion
The grip force tasks and instrumentation used in this study were developed based on impairments in upper extremity movement control that are well established in the literature. Previous research shows that people with PD demonstrate deficits in force control,5,25–27 kinematic parameters,28,29 and temporal control25 during upper extremity movements. Sequential movements are more affected than repetitive movements,19,30 and performance of a concurrent task exacerbates these deficits.31 Thus, this study used a novel instrumented device to examine force modulation during a challenging fine motor task, with and without a cognitive dual task, in people with early PD compared with age-matched controls.
Under single-task conditions, individuals with PD demonstrated impairments in movement quality for both precision and power grasp tasks. Movement quality deficits persisted under dual-task conditions, despite prioritization of the fine motor task. People with PD had impairments in temporal parameters during single- and dual-task performance of power grasp task. When a concurrent task was added, people with PD demonstrated additional deficits in force parameters, including lower rates of force production during precision grasp and higher peak force in power grasp. The MDS-UPDRS motor subscale score was significantly associated with precision grasp control, specifically the total arrests in movement during the single- and dual-task conditions and the total movement time under dual-task conditions. These differences are notable especially because our sample consisted of individuals in the early stages of the disease, with more than half in Hoehn and Yahr stage 1 and the rest in Hoehn and Yahr stage 2. Thus, these findings illustrate consistent deficits in movement quality during a challenging, fine motor task that are evident even in the early stages of PD and associated with motor symptom severity. The use of instrumented devices and the addition of a dual-task condition may be critical to provide sufficient challenge and unmask these deficits in force control during early PD.
Our results concur somewhat with previous findings, but there are some notable contrasts, as outlined below. Much of the previous research examined individuals at a more advanced stage of PD than those in this sample. The results of this study suggest that even very early in the disease process, deficits in grip force are present in people with PD. In the current study, force control parameters were not consistently impaired in the PD group compared with the control group. Average tangential forces were similar between groups during both precision and power grasp tasks. However, peak tangential force was higher among people with PD during the dual-task power grasp task, in agreement with previous findings in the literature.5 Although previous studies have documented that individuals with PD show slower rates of force production,26,27 we found that individuals with PD in our study generally demonstrated similar rates of force production, with the exception of lower rates of force production only in the dual-task precision grasp condition. For example, Park and Stelmach27 demonstrated a slower rate of force production in people with PD compared with healthy older adults during an isometric force production task, and this difference was more pronounced at higher force levels (55% of maximum voluntary contraction) than at lower force levels (15% of maximum voluntary contraction). The amount of force required for the precision and power grasp tasks in the current study was relatively small (5–9 N), and rates of force production may be more similar between people with PD and healthy older adults at such low force levels. The low levels of force required to successfully complete this task also may have contributed to similar average and peak forces between groups. Finally, the sample of individuals with PD in the current study represented those in the early stages of the disease relative to other studies,26,32 suggesting that deficits in force generation and rates of force production may increase with disease progression.
Some studies have demonstrated upper extremity freezing of movement,33–35 which is an upper extremity analog to freezing of gait.36–38 Freezing in the upper extremity is defined as a complete halt or a severely disrupted motion with a nearly complete loss of movement, typically preceded by small-amplitude and hastened movements similar to festination preceding freezing of gait.34 The movement arrests seen during this fine motor control task, which were brief interruptions in force production, differed from freezing in the upper extremity because the arrests in movement were abrupt and there were no high frequency components just prior to the occurrences of the arrest. Although upper extremity freezing is correlated with freezing of gait,35 none of the participants in our sample exhibited or reported freezing of gait. In previous studies, participants reporting upper extremity freezing were at a more clinically advanced stage of the disease than the sample in the current study.34,39,40 Whether individuals with PD who exhibit movement arrests develop freezing of upper extremity movements or gait remains to be determined based on prospective follow-up of these individuals. If so, it would provide further validation of instrumented fine motor control tests as a means of detecting subtle but meaningful deficits in the very early stages of the disease.
Despite the documented role of the basal ganglia in precision grip force control,41 people with PD in our sample demonstrated more impairments in force control, temporal parameters, and movement quality during the power grasp compared with the precision grasp task. This finding may be related to strength deficits observed in the early stages of PD when instrumented devices are used.42,43 It has been suggested that strength deficits in this population are not detectable on standard clinical muscle testing and instrumented devices are necessary to unmask such deficits,44 and the PD sample in the current study did not demonstrate strength deficits on clinical examination.
When examining grip force deficits in early PD, sufficiently challenging tasks may be necessary to unmask subtle deficits. The precision and power grasp tasks incorporated repeated, sequential clockwise and counterclockwise rotations. Individuals with PD demonstrate deficits in motor control when switching between motor programs45 and when multiple motor acts are performed in sequence.29,30,46 These deficits in motor control may be attributed to difficulty switching from one motor program to another while transitioning between movement components.21
The grip force protocol used in the current study also was challenging due to the addition of a concurrent cognitive task. In the current study, people with PD generally demonstrated the ability to prioritize the fine motor control task, as evidenced by similar force and temporal control under single- and dual-task conditions. However, some force control deficits emerged only under dual-task conditions, with slower rates of force production for the precision task and higher peak force in the power task. These findings suggest that if upper extremity assessments occur under single-task conditions, people with PD may be able to adequately compensate by using increased attention to movement.
The participants in our study had low scores on the MDS-UPDRS motor subscale, indicating only mild clinical deficits. Although force control parameters did not show an association with motor symptom severity (based on MDS-UPDRS motor subscale scores), the number of movement arrests in the precision grasp task was positively correlated with motor symptom severity. This finding may be representative of the self-reported clumsiness, or decline in movement quality, that is not evident on clinical examination. As this decline in movement quality is associated with motor symptom severity, it may have potential as a clinical marker of progression of motor symptoms. The only other clinical assessment that has shown to be a significant predictor of disease progression is the grooved pegboard test.47 These scores represent only a measure of movement speed, however, and fail to capture the quality of movement.
Upper extremity dysfunction in the form of subjectively reported clumsiness is a common early occurrence in people with PD,13 but upper extremity impairments may be difficult to demonstrate on clinical examination. Instrumented devices may be useful when quantifying upper extremity dysfunction in people with PD and have several additional strengths over traditional clinical outcome measures. One of major strengths is that instrumentation allows for objective and precise movement quantification due to the high measurement resolution of such devices. Furthermore, the current study demonstrated that individuals with early-stage PD may have subtle deficits in movement quality that may not be detected on clinical examination and that occur in the absence of force control or speed impairments. Because movement quality deficits were associated with disease severity, they may be useful to track changes in fine motor control in early PD. Increasingly, rehabilitation interventions in PD are being implemented soon after diagnosis, as the potential for neuroprotection appears to be greatest in the early stages of the disease.48,49 Thus, there is a need for sensitive and objective measures that can document and track deficits in fine motor control early in the disease process.
Several limitations of our study should be acknowledged. Our sample primarily consisted of individuals with early PD and mild motor symptoms. Whether these deficits worsen as the disease progresses remains to be determined with longitudinal follow-up of individuals with PD. Another consideration was the use of a visual indicator to cue changes in movement direction. Research suggests that the basal ganglia are preferentially involved in the control of internally generated movements, and these types of movement are likely to be affected in people with PD.50 The fine motor control task used in this study would likely be more challenging if this external cue were eliminated. A third limitation was lack of sex-matched control participants, although participants were matched for age and hand dominance. Ten of the 14 participants in the PD group were male, and 11 of the 14 control group participants were female, and we acknowledge that sex-based differences may appear with a larger sample. Finally, replication of these results in a larger sample is necessary. The association between arrests in movement and the MDS-UPDRS motor subscale score needs to be examined prospectively to determine if these deficits may be predictive of disease progression.
This study represents the first step in the development of a quantitative clinical assessment of upper extremity fine motor control that can be used to detect, monitor, and track early motor deficits in PD that may not be evident on clinical examination. Future investigations with this protocol could examine the diagnostic and prognostic value of early deficits in movement quality, and this type of assessment may be useful as a screening tool for older individuals or individuals at risk for developing PD. Although there are known benefits of exercise in the early phase of the disease,51,52 newly diagnosed individuals are not consistently referred for regular physical rehabilitation before balance and gait are affected. One factor that may contribute to limited rehabilitation referrals is the inability to demonstrate and document motor performance impairments using clinical measures of function. The identification and quantification of subtle deficits early in the disease process of PD are critical to demonstrating the efficacy of early intervention strategies, including exercise and pharmacological treatments. In the current study, deficits in movement quality were detected even in early PD and were associated with motor symptom severity, supporting the utility of a quantitative assessment combining sensitive instrumentation and challenging fine motor control tasks in early detection and monitoring of symptoms in PD.
Footnotes
All authors provided concept/idea/research design. Dr Pradhan, Dr Scherer, and Dr Kelly provided writing and data analysis. Dr Pradhan provided data collection and project management. Dr Scherer and Dr Matsuoka provided consultation (including review of manuscript before submission). The authors thank Marcia Ciol, PhD, for her valuable statistical consultation on the project and the Washington Parkinson Disease Registry for their assistance with recruitment.
The research protocol was approved by the University of Washington Institutional Review Board's Human Subjects Division.
This work is supported by the University of Washington–Advanced Rehabilitation Research Training (NIDRR training grant number H133P080008) through a postdoctoral fellowship awarded to Dr Pradhan and the Neurobotics Laboratory, Department of Computer Science and Engineering, University of Washington.
References
- 1. de Lau LM, Breteler MM. Epidemiology of Parkinson's disease. Lancet Neurol. 2006;5:525–535. [DOI] [PubMed] [Google Scholar]
- 2. Koller WC, Vetere-Overfield B, Barter R. Tremors in early Parkinson's disease. Clin Neuropharmacol. 1989;12:293–297. [DOI] [PubMed] [Google Scholar]
- 3. Vaillancourt DE, Mayka MA, Thulborn KR, Corcos DM. Subthalamic nucleus and internal globus pallidus scale with the rate of change of force production in humans. Neuroimage. 2004;23:175–186. [DOI] [PubMed] [Google Scholar]
- 4. Vaillancourt DE, Yu H, Mayka MA, Corcos DM. Role of the basal ganglia and frontal cortex in selecting and producing internally guided force pulses. Neuroimage. 2007;36:793–803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Fellows SJ, Noth J. Grip force abnormalities in de novo Parkinson's disease. Mov Disord. 2004;19:560–565. [DOI] [PubMed] [Google Scholar]
- 6. Hejdukova B, Hosseini N, Johnels B, et al. Manual transport in Parkinson's disease. Mov Disord. 2003;18:565–572. [DOI] [PubMed] [Google Scholar]
- 7. Agostino R, Curra A, Giovannelli M, et al. Impairment of individual finger movements in Parkinson's disease. Mov Disord. 2003;18:560–565. [DOI] [PubMed] [Google Scholar]
- 8. Teulings HL, Contreras-Vidal JL, Stelmach GE, Adler CH. Parkinsonism reduces coordination of fingers, wrist, and arm in fine motor control. Exp Neurol. 1997;146:159–170. [DOI] [PubMed] [Google Scholar]
- 9. Homberg V, Stephan KM, Netz J. Transcranial stimulation of motor cortex in upper motor neurone syndrome: Its relation to the motor deficit. Electroencephalogr Clin Neurophysiol. 1991;81:377–388. [DOI] [PubMed] [Google Scholar]
- 10. Ehrsson HH, Fagergren A, Jonsson T, et al. Cortical activity in precision- versus power-grip tasks: an fMRI study. J Neurophysiol. 2000;83:528–536. [DOI] [PubMed] [Google Scholar]
- 11. Ehrsson HH, Fagergren E, Forssberg H. Differential fronto-parietal activation depending on force used in a precision grip task: an fMRI study. J Neurophysiol. 2001;85:2613–2623. [DOI] [PubMed] [Google Scholar]
- 12. Catalan MJ, Ishii K, Honda M, et al. A PET study of sequential finger movements of varying length in patients with Parkinson's disease. Brain. 1999;122(pt 3):483–495. [DOI] [PubMed] [Google Scholar]
- 13. Uitti RJ, Baba Y, Wszolek ZK, Putzke DJ. Defining the Parkinson's disease phenotype: initial symptoms and baseline characteristics in a clinical cohort. Parkinsonism Relat Disord. 2005;11:139–145. [DOI] [PubMed] [Google Scholar]
- 14. Kelly VE, Eusterbrock AJ, Shumway-Cook A. The effects of instructions on dual-task walking and cognitive task performance in people with Parkinson's disease. Parkinsons Dis. 2012;2012:671261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Kelly VE, Eusterbrock AJ, Shumway-Cook A. A review of dual-task walking deficits in people with Parkinson's disease: motor and cognitive contributions, mechanisms, and clinical implications. Parkinsons Dis. 2012;2012:918719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kelly VE, Janke AA, Shumway-Cook A. Effects of instructed focus and task difficulty on concurrent walking and cognitive task performance in healthy young adults. Exp Brain Res. 2010;207:65–73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Kelly VE, Shumway-Cook A. The ability of people with Parkinson's disease to modify dual-task performance in response to instructions during simple and complex walking tasks. Exp Brain Res. 2014;232:263–271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Broeder S, Nackaerts E, Nieuwboer A, et al. The effects of dual tasking on handwriting in patients with Parkinson's disease. Neuroscience. 2014;263:193–202. [DOI] [PubMed] [Google Scholar]
- 19. Benecke R, Rothwell JC, Dick JP, et al. Simple and complex movements off and on treatment in patients with Parkinson's disease. J Neurol Neurosurg Psychiatry. 1987;50:296–303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Agostino R, Berardelli A, Formica A, et al. Sequential arm movements in patients with Parkinson's disease, Huntington's disease and dystonia. Brain. 1992;115(pt 5):1481–1495. [DOI] [PubMed] [Google Scholar]
- 21. Weiss P, Stelmach GE, Hefter H. Programming of a movement sequence in Parkinson's disease. Brain. 1997;120(pt 1):91–102. [DOI] [PubMed] [Google Scholar]
- 22. Nasreddine ZS, Phillips N, Chertkow H. Normative data for the Montreal Cognitive Assessment (MoCA) in a population-based sample. Neurology. 2012;78:765–766; author reply 766. [DOI] [PubMed] [Google Scholar]
- 23. Dalrymple-Alford JC, MacAskill MR, Nakas CT, et al. The MoCA: well-suited screen for cognitive impairment in Parkinson disease. Neurology. 2010;75:1717–1725. [DOI] [PubMed] [Google Scholar]
- 24. Goetz CG, Tilley BC, Shaftman SR, et al. Movement Disorder Society–sponsored revision of the Unified Parkinson's Disease Rating Scale (MDS-UPDRS): scale presentation and clinimetric testing results. Mov Disord. 2008;23:2129–2170. [DOI] [PubMed] [Google Scholar]
- 25. Fellows SJ, Noth J, Schwarz M. Precision grip and Parkinson's disease. Brain. 1998;121(pt 9):1771–1784. [DOI] [PubMed] [Google Scholar]
- 26. Corcos DM, Chen CM, Quinn NP, et al. Strength in Parkinson's disease: relationship to rate of force generation and clinical status. Ann Neurol. 1996;39:79–88. [DOI] [PubMed] [Google Scholar]
- 27. Park JH, Stelmach GE. Force development during target-directed isometric force production in Parkinson's disease. Neurosci Lett. 2007;412:173–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Contreras-Vidal JL, Stelmach GE. Effects of parkinsonism on motor control. Life Sci. 1996;58:165–176. [DOI] [PubMed] [Google Scholar]
- 29. Desmurget M, Gaveau V, Vindras P, et al. On-line motor control in patients with Parkinson's disease. Brain. 2004;127:1755–1773. [DOI] [PubMed] [Google Scholar]
- 30. Benecke R, Rothwell JC, Dick JP, et al. Disturbance of sequential movements in patients with Parkinson's disease. Brain. 1987;110(pt 2):361–379. [DOI] [PubMed] [Google Scholar]
- 31. Proud EL, Morris ME. Skilled hand dexterity in Parkinson's disease: effects of adding a concurrent task. Arch Phys Med Rehabil. 2010;91:794–799. [DOI] [PubMed] [Google Scholar]
- 32. Neely KA, Planetta PJ, Prodoehl J, et al. Force control deficits in individuals with Parkinson's disease, multiple systems atrophy, and progressive supranuclear palsy. PLoS One. 2013;8:e58403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Vercruysse S, Spildooren J, Heremans E, et al. The neural correlates of upper limb motor blocks in Parkinson's disease and their relation to freezing of gait. Cereb Cortex. 2014;24:3154–3166. [DOI] [PubMed] [Google Scholar]
- 34. Vercruysse S, Spildooren J, Heremans E, et al. Freezing in Parkinson's disease: a spatiotemporal motor disorder beyond gait. Mov Disord. 2012;27:254–263. [DOI] [PubMed] [Google Scholar]
- 35. Nieuwboer A, Vercruysse S, Feys P, et al. Upper limb movement interruptions are correlated to freezing of gait in Parkinson's disease. Eur J Neurosci. 2009;29:1422–1430. [DOI] [PubMed] [Google Scholar]
- 36. Kemoun G, Defebvre L. Gait disorders in Parkinson disease—gait freezing and falls: therapeutic management. Presse Med. 2001;30:460–468. [PubMed] [Google Scholar]
- 37. Leentjens AF, Dujardin K, Marsh L, et al. Anxiety rating scales in Parkinson's disease: critique and recommendations. Mov Disord. 2008;23:2015–2025. [DOI] [PubMed] [Google Scholar]
- 38. Hong M, Earhart GM. Rotating treadmill training reduces freezing in Parkinson disease: preliminary observations. Parkinsonism Relat Disord. 2008;14:359–363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Popovic MB, Dzoljic E, Kostic V. A method to assess hand motor blocks in Parkinson's disease with digitizing tablet. Tohoku J Exp Med. 2008;216:317–324. [DOI] [PubMed] [Google Scholar]
- 40. Williams AJ, Peterson DS, Ionno M, et al. Upper extremity freezing and dyscoordination in Parkinson's disease: effects of amplitude and cadence manipulations. Parkinsons Dis. 2013;2013:595378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Prodoehl J, Corcos DM, Vaillancourt DE. Basal ganglia mechanisms underlying precision grip force control. Neurosci Biobehav Rev. 2009;33:900–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Kakinuma S, Nogaki H, Pramanik B, Morimatsu M. Muscle weakness in Parkinson's disease: isokinetic study of the lower limbs. Eur Neurol. 1998;39:218–222. [DOI] [PubMed] [Google Scholar]
- 43. Nogaki H, Fukusako T, Sasabe F, et al. Muscle strength in early Parkinson's disease. Mov Disord. 1995;10:225–226. [DOI] [PubMed] [Google Scholar]
- 44. Cano-de-la-Cuerda R, Perez-de-Heredia M, et al. Is there muscular weakness in Parkinson's disease? Am J Phys Med Rehabil. 2010;89:70–76. [DOI] [PubMed] [Google Scholar]
- 45. Krebs HI, Hogan N, Hening W, et al. Procedural motor learning in Parkinson's disease. Exp Brain Res. 2001;141:425–437. [DOI] [PubMed] [Google Scholar]
- 46. Agostino R, Berardelli A, Curra A, et al. Clinical impairment of sequential finger movements in Parkinson's disease. Mov Disord. 1998;13:418–421. [DOI] [PubMed] [Google Scholar]
- 47. Bohnen NI, Kuwabara H, Constantine GM, et al. Grooved pegboard test as a biomarker of nigrostriatal denervation in Parkinson's disease. Neurosci Lett. 2007;424:185–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Cohen AD, Tillerson JL, Smith AD, et al. Neuroprotective effects of prior limb use in 6-hydroxydopamine-treated rats: possible role of GDNF. J Neurochem. 2003;85:299–305. [DOI] [PubMed] [Google Scholar]
- 49. Tillerson JL, Cohen AD, Philhower J, et al. Forced limb-use effects on the behavioral and neurochemical effects of 6-hydroxydopamine. J Neurosci. 2001;21:4427–4435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Taniwaki T, Okayama A, Yoshiura T, et al. Reappraisal of the motor role of basal ganglia: a functional magnetic resonance image study. J Neurosci. 2003;23:3432–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Fisher BE, Wu AD, Salem GJ, et al. The effect of exercise training in improving motor performance and corticomotor excitability in people with early Parkinson's disease. Arch Phys Med Rehabil. 2008;89:1221–1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Fisher BE, Li Q, Nacca A, et al. Treadmill exercise elevates striatal dopamine D2 receptor binding potential in patients with early Parkinson's disease. Neuroreport. 2013;24:509–514. [DOI] [PubMed] [Google Scholar]