Abstract
AIM: To determine the significance of cholesteryl ester transfer protein (CETP) in lipoprotein abnormalities in chronic hepatitis C virus (HCV) infection.
METHODS: We evaluated the significance of the serum concentration of CETP in 110 Japanese patients with chronic HCV infection. Fifty-five patients had active HCV infection, and HCV eradication had been achieved in 55. The role of CETP in serum lipoprotein abnormalities, specifically, in triglyceride (TG) concentrations in the four major classes of lipoproteins, was investigated using Pearson correlations in conjunction with multiple regression analysis and compared them between those with active HCV infection and those in whom eradication had been achieved.
RESULTS: The serum CETP levels of patients with active HCV infection were significantly higher than those of patients in whom HCV eradication was achieved (mean ± SD, 2.84 ± 0.69 μg/mL vs 2.40 ± 1.00 μg/mL, P = 0.008). In multiple regression analysis, HCV infection status (active or eradicated) was an independent factor significantly associated with the serum CETP level. TG concentrations in low-density lipoprotein (mean ± SD, 36.25 ± 15.28 μg/mL vs 28.14 ± 9.94 μg/mL, P = 0.001) and high-density lipoprotein (HDL) (mean ± SD, 25.9 ± 7.34 μg/mL vs 17.17 ± 4.82 μg/mL, P < 0.001) were significantly higher in patients with active HCV infection than in those in whom HCV eradication was achieved. The CETP level was strongly correlated with HDL-TG in patients with active HCV infection (R = 0.557, P < 0.001), whereas CETP was not correlated with HDL-TG in patients in whom HCV eradication was achieved (R = -0.079, P = 0.56).
CONCLUSION: Our results indicate that CETP plays a role in abnormalities of lipoprotein metabolism in patients with chronic HCV infection.
Keywords: Hepatitis C virus, Cholesteryl ester transfer protein, High-density lipoprotein triglyceride, Case control study, Lipoprotein metabolism
Core tip: Cholesteryl ester transfer protein (CETP) mediates the transfer of neutral lipids between lipoproteins. Although lipoprotein metabolism abnormalities have been extensively studied, the role of CETP in abnormal lipoprotein profiles in patients with hepatitis C virus (HCV) infection is unknown. Accordingly, we investigated, for the first time, high serum CETP level in patients with active HCV infection. HCV infection was a determinant of the serum CETP level in multiple regression analysis. A high CETP concentration in HCV infection was strongly correlated with excessive triglyceride accumulation in high-density lipoprotein. Thus, CETP may contribute to abnormal lipoprotein metabolism in HCV infection.
INTRODUCTION
Chronic hepatitis C virus (HCV) infection is one of the most important etiologies of chronic hepatitis, liver cirrhosis, and hepatocellular carcinoma worldwide[1-3]. HCV is a unique virus; its use of host lipid metabolism results in a persistent infection. Therefore, it is very important to understand how HCV uses host lipid metabolism and how host lipid metabolism is affected by HCV infection, because HCV infection represents a unique model in which the virus causes chronic infection while coexisting with the host, simultaneously taking over the host’s metabolism[4].
Infectious HCV forms a lipoviral particle that can enter into hepatocytes in the blood[5]. The characteristics of HCV lipoviral particles are similar to those of very-low-density lipoproteins (VLDLs)[6]. This suggests a close association between HCV infection and VLDL. Both VLDL and HCV lipoviral particles are synthesized, assembled, and secreted from hepatocytes via similar metabolic pathways[7]. Consequently, dysregulated lipid metabolism in chronic HCV infection may primarily be caused by VLDL abnormalities. According to some in vitro studies, HCV core protein suppressed VLDL production and secretion from the liver by inhibiting microsomal triglyceride (TG) transfer protein[8,9].
In clinical situations, chronic HCV infection alters serum lipid profiles by decreasing the low-density lipoprotein cholesterol (LDL-C) level[10] and the VLDL-TG/non-VLDL-TG ratio[11]. However, the abnormalities of lipoproteins as a whole in patients with chronic HCV infection have not been clarified. In particular, the abnormal distribution of TGs among lipoprotein subclasses has not been extensively studied, because TG content in each lipoprotein subclass cannot be measured easily by routine laboratory tests.
Cholesterol ester transfer protein (CETP) is a plasma glycoprotein that facilitates the transfer of cholesteryl ester (CE) from high-density lipoprotein (HDL) to other subclasses of lipoprotein [chylomicrons (CM), VLDL, and LDL][12]. The principal effect of CETP on lipoproteins is considered to be the reduction of HDL-C levels and facilitation of reverse cholesterol transport to the liver[13]. Accordingly, CETP adjusts the distribution of TG among the different lipoprotein subclasses. Therefore, we speculated that CETP may play an important role in the abnormalities of lipoprotein metabolism in patients with active HCV infection.
In this study, we determined the serum concentration of CETP in patients with HCV infection and in those in whom HCV was eradicated, to determine the significance of CETP in HCV infection. Furthermore, we investigated the influence of CETP on lipoprotein abnormalities in HCV infection, with particular attention to TG concentrations.
MATERIALS AND METHODS
The protocol of this case control study was in accordance with the 2004 standards of the Declaration of Helsinki and current ethical guidelines, and was approved by the human ethics review committee of the Jikei University School of Medicine. Written informed consent was obtained from all patients who enrolled in this study.
Patient population
Japanese patients with active chronic HCV infection (active HCV group) or successfully eradicated chronic HCV infection [negative serum HCV-RNA 6 mo after the end of interferon (IFN)-based therapy] (eradication group) who had been followed up at Jikei University Katsushika Medical Center between September 2013 and October 2014 were randomly considered for enrollment. Patients receiving treatment for diabetes (DM) or hyperlipidemia or hormone replacement therapy and those with hepatitis B virus or human immunodeficiency virus infection were excluded. Additionally, patients who had received IFN within 6 mo or who had been diagnosed with hepatocellular carcinoma or decompensated cirrhosis were excluded.
Laboratory tests and demographic data
Demographic data, including age, sex, and body mass index (BMI), and basic laboratory data were obtained from the medical records. The collected basic laboratory data included aspartate 2-oxoglutarate aminotransferase (AST), alanine 2-oxoglutarate aminotransferase (ALT), gamma-glutamyl transpeptidase (γ-GTP), albumin, total bilirubin, fasting blood glucose (FBG), and hemoglobin A1c (HbA1c) levels, hemoglobin (Hb) levels and the platelet count. In addition, basic serum lipid data, including total cholesterol, TG, HDL-cholesterol (HDL-C), and LDL-C were collected. HDL-C had been directly measured by a commercial kit (Kyowa Medex, Tokyo, Japan), and the LDL-C level had been calculated by the Friedewald equation.
CEPT measurement
The CETP concentration was measured in sera collected after at least a 10-h overnight fast. The CETP mass concentration was measured using a sandwich enzyme-linked immunosorbent assay with two monoclonal antibodies specific to human CETP, JHC1, and JHC2, as previously described[14]. The assay was performed in duplicate, and the mean was adopted as the measured value[15].
Measurement of cholesterol and TG concentration in the major classes of serum lipoproteins
To examine the distribution of cholesterol and TG in lipoprotein fractions, fresh sera from the collected patient samples were fractionated by high-performance liquid chromatography, and the cholesterol and TG concentration in the major four lipoprotein classes was measured using the online detection system (Skylight Biotech, Inc., Akita, Japan)[16,17]. Serum lipoproteins were classified into four classes according to particle size: CM (> 80 nm), VLDL (30-80 nm), LDL (16-30 nm), and HDL (< 16 nm).
Biostatistics
A statistical review of the study was performed by a biomedical statistician.
Statistical analysis
Continuous data are expressed as mean ± SD. Categorical data are expressed as numbers (%). We used Welch’s t test or the χ2 test for comparisons between the two groups. Correlations between two parameters were evaluated by the Pearson product-moment correlation coefficient. To determine the significance of HCV infection on the serum CETP level, multiple regression analysis was performed, with demographic and basic laboratory data including the HCV infection status (active infection or HCV eradication) as independent variables. The most suitable regression model for explanation of the serum CETP level was constructed by backward elimination of candidate variables.
We performed statistical analyses using STATISTICA software, version 6 (StatSoft Japan Inc. Tokyo, Japan), and two-tailed P values of ≤ 0.05 were considered significant; P values > 0.05 but ≤ 0.1 were considered to indicate marginal significance. P values less than 0.001 are expressed as P < 0.001. We determined the multicollinearity of the multiple regression analysis to verify the reliability; the variance inflation factor was < 5, indicating that our models were reliable.
RESULTS
Study population
In total, 110 patients were included in the study. Fifty-five had active HCV infection, and HCV eradication with IFN-based anti-viral therapy had been achieved in the remaining 55 patients. In the active HCV group, 48 (87%) had HCV genotype (G) 1b infection and 7 (13%) had HCV G2 infection. In the eradication group, 34 (62%) were previously infected with HCV G1b and 21 (38%) were previously infected with HCV G2.
Increase in the CETP level in active chronic HCV infection
The serum CETP level was significantly higher in the active HCV group than in the eradication group (2.84 ± 1.00 μg/mL vs 2.40 ± 0.70 μg/mL, P = 0.008, Figure 1).
Figure 1.
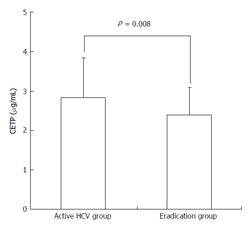
Comparison of the serum cholesteryl ester transfer protein level between the active hepatitis C virus infection group and the hepatitis C virus eradication group. The serum CETP level was significantly higher in patients with active HCV infection than those in whom HCV eradication was achieved (2.84 ± 1.00 μg/mL vs 2.40 ± 0.70 μg/mL, P = 0.008). CETP: Cholesterol ester transfer protein; HCV: Hepatitis C virus.
Characteristics of the active HCV group and the eradication group
The clinical features of patients in the active HCV group and those in the HCV eradication group are summarized in Table 1. There were significant differences in the proportion of patients with HCV G1b infection. There were no significant differences in BMI, FBG levels, and HbA1c levels, whereas AST, ALT, and albumin levels differed significantly. TC and LDL-C levels were significantly lower in patients with active infection than in patients whose infection was eradicated, whereas TG and HDL-C levels were similar between the two groups.
Table 1.
Clinical features of patients with active hepatitis C virus infection (active hepatitis C virus group) and of those in whom hepatitis C virus infection was eradicated (eradication group) n (%)
| Discrete traits | Active HCV group (n = 55) | Eradication group (n = 55) | P value |
| Sex | 0.254 | ||
| Male | 22 (40) | 28 (51) | |
| Female | 33 (60) | 27 (49) | |
| HCV genotype | 0.003 | ||
| 1b | 48 (87) | 36 (65) | |
| 2 | 7 (13) | 19 (35) | |
| Quantitative traits | Mean ± SD | Mean ± SD | |
| Age (yr) | 66.9 ± 11.2 | 64.3 ± 12.1 | 0.200 |
| BMI (kg/m2) | 22.5 ± 3.2 | 22.8 ± 3.4 | 0.631 |
| AST (IU/L) | 53.1 ± 27.5 | 24.0 ± 7.2 | < 0.001 |
| ALT (IU/L) | 49.9 ± 37.1 | 20.0 ± 11.7 | < 0.001 |
| Total bilirubin (mg/dL) | 0.8 ± 0.3 | 0.7 ± 0.3 | 0.242 |
| γ-GTP (IU/L) | 50.4 ± 64 | 27.1 ± 20 | 0.011 |
| Albumin (g/dL) | 3.9 ± 0.4 | 4.4 ± 0.3 | < 0.001 |
| Hb (g/dL) | 13.4 ± 1.7 | 14.2 ± 1.5 | 0.010 |
| Platelet (104/μL) | 15.0 ± 6.3 | 20.0 ± 17.8 | 0.050 |
| FBG (mg/dL) | 108 ± 32 | 107 ± 16 | 0.740 |
| HbA1c (%) | 5.4 ± 0.6 | 6.1 ± 0.5 | 0.030 |
| Lipid profiles | |||
| Total cholesterol (mg/dL) | 173.6 ± 31 | 200.5 ± 37.8 | < 0.001 |
| Triglyceride (mg/dL) | 107.5 ± 52.0 | 103 ± 53.1 | 0.712 |
| LDL cholesterol (mg/dL) | 92.1 ± 25.7 | 117 ± 29.9 | < 0.001 |
| HDL cholesterol (mg/dL) | 59.9 ± 17.4 | 64.9 ± 19.0 | 0.159 |
| CETP level (μg/mL) | 2.84 ± 1.00 | 2.40 ± 0.69 | 0.008 |
There were significant differences in the proportion of patients with HCV G1b infection. There were no significant differences in BMI, FBG levels, and HbA1c levels, whereas AST, ALT, and albumin levels differed significantly. TC and LDL-C levels were significantly lower in the active infection group than in the eradication group, whereas TG and HDL-C levels were similar in the two groups. HCV: Hepatitis C virus; BMI: Body mass index; AST: Aspartate-2-oxoglutarate aminotransferase; ALT: Alanine-2-oxoglutarate aminotransferase; γ-GTP: Gamma-glutamyl transpeptidase; Hb: Hemoglobin; FBG: Fasting blood glucose; HbA1c: Glycosylated hemoglobin; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; CETP: Cholesterol ester transfer protein; LDL-C: Low-density lipoprotein cholesterol.
HCV infection status (active infection or eradication) as an independent determinant that significantly contributed to the serum CETP level
To construct a multiple regression model that can suitably explain the serum CETP concentration, six variables were selected as candidate independent variables. Among categorical data, HCV infection status and sex were selected, because the serum CETP level was significantly higher in female than in male patients (2.90 ± 0.91 μg/mL vs 2.28 ± 0.73 μg/mL, P < 0.001). However, difference in HCV genotype was not selected as a candidate variable because there was no difference in serum CETP level between HCV G1b and G2 patients (2.51 ± 0.68 vs 2.66 ± 0.94, P = 0.472). Furthermore, the serum CETP level was similar between HCV G1b patients and G2 patients in the active group (2.82 ± 1.02 vs 2.99 ± 0.76, P = 0.685) and the eradication group (2.42 ± 0.75 vs 2.36 ± 0.57, P = 0.734).
Among the continuous variables, age (R = 0.246, P = 0.010), albumin level (R = -0.194, P = 0.046), Hb level (R = 0.249, P = 0.009), and HDL-C level (R = 0.236, P = 0.014) were significantly correlated with the serum CETP level and were thus selected as candidates. Other factors including HbA1c (R = 0.084, P = 0.538) and FBS (R = 0.074, P = 0.589) were not selected as candidates, because significant correlation was not verified.
Of these six candidates, HCV infection status, age, sex, and HDL-C level were selected as independent variables for the most suitable multiple regression model to explain the serum CETP level. As shown in Table 2, HCV infection status was an independent factor that significantly influenced the serum CETP level. The R2 value of this model was 0.221, and the adjusted value was 0.191. The R2 value did not improve significantly by the addition of any other candidate factors. However, elimination of any of these four factors significantly decreased the R2 values.
Table 2.
Influence of the hepatitis C virus infection status on the serum cholesterol ester transfer protein level as analyzed by multiple regression analysis
| B | SE | P value | |
| Constant | 0.770 | 0.541 | 0.157 |
| HCV infection | 0.415 | 0.158 | 0.010 |
| Age | 0.014 | 0.006 | 0.042 |
| Female sex | 0.391 | 0.182 | 0.034 |
| HDL | 0.008 | 0.004 | 0.099 |
HCV infection status was an independent factor that significantly influenced the serum cholesterol ester transfer protein level. HCV: Hepatitis C virus; HDL: High-density lipoprotein.
The most suitable regression equation was as follows: Serum CETP (μg/mL) = 0.770 + 0.0139 (age) + 0.391 (sex: male: 0, female: 1) + 0.00818 HDL-C (mg/dL) + 0.416 HCV infection status (eradication: 0, active infection: 1).
Differences in the TG concentration in the four major classes of lipoproteins according to HCV infection status
The cholesterol concentration in LDL-C was significantly lower and the HDL-C concentration was marginally lower in the active HCV group compared to those in the eradication group, whereas cholesterol concentrations in CM and VLDL were similar between the groups.
The TG concentrations in LDL-TG and HDL-TG were significantly higher in the active HCV group compared to the eradication group, whereas TG concentrations in CM and VLDL were similar between groups (Table 3).
Table 3.
Comparison of the cholesterol and triglyceride concentrations in the four major lipoprotein fractions according to hepatitis C virus infection status
| Major class |
Active HCV group |
Eradication group |
P value |
Active HCV group |
Eradication group |
P value |
| Cholesterol (mg/dL) | Triglyceride (mg/dL) | |||||
| CM | 0.19 ± 0.19 | 0.19 ± 0.18 | 0.86 | 1.21 ± 1.53 | 1.28 ± 1.31 | 0.774 |
| VLDL | 29.04 ± 12.31 | 26.12 ± 10.66 | 0.187 | 57.66 ± 42.06 | 65.97 ± 44.18 | 0.314 |
| LDL | 87.94 ± 24.11 | 117.31 ± 26.91 | < 0.001 | 36.25 ± 15.28 | 28.14 ± 9.94 | 0.001 |
| HDL | 49.58 ± 15.19 | 55.19 ± 16.29 | 0.064 | 25.9 ± 7.34 | 17.17 ± 4.82 | < 0.001 |
The cholesterol concentration in LDL-C was significantly lower and HDL-C was marginally lower in the active HCV group, whereas cholesterol concentrations in CM and VLDL were similar between groups. The TG concentrations in LDL-TG and HDL-TG were significantly higher in the active HCV group, whereas TG concentrations in CM and VLDL were similar between groups. HCV: Hepatitis C virus; CM: Chylomicron; VLDL: Very-low-density lipoprotein; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; TG: Triglyceride.
Correlation between the serum CETP concentration and TG concentration in the four major lipoprotein classes
The CETP level had a weak, inverse correlation with CM-C (R = -0.290, P = 0.031) in the eradication group; it had a positive correlation with HDL-C in the active HCV group (R = 0.324, P = 0.015), but this correlation was marginal in the eradication group (R = 0.250, P = 0.064). Significant correlations were not found between groups for the other lipoprotein fractions (Figure 2A).
Figure 2.
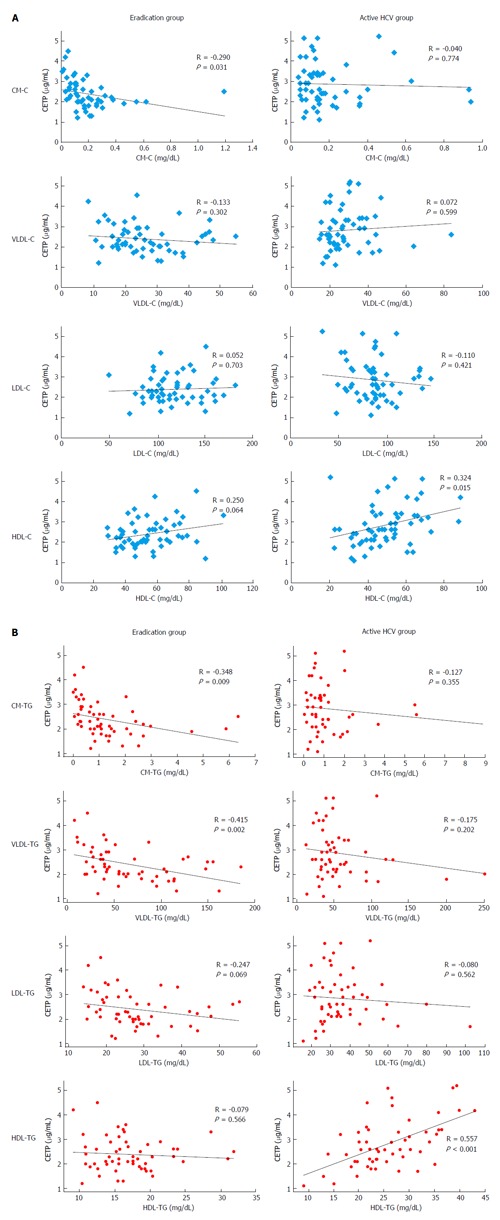
Correlation between the cholesteryl ester transfer protein level and triglyceride concentration in the four major lipoprotein classes in the hepatitis C virus eradication group and the active hepatitis C virus infection group. A: The CETP level had a weak correlation with HDL cholesterol in the active HCV infection group (R = 0.324, P = 0.015) and a weak, inverse correlation with chylomicron-cholesterol (R = -0.290, P = 0.031); B: The CETP level had a strong correlation with HDL-TG in the active HCV infection group (R = 0.557, P < 0.001). However, significant correlations with TG for other lipoprotein classes were not detected in the active HCV group. HCV: Hepatitis C virus; CM: Chylomicron; VLDL: Very-low-density lipoprotein; LDL: Low-density lipoprotein; HDL: High-density lipoprotein; TG: Triglyceride.
According to the TG concentration, the CETP level was inversely correlated with chylomicron-TG (R = -0.348, P = 0.009) and VLDL-TG (R = -0.415, P = 0.002) and marginally correlated with LDL-TG (R = -0.247, P = 0.069), but was not correlated with HDL-TG (R = -0.079, P = 0.566) in the eradication group. In contrast, the CETP level was strongly correlated with HDL-TG in the active HCV group (R = 0.557, P < 0.001). However, significant correlations with TG for other lipoprotein classes were not detected in the active HCV group (Figure 2B).
DISCUSSION
CETP is a glycoprotein that mediates the exchange of CE in HDL for TG in other lipoproteins. In the present study, we investigated the serum concentration of CETP and found that serum CETP levels were significantly higher in the active group than in the eradication group. Moreover, HCV infection was found to be an independent factor in determining the serum CETP level in multiple regression analysis. This suggests that HCV infection promotes the exchange of CE for TG in HDL by increasing the serum CETP concentration.
Although we did not evaluate the activity of CETP, it has generally been accepted that the serum CETP concentration reflects CETP activity[18]. Thus, an increase in the CETP level in patients with active HCV infection may indicate enhanced CETP activity.
CETP activity is regulated by the amount of dietary cholesterol[19,20] and hypertriglyceridemia[21]. In addition, CETP activity is dependent on genetic variations in the CETP gene[22-24]. However, the effect of HCV infection on the activity of CETP has not been investigated previously. Our results indicate that active HCV infection may promote CETP activity. This enhanced CETP activity may play a role in lipoprotein metabolism abnormalities in patients with active HCV infection[22-24].
An unexpected finding in our study was a positive correlation between the serum CETP level and HDL-C, although the correlation was relatively weak and did not reach the level of significance in the eradication group. One of the major functions of CETP is the removal of CE from HDL. In fact, CETP inhibitors substantially increase HDL-C levels and moderately decrease LDL-C levels in humans[25]. There may be a weak negative correlation between CETP and HDL-C in patients with type 2 DM[26].
However, the correlation between serum level of HDL-C and CETP in a healthy population has not been observed[27]. Therefore, the HDL-C level is not simply determined by the function of CETP because the serum level of HDL-C may be dynamically controlled by the balance between HDL synthesis and catabolism, which is not mediated by CETP.
Although there is a consensus that a decrease in serum TC[28] and LDL-C[10] is a feature of HCV infection, little is known about TG abnormalities. A previous study reported that chronic hepatitis C patients had a lower serum VLDL-TG/non-VLDL-TG ratio[11]. Moreover, there was a reported increase in the TG concentration in HDL and LDL in patients with active HCV infection, although the total serum TG level was similar in the active HCV and eradication groups[29].
In a correlation study between CETP and TG concentration in four lipoprotein classes, we found positive and strong correlations between CETP and HDL-TG in the active HCV group. However, this correlation was not found in the eradication group. Conversely, CETP was correlated with CM-TG and VLDL-TG in the eradication group, but was not significantly correlated with CM-TG or VLDL-TG in the active HCV group. These findings indicate that the significance of CETP in the regulation of TG concentration differs according to the HCV infection status. The most striking difference was found in HDL-TG.
The major source of serum TG is a TG-enriched VLDL that is secreted from the liver. TG in VLDL or LDL is transferred to HDL by the action of CETP. In active HCV infection, an increased serum CETP may enhance the transport of TG to HDL. Therefore, the increase in CETP and the strong positive correlation between CETP and HDL-TG in active HCV infection may indicate that HDL has abundant TG, promptly transferred from VLDL, but is not effectively catabolized and eliminated from the serum. Accordingly, as the major metabolic pathway that degrades and eliminates TG in HDL is mediated by hepatic lipase (HL)[30], we speculate that HL activity in active HCV infection is impaired. Our hypothesis of reduced HL activity in active HCV infection is concordant with a previous result that the HL messenger RNA level is lower in the liver of patients with chronic hepatitis C than in the liver of patients with other etiologies and similar disease progression[31]. As a consequence of the abnormal retention of TG in HDL, the multifaceted functions of HDL on atherosclerosis[32] may be affected, and this could contribute to the progression of atherosclerosis in patients with active HCV infection[33]. Furthermore, dyslipidemia, which is caused by high serum CETP activity in active HCV infection, may contribute to intravascular lipoviral particle formation and thus for sustaining HCV infection[34,35].
Our study had some limitations. It included a relatively small sample size, and the degree of atherosclerosis was not determined in the enrolled patients. In addition, we did not examine lipoviral particle and non-lipoviral particle viral load in the active HCV patients. To strengthen our hypothesis that an increase of TG in HDL contributes to atherosclerosis, further large-scale studies including the evaluation of the anti-inflammatory[36,37] and proinflammatory[38] functions of HDL and measurement of the degree of atherosclerosis are warranted.
In summary, HCV infection was an independent factor contributing to the increase in serum CETP. The increase in CETP resulted in abnormal retention of TG in HDL. These findings suggest that CETP is one of the factors that contribute to abnormal lipoprotein metabolism in patients with active HCV infection.
COMMENTS
Background
Hepatitis C virus (HCV) is a unique virus; its use of host lipid metabolism results in a persistent infection. It is important to understand how HCV uses host lipid metabolism and how host lipid metabolism is affected by HCV infection, because HCV infection represents a unique model in which the virus causes chronic infection while coexisting with the host by taking over the host’s metabolism.
Research frontiers
The effect of HCV infection on the activity of cholesteryl ester transfer protein (CETP) has not been investigated previously. The authors have been the first to clarify that CETP may be increased with HCV.
Innovations and breakthroughs
The authors confirmed that CETP plays a role in abnormal lipoprotein metabolism in patients with HCV infection.
Applications
An increase of triglyceride (TG) in high-density lipoprotein (HDL) as a consequence of activated CETP may contribute to progression of atherosclerosis in HCV infection. Furthermore, disturbed lipoprotein metabolism induced by activated CETP may contribute to intravascular formation of HCV lipoviral particles.
Terminology
CETP is a glycoprotein mediating the exchange of cholesteryl ester in HDL for TG in other lipoproteins.
Peer-review
In this study, the authors found that HCV infection was an independent factor contributing to the increase in serum CETP, the increase in CETP resulted in abnormal retention of TG in HDL. These findings suggest that CETP is one of the factors that contribute to abnormal lipoprotein metabolism in patients with active HCV infection. This study has scientific basis and is interesting.
Footnotes
Institutional review board statement: The study was approved by the ethics committee of Jikei University School of Medicine (Tokyo, Japan).
Informed consent statement: All patients gave informed consent.
Conflict-of-interest statement: There were no conflict-of-interests which must be declared.
Data sharing statement: The technical appendix, statistical code, and dataset are available from the corresponding author at satoken@jikei.ac.jp.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: August 31, 2015
First decision: September 28, 2015
Article in press: January 29, 2016
P- Reviewer: Felmlee DJ, Wang Y, Zeng Z S- Editor: Song XX L- Editor: A E- Editor: Liu SQ
References
- 1.Seeff LB. Natural history of chronic hepatitis C. Hepatology. 2002;36:S35–S46. doi: 10.1053/jhep.2002.36806. [DOI] [PubMed] [Google Scholar]
- 2.Hoofnagle JH. Course and outcome of hepatitis C. Hepatology. 2002;36:S21–S29. doi: 10.1053/jhep.2002.36227. [DOI] [PubMed] [Google Scholar]
- 3.Casiraghi MA, De Paschale M, Romanò L, Biffi R, Assi A, Binelli G, Zanetti AR. Long-term outcome (35 years) of hepatitis C after acquisition of infection through mini transfusions of blood given at birth. Hepatology. 2004;39:90–96. doi: 10.1002/hep.20030. [DOI] [PubMed] [Google Scholar]
- 4.Bassendine MF, Sheridan DA, Bridge SH, Felmlee DJ, Neely RD. Lipids and HCV. Semin Immunopathol. 2013;35:87–100. doi: 10.1007/s00281-012-0356-2. [DOI] [PubMed] [Google Scholar]
- 5.Barth H, Liang TJ, Baumert TF. Hepatitis C virus entry: molecular biology and clinical implications. Hepatology. 2006;44:527–535. doi: 10.1002/hep.21321. [DOI] [PubMed] [Google Scholar]
- 6.Scholtes C, Ramière C, Rainteau D, Perrin-Cocon L, Wolf C, Humbert L, Carreras M, Guironnet-Paquet A, Zoulim F, Bartenschlager R, et al. High plasma level of nucleocapsid-free envelope glycoprotein-positive lipoproteins in hepatitis C patients. Hepatology. 2012;56:39–48. doi: 10.1002/hep.25628. [DOI] [PubMed] [Google Scholar]
- 7.Huang H, Sun F, Owen DM, Li W, Chen Y, Gale M, Ye J. Hepatitis C virus production by human hepatocytes dependent on assembly and secretion of very low-density lipoproteins. Proc Natl Acad Sci USA. 2007;104:5848–5853. doi: 10.1073/pnas.0700760104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Perlemuter G, Sabile A, Letteron P, Vona G, Topilco A, Chrétien Y, Koike K, Pessayre D, Chapman J, Barba G, et al. Hepatitis C virus core protein inhibits microsomal triglyceride transfer protein activity and very low density lipoprotein secretion: a model of viral-related steatosis. FASEB J. 2002;16:185–194. doi: 10.1096/fj.01-0396com. [DOI] [PubMed] [Google Scholar]
- 9.Yamaguchi A, Tazuma S, Nishioka T, Ohishi W, Hyogo H, Nomura S, Chayama K. Hepatitis C virus core protein modulates fatty acid metabolism and thereby causes lipid accumulation in the liver. Dig Dis Sci. 2005;50:1361–1371. doi: 10.1007/s10620-005-2788-1. [DOI] [PubMed] [Google Scholar]
- 10.Petit JM, Benichou M, Duvillard L, Jooste V, Bour JB, Minello A, Verges B, Brun JM, Gambert P, Hillon P. Hepatitis C virus-associated hypobetalipoproteinemia is correlated with plasma viral load, steatosis, and liver fibrosis. Am J Gastroenterol. 2003;98:1150–1154. doi: 10.1111/j.1572-0241.2003.07402.x. [DOI] [PubMed] [Google Scholar]
- 11.Nishimura M, Yamamoto H, Yoshida T, Seimiya M, Sawabe Y, Matsushita K, Umemura H, Sogawa K, Takizawa H, Yokosuka O, et al. Decreases in the serum VLDL-TG/non-VLDL-TG ratio from early stages of chronic hepatitis C: alterations in TG-rich lipoprotein levels. PLoS One. 2011;6:e17309. doi: 10.1371/journal.pone.0017309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kuivenhoven JA, Jukema JW, Zwinderman AH, de Knijff P, McPherson R, Bruschke AV, Lie KI, Kastelein JJ. The role of a common variant of the cholesteryl ester transfer protein gene in the progression of coronary atherosclerosis. The Regression Growth Evaluation Statin Study Group. N Engl J Med. 1998;338:86–93. doi: 10.1056/NEJM199801083380203. [DOI] [PubMed] [Google Scholar]
- 13.deGoma EM, deGoma RL, Rader DJ. Beyond high-density lipoprotein cholesterol levels evaluating high-density lipoprotein function as influenced by novel therapeutic approaches. J Am Coll Cardiol. 2008;51:2199–2211. doi: 10.1016/j.jacc.2008.03.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Nagano M, Yamashita S, Hirano K, Kujiraoka T, Ito M, Sagehashi Y, Hattori H, Nakajima N, Maruyama T, Sakai N, et al. Point mutation (-69 G--> A) in the promoter region of cholesteryl ester transfer protein gene in Japanese hyperalphalipoproteinemic subjects. Arterioscler Thromb Vasc Biol. 2001;21:985–990. doi: 10.1161/01.atv.21.6.985. [DOI] [PubMed] [Google Scholar]
- 15.Nagano M, Yamashita S, Hirano K, Ito M, Maruyama T, Ishihara M, Sagehashi Y, Oka T, Kujiraoka T, Hattori H, et al. Two novel missense mutations in the CETP gene in Japanese hyperalphalipoproteinemic subjects: high-throughput assay by Invader assay. J Lipid Res. 2002;43:1011–1018. doi: 10.1194/jlr.m200024-jlr200. [DOI] [PubMed] [Google Scholar]
- 16.Usui S, Hara Y, Hosaki S, Okazaki M. A new on-line dual enzymatic method for simultaneous quantification of cholesterol and triglycerides in lipoproteins by HPLC. J Lipid Res. 2002;43:805–814. [PubMed] [Google Scholar]
- 17.Okazaki M, Usui S, Ishigami M, Sakai N, Nakamura T, Matsuzawa Y, Yamashita S. Identification of unique lipoprotein subclasses for visceral obesity by component analysis of cholesterol profile in high-performance liquid chromatography. Arterioscler Thromb Vasc Biol. 2005;25:578–584. doi: 10.1161/01.ATV.0000155017.60171.88. [DOI] [PubMed] [Google Scholar]
- 18.Yamashita S, Hui DY, Wetterau JR, Sprecher DL, Harmony JA, Sakai N, Matsuzawa Y, Tarui S. Characterization of plasma lipoproteins in patients heterozygous for human plasma cholesteryl ester transfer protein (CETP) deficiency: plasma CETP regulates high-density lipoprotein concentration and composition. Metabolism. 1991;40:756–763. doi: 10.1016/0026-0495(91)90097-g. [DOI] [PubMed] [Google Scholar]
- 19.Quinet EM, Agellon LB, Kroon PA, Marcel YL, Lee YC, Whitlock ME, Tall AR. Atherogenic diet increases cholesteryl ester transfer protein messenger RNA levels in rabbit liver. J Clin Invest. 1990;85:357–363. doi: 10.1172/JCI114446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jiang XC, Moulin P, Quinet E, Goldberg IJ, Yacoub LK, Agellon LB, Compton D, Schnitzer-Polokoff R, Tall AR. Mammalian adipose tissue and muscle are major sources of lipid transfer protein mRNA. J Biol Chem. 1991;266:4631–4639. [PubMed] [Google Scholar]
- 21.Rashid S, Sniderman A, Melone M, Brown PE, Otvos JD, Mente A, Schulze K, McQueen MJ, Anand SS, Yusuf S. Elevated cholesteryl ester transfer protein (CETP) activity, a major determinant of the atherogenic dyslipidemia, and atherosclerotic cardiovascular disease in South Asians. Eur J Prev Cardiol. 2015;22:468–477. doi: 10.1177/2047487314528461. [DOI] [PubMed] [Google Scholar]
- 22.Nakamura A, Niimura H, Kuwabara K, Takezaki T, Morita E, Wakai K, Hamajima N, Nishida Y, Turin TC, Suzuki S, et al. Gene-gene combination effect and interactions among ABCA1, APOA1, SR-B1, and CETP polymorphisms for serum high-density lipoprotein-cholesterol in the Japanese population. PLoS One. 2013;8:e82046. doi: 10.1371/journal.pone.0082046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Suhy A, Hartmann K, Papp AC, Wang D, Sadee W. Regulation of cholesteryl ester transfer protein expression by upstream polymorphisms: reduced expression associated with rs247616. Pharmacogenet Genomics. 2015;25:394–401. doi: 10.1097/FPC.0000000000000151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zhong S, Sharp DS, Grove JS, Bruce C, Yano K, Curb JD, Tall AR. Increased coronary heart disease in Japanese-American men with mutation in the cholesteryl ester transfer protein gene despite increased HDL levels. J Clin Invest. 1996;97:2917–2923. doi: 10.1172/JCI118751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Barter PJ, Kastelein JJ. Targeting cholesteryl ester transfer protein for the prevention and management of cardiovascular disease. J Am Coll Cardiol. 2006;47:492–499. doi: 10.1016/j.jacc.2005.09.042. [DOI] [PubMed] [Google Scholar]
- 26.Inukai Y, Ito K, Hara K, Yamazaki A, Takebayashi K, Aso Y, Inukai T. Serum cholesteryl ester transfer protein concentrations are associated with serum levels of total cholesterol, beta-lipoprotein and apoproteins in patients with type 2 diabetes mellitus. Med Princ Pract. 2007;16:367–372. doi: 10.1159/000104810. [DOI] [PubMed] [Google Scholar]
- 27.Jones RJ, Owens D, Brennan C, Collins PB, Johnson AH, Tomkin GH. Increased esterification of cholesterol and transfer of cholesteryl ester to apo B-containing lipoproteins in Type 2 diabetes: relationship to serum lipoproteins A-I and A-II. Atherosclerosis. 1996;119:151–157. doi: 10.1016/0021-9150(95)05639-4. [DOI] [PubMed] [Google Scholar]
- 28.Maggi G, Bottelli R, Gola D, Perricone G, Posca M, Zavaglia C, Ideo G. Serum cholesterol and chronic hepatitis C. Ital J Gastroenterol. 1996;28:436–440. [PubMed] [Google Scholar]
- 29.Nagano T, Seki N, Tomita Y, Sugita T, Aida Y, Itagaki M, Sutoh S, Abe H, Tsubota A, Aizawa Y. Impact of Chronic Hepatitis C Virus Genotype 1b Infection on Triglyceride Concentration in Serum Lipoprotein Fractions. Int J Mol Sci. 2015;16:20576–20594. doi: 10.3390/ijms160920576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clay MA, Newnham HH, Barter PJ. Hepatic lipase promotes a loss of apolipoprotein A-I from triglyceride-enriched human high density lipoproteins during incubation in vitro. Arterioscler Thromb. 1991;11:415–422. doi: 10.1161/01.atv.11.2.415. [DOI] [PubMed] [Google Scholar]
- 31.Shinohara Y, Imajo K, Yoneda M, Tomeno W, Ogawa Y, Fujita K, Kirikoshi H, Takahashi J, Funakoshi K, Ikeda M, et al. Hepatic triglyceride lipase plays an essential role in changing the lipid metabolism in genotype 1b hepatitis C virus replicon cells and hepatitis C patients. Hepatol Res. 2013;43:1190–1198. doi: 10.1111/hepr.12072. [DOI] [PubMed] [Google Scholar]
- 32.Ansell BJ, Watson KE, Fogelman AM, Navab M, Fonarow GC. High-density lipoprotein function recent advances. J Am Coll Cardiol. 2005;46:1792–1798. doi: 10.1016/j.jacc.2005.06.080. [DOI] [PubMed] [Google Scholar]
- 33.Olubamwo OO, Onyeka IN, Miettola J, Kauhanen J, Tuomainen TP. Hepatitis C as a risk factor for carotid atherosclerosis - a systematic review. Clin Physiol Funct Imaging. 2015:Epub ahead of print. doi: 10.1111/cpf.12229. [DOI] [PubMed] [Google Scholar]
- 34.Felmlee DJ, Sheridan DA, Bridge SH, Nielsen SU, Milne RW, Packard CJ, Caslake MJ, McLauchlan J, Toms GL, Neely RD, et al. Intravascular transfer contributes to postprandial increase in numbers of very-low-density hepatitis C virus particles. Gastroenterology. 2010;139:1774–1783, 1783.e1-6. doi: 10.1053/j.gastro.2010.07.047. [DOI] [PubMed] [Google Scholar]
- 35.Bridge SH, Sheridan DA, Felmlee DJ, Nielsen SU, Thomas HC, Taylor-Robinson SD, Neely RD, Toms GL, Bassendine MF. Insulin resistance and low-density apolipoprotein B-associated lipoviral particles in hepatitis C virus genotype 1 infection. Gut. 2011;60:680–687. doi: 10.1136/gut.2010.222133. [DOI] [PubMed] [Google Scholar]
- 36.Nofer JR, Kehrel B, Fobker M, Levkau B, Assmann G, von Eckardstein A. HDL and arteriosclerosis: beyond reverse cholesterol transport. Atherosclerosis. 2002;161:1–16. doi: 10.1016/s0021-9150(01)00651-7. [DOI] [PubMed] [Google Scholar]
- 37.Zhang H, Reilly MP. Anti-inflammatory effects of high-density lipoprotein through activating transcription factor 3: benefit beyond cholesterol transport-dependent processes. Arterioscler Thromb Vasc Biol. 2014;34:e11–e12. doi: 10.1161/ATVBAHA.114.303553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fogelman AM. When good cholesterol goes bad. Nat Med. 2004;10:902–903. doi: 10.1038/nm0904-902. [DOI] [PubMed] [Google Scholar]


