Abstract
Osteochondritis dissecans (OCD) is a disorder of articular cartilage and subchondral bone. In the elbow, an OCD is localized most commonly at the humeral capitellum. Teenagers engaged in sports that involve repetitive stress on the elbow are at risk. A high index of suspicion is warranted to prevent delay in the diagnosis. Plain radiographs may disclose the lesion but computed tomography and magnetic resonance imaging are more accurate in the detection of OCD. To determine the best treatment option it is important to differentiate between stable and unstable OCD lesions. Stable lesions can be initially treated nonoperatively with elbow rest or activity modification and physical therapy. Unstable lesions and stable lesions not responding to conservative therapy require a surgical approach. Arthroscopic debridement and microfracturing has become the standard initial procedure for treatment of capitellar OCD. Numerous other surgical options have been reported, including internal fixation of large fragments and osteochondral autograft transfer. The aim of this article is to provide a current concepts review of the etiology, clinical presentation, diagnosis, treatment, and outcomes of elbow OCD.
Keywords: Osteochondritis dissecans, Cartilage, Elbow, Capitellum, Athletes, Overhead sports, Arthroscopy, Bone marrow stimulation, Adolescent, Osteoarthritis
Core tip: The aim of this article is to provide a current concepts review of the etiology, clinical presentation, diagnosis, treatment, and outcomes of elbow osteochondritis dissecans. This well illustrated paper highlights the need for a high index of suspicion to prevent delay in the diagnosis. Various imaging methods are outlined. Current treatment options are discussed and future directions are provided.
INTRODUCTION
Osteochondritis dissecans (OCD) is a process in which a segment of articular cartilage separates from the subchondral bone. In the human body, OCD lesions are most commonly found in the knee, followed by the ankle and the elbow[1]. OCD of the elbow typically affects the capitellum of the humerus. It can be a debilitating injury in a young patient population.
Epidemiology
Elbow OCD presents typically in adolescent athletes engaged in repetitive overhead or upper extremity weight-bearing activities (e.g., baseball, tennis, volleyball, weight lifting and gymnastics). The prevalence of OCD of the humeral capitellum was 3.4% among more than 2000 adolescent baseball players[2]. Not all of these patients had symptoms[2]. Patients with an OCD usually are in their second decade of life, with an age ranging from 11 to 23 years. Boys are affected more commonly than girls. The capitellum of the dominant elbow is mostly affected. Bilateral involvement is seen in up to 20% of the patients[3].
Elbow OCD should be distinguished from Panner’s disease or osteochondrosis of the capitellum. Panner’s disease is encountered in younger children (aged 4-12 years), and characterized by ischemia and necrosis of the capitellar epiphysis, followed by regeneration and recalcification. It is a self-limiting, benign disorder that usually resolves with rest.
Etiology
The exact etiology of OCD is unknown. A genetic predisposition has been suggested in twin studies[4]. The main cause, however, is thought to be excessive repetitive valgus compression across the elbow joint with immature articular cartilage[5,6]. Repetitive stress to the lateral elbow compartment could lead to localized injury of subchondral bone of the poorly vascularized humeral capitellum (Figure 1), characterized by focal avascular necrosis and subchondral bone changes. Subsequently, this could result in loss of support for the overlying articular cartilage and eventually breakdown and formation of loose fragments once the mechanical support of the articular cartilage is compromised[5,6].
Figure 1.
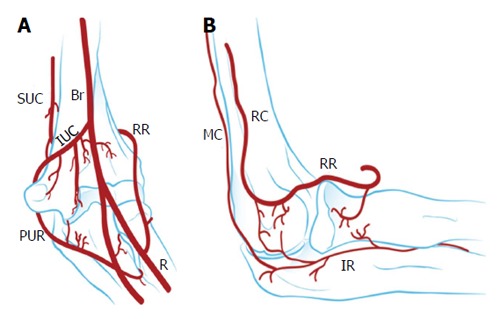
Vascularisation of the capitellum. A: Anterior view; B: Lateral view. Br: Brachial; IR: Interosseous recurrent; IUC: Inferior ulnar collateral; MC: Middle collateral; PUR: Posterior ulnar recurrent; R: Radial; RC: Radial collateral; RR: Radial recurrent; SUC: Superior ulnar collateral artery.
Pathology
OCD usually evolves through three stages[3,6]. In stage 1, hyperemic bone and edematous periarticular soft tissues are found. In stage 2, the epiphysis deforms, sometimes with fragmentation. In stage 3, necrotic bone is replaced by granulation tissue. The articular surface may separate and form a loose body as the bone heals.
Natural history
It seems logical to assume that patients with OCD are predisposed to early osteoarthritis of the elbow. However, the relation between cartilage defects in general and the development of osteoarthritis in the long term has not been elucidated to date. Most evidence is available for cartilage lesions in the knee and ankle[7,8]. Large chondral and osteochondral lesions of the knee are presumed to predispose to osteoarthritis, although the scientific evidence is limited[7]. In the ankle, however, a relation between OCD and osteoarthritis has not been shown[8]. Only 4% of ankle OCDs develop a narrowed joint space up to 20 years of follow-up[9].
With regard to the elbow, little is known about the risk of developing degenerative changes in the long term. Bauer et al[10] investigated elbow degeneration amongst 31 OCD patients at a mean follow-up of 23 years. One-third had radiographic degenerative changes and 42% of patients complained of pain and/or reduced range of motion at the time of follow-up. Younger patients had better odds of having a pain-free elbow without radiographic signs of degeneration in the long term. In addition, larger lesions may be more prone to degenerative changes over time. Takahara et al[11] noted a poorer long-term outcome of patients with large cartilage lesions compared to those with small lesions. There is no evidence that surgical debridement with or without microfracturing protects against degeneration.
CLINICAL PRESENTATION
Patient’s delay and doctor’s delay are very common in elbow OCD. Therefore, a high index of suspicion and directed imaging studies are necessary. In fact, any teenager presenting with lateral elbow pain should be suspected of having an OCD lesion. The typical patient is a young male sports person, initially presenting with pain, tenderness, and swelling over the lateral aspect of the elbow[12]. In a later stage, there may be loss of extension and intermittent catching and locking of the elbow, but physical examination findings are not very distinct in the early stage of OCD. Yet, it is important to detect OCD as early as possible to prevent expansion of the lesion and possible degeneration of the joint.
IMAGING
Plain anteroposterior and lateral radiographs are often used as an initial screening method (Figure 2). Radiographic signs of an OCD are flattening of the capitellum, a focal defect of the articular surface, and loose bodies. However, routine radiographs of the elbow are insensitive in identifying OCD of the capitellum[13]. In fact, approximately half of the radiographs of patients with a capitellar OCD appear normal[13]. An anteroposterior view with the elbow in 45° of flexion may better depict the lesion[14].
Figure 2.
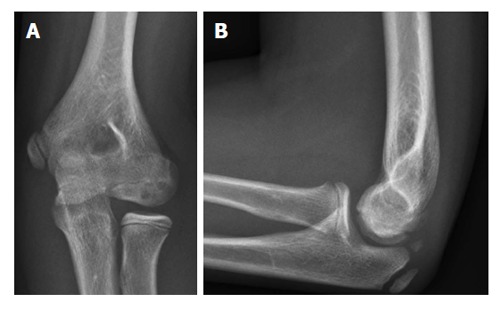
Plain radiography of the elbow showing an osteochondritis dissecans lesion of the capitellum. A: Anteroposterior; B: Lateral.
Because of the low sensitivity of plain radiography, additional imaging is indicated when an OCD is suspected. Ultrasound of the elbow has been described to detect capitellar OCD[15-17]. However, the capitellum is partially obscured by the radial head[17]. Computed tomography (CT) and magnetic resonance imaging (MRI) are most useful in diagnosing an OCD. MRI demonstrates early OCD and is valuable in determining the stability and viability of the OCD fragment (Figure 3)[10,17,18]. Magnetic resonance arthrography utilizes intra-articular gadolinium contrast agent to detect OCD and loose bodies[18]. However, the addition of intra-articular contrast agent does not improve the sensitivity of MRI without contrast agent in detecting cartilage lesions in the elbow[19]. CT scans might be more sensitive and better depict loose bodies (Figure 4). We studied 25 patients with an OCD proven by arthroscopy who all had preoperative radiographs, MRI and CT. The OCD was visible on 25 CT scans (sensitivity, 100%), on 24 MRI scans (sensitivity, 96%), and on 19 radiographs (sensitivity, 76%). Arthroscopy identified loose bodies in 20 cases. These were visible in 18 CT scans (90%), 13 MRI scans (65%) and 11 radiographs (55%). Based on these preliminary data, one might carefully conclude that CT seems to be the optimal imaging technique to diagnose OCD and loose bodies.
Figure 3.
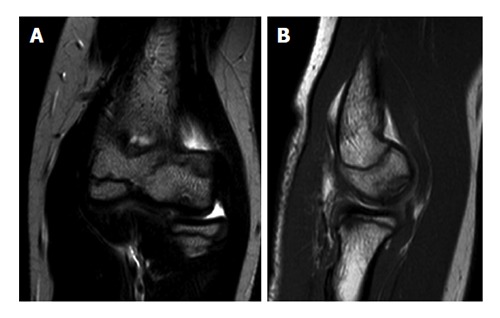
Magnetic resonance images of an elbow affected with osteochondritis dissecans of the capitellum. A: Coronal view; B: Sagittal view.
Figure 4.
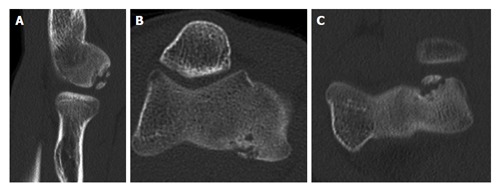
Computed tomography scans showing capitellar osteochondritis dissecans (A-C).
Classification
Although the value of grading capitellar OCD seems limited, various classifications have been described. Most are based on radiography, MRI, or arthroscopy.
Minami et al[20] in 1979 described a classification based on anteroposterior radiography. Grade 1 describes a stable lesion with a translucent cystic shadow in the capitellum; grade 2, a clear zone between the OCD and adjacent subchondral bone; and grade 3, loose bodies.
Itsubo et al[21] recently introduced a T2-weighted MRI staging system that provides accurate and reliable estimation of stability of OCD. The following stages are distinguished: Stage 1, normally shaped capitellum with several spotted areas of high signal intensity that is lower than that of cartilage; stage 2, as with stage 1 but with several spotted areas of higher intensity than that of cartilage; stage 3, as with stage 2 but with both discontinuity and noncircularity of the chondral surface signal of the capitellum and no high signal interface apparent between the lesion and the floor; stage 4, lesion separated by a high intensity line in comparison with cartilage; and stage 5, capitellar lesion displaced from the floor or defect of the capitellar lesion noted. Stages 1 and 2 are considered stable. Stages 3, 4 and 5 are considered unstable[21].
The International Cartilage Repair Society has proposed an arthroscopic classification system for OCD lesions[22]. Grade 1 indicates a stable lesion with a continuous but softened area covered by intact cartilage; grade 2, a lesion with partial discontinuity that is stable when probed; grade 3, a lesion with a complete discontinuity that is not yet dislocated; and grade 4, an empty defect as well as a defect with a dislocated fragment or a loose fragment lying within the bed.
TREATMENT AND OUTCOMES
The treatment choice depends on several aspects, including the severity of symptoms and the size, location and stability of the lesion. It is important to differentiate between stable and unstable OCD lesions. In general, stable lesions may be reversible and can heal completely with nonoperative management, while unstable lesions need surgical treatment[23]. Stable lesions are characterized by an immature capitellum with an open growth plate, and flattening or radiolucency of the subchondral bone, in a patient with (almost) normal elbow motion[23,24]. Unstable lesions have at least one of the following findings: A capitellum with a closed growth plate, fragmentation, or restriction of elbow motion 20 degrees or more[23,25]. On MRI, unstable lesions are characterized by a high signal intensity line through the articular cartilage, a high signal intensity interface, and an articular defect[21,26].
Nonoperative treatment
Nonoperative measures consist of rest or sports restriction (cessation of repetitive stress on the elbow), muscle strengthening exercises, non-steroidal anti-inflammatory drugs, and/or a short course of immobilization[23,24,27]. The minority of OCD lesions are classified as stable and the initial success rates of nonoperative treatment were poor[23,28]. Takahara et al[28] in 1999 reported a success rate of only 50% after an average follow-up of 12.6 years. Factors that are associated with the outcomes of nonoperative treatment were identified later. Bradley and Petrie reported that most patients fully recovered with complete return to sports with rest alone if they had a lesions with all of the following conditions: (1) open capitellar growth plate; (2) localized flattening or radiolucency of the subchondral bone; and (3) good elbow motion[27]. Likewise, Mihara et al[24] showed that spontaneous healing potential of OCD in patients with open capitellar growth plates appears high. Conversely, healing potential with nonoperative management is extremely low in advanced OCD lesions with closed growth plates and in those that are unstable, even if they are undisplaced[6,10,24,27,28].
Operative treatment
Although outcome studies on surgical treatment lack long-term follow-up and have limited methodologic quality, they generally show satisfactory results regarding pain, return to sports, and elbow function[29]. Surgical intervention is therefore indicated for lesions that do not respond to initial nonoperative treatment and for unstable lesions[29].
Primary surgical management most commonly consists of arthroscopic debridement of the lesion, bone marrow stimulation (by microfracturing the subchondral bone) and removal of loose fragments. Alternatively to arthroscopic surgery or for lesions after failed previous surgery, numerous open surgical approaches have been reported, including internal fixation of large fragments and osteochondral autograft transfer[14,30-33].
Arthroscopic treatment
Arthroscopic surgery has become the standard procedure for the treatment of capitellar OCD[34]. It offers the advantage of direct visualization of the pathology and the ability to treat the lesion through small stab incisions. This minimally invasive approach reduces the risk of operative morbidity and allows the patient to start rehabilitation directly after surgery[34].
Arthroscopic treatment consists of debridement of the lesion to achieve a stable rim, followed by bone marrow stimulation, and removal of any loose fragments and osteophytes[35,36]. The patient is placed in the lateral decubitus position on the operating table. A tourniquet is placed around the upper arm, which rests on a padded arm holder that is attached to the side of the table (Figure 5). The portal sites and the ulnar nerve are marked, and the elbow is disinfected and draped. The joint is injected with 20 mL of saline solution. The complete elbow joint is inspected from anterior and posterior with use of five to six portals. A distal ulnar portal allows for ergonomic exposure to the posterolateral capitellum providing easy access for drilling, burring and local debridement[34]. A bonecutter shaver or curette is brought into the posterolateral capitellar joint space through the standard soft-spot lateral portal. All unstable cartilage and necrotic bone are removed. Any cysts underlying the defect are opened and curetted. After debridement, several connections with the subchondral bone are created by drilling with a Kirschner wire or microfracturing with an awl (Figure 6). The objective is to partially destroy the calcified zone that is often present and to create openings into the subchondral bone. Intraosseous blood vessels are disrupted and the release of growth factors leads to the formation of a fibrin clot. The formation of local new blood vessels is stimulated, marrow cells are introduced in the defect, and fibrocartilaginous tissue is formed[37,38].
Figure 5.
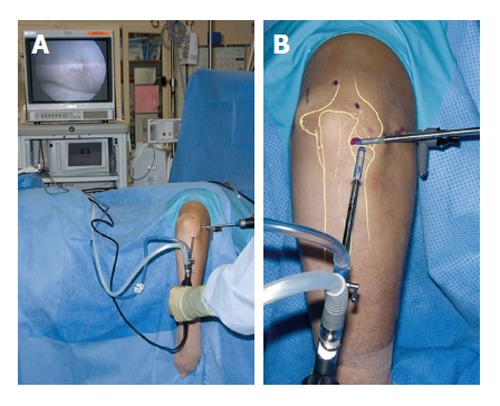
Patient positioning for arthroscopy of a right elbow (A and B). The patient is in the lateral decubitus position and the arm rests on a support.
Figure 6.
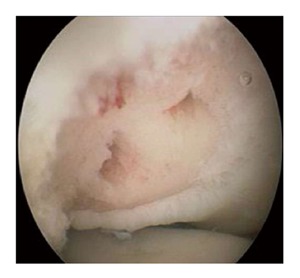
Arthroscopic picture showing an osteochondritis dissecans lesion after debridement and microfracturing.
Arthroscopic treatment has shown encouraging results at intermediate follow-up[35,36,38,39]. Most studies report significant improvement in clinical outcome scores up to 9 years of follow-up[35,36,38-40]. Approximately 80% to 90% of patients return to sports. Time to return to sports varies from 1 to 5 mo[41-43]. Complications of elbow arthroscopy are seen in 7% to 14% of cases[44,45]. Most complications are minor, e.g., superficial wound problems and transient nerve palsies not affecting clinical outcome. Major complications occur in 0.5% to 5% of cases (e.g., deep infection, permanent nerve injury, or complications requiring additional anesthesia)[44,45].
Open surgical treatment
Refixation of the lesion can be indicated for large and (sub)acute osteochondral fragments[31,32,46]. Different fixation techniques are available, including metal and bioresorbable screws[31], pull-out wiring[32,47], and corticocancellous bone pegs from the iliac crest or olecranon process[3]. Cancellous bone can be additionally grafted into the defect to enhance union of the fragment[31,32].
In follow-up studies, the clinical success rate of refixation is approximately 80%[46-48]. Reossification is observed in 44% to 100% at follow-up[31,32,47]. An intact lateral wall of the capitellum appears to be important for fixation to be successful[48]. Complications have been observed in terms of intra-articular protrusion and loosening of screws[27].
After successful application in the knee and ankle[49], autologous osteochondral transplantation (or mosaicplasty) has been used in repairing OCD lesions of the humeral capitellum. With this technique, cylindrical osteochondral grafts are harvested from a non-weight-bearing area at the proximal aspect of the lateral femoral condyle and transplanted to the elbow to resurface the capitellar OCD.
Several authors have evaluated the technique[30,33,50-52]. In a series of 10 patients, eight were completely pain free after a mean follow-up of 30 mo[51]. In a recent investigation of 33 patients who were allowed to begin throwing after 3 mo and to return to sports after 6 mo, 31 patients returned to a competitive level at which they had previously played after a mean of 7 mo[50]. Although the clinical outcomes are encouraging, the grafting technique implies damaging a healthy knee joint, possibly leading to donor-site morbidity. In a study that addressed the effect of the harvesting on donor knee function in young athletes, a time lag was evident in recovery between postoperative symptoms and muscle power at 3 mo[53]. However, harvesting osteochondral grafts did not exert adverse effects at 2 years after the procedure[53].
Osteochondral autograft transfer has the advantage of replacing the affected articular surface with hyaline cartilage, but is an invasive procedure with possible donor-site morbidity. Therefore, we recommend reserving this method for revision cases after failed primary arthroscopic treatment.
Other open procedures in the literature include rib osteochondral autograft and capitellar correction osteotomy[54-56]. Rib autografting provided satisfactory results after a follow-up of 1 to 6 years for advanced OCD with extensive lesions ≥ 15 mm and those affecting the lateral wall[55,56]. Closed-wedge osteotomy of the capitellum has been described to widen the radiohumeral joint space, reduce compression, and stimulate revascularization and remodeling of the area of the lesion in the capitellum[54]. Although almost all patients returned to full athletic activity, postoperative osteoarthritic changes and enlargement of the radial head occurred in all patients. Because of the few scientific data, the place of these experimental treatment methods is unclear until more evidence is available.
Postoperative rehabilitation
A physical therapist supervises the rehabilitation after surgery. Rehabilitation is aimed at reducing pain and swelling and restoring range of motion. The recovery after arthroscopic treatment is usually faster than after open surgery[3]. Active-assisted motion exercises are started within a couple of days after surgery. After arthroscopy, the range of motion is unrestricted as pain tolerates. For patients who were treated by mosaicplasty, flexion is restricted for the first 6 wk. Resistive exercises are begun at 8 wk after arthroscopic treatment and at 12 wk after open treatment. If the patient has no pain and normal range of motion, an interval throwing program is initiated before the patient returns to sports[3].
FUTURE DIRECTIONS
Investigations in the near future primarily should be focused on improvement of awareness and early recognition of OCD, since early intervention may prevent expansion of the lesion and future degeneration. Future studies should also aim for improvement of current treatment and development of new treatment modalities. Although outcomes of arthroscopic treatment are encouraging, larger, randomized, prospective trials are required[29]. Likewise, promising outcomes have been described following autologous osteochondral transplantation[50-52], but future studies should be performed with a longer follow-up time to evaluate intra-articular changes on the long term.
CONCLUSION
OCD of the elbow typically affects the humeral capitellum of adolescent throwing athletes and leads to pain on the lateral aspect of the joint. CT or MRI are indicated to confirm the diagnosis and to address stability of the lesion. Nonoperative treatment can be initiated for stable lesions. Arthroscopic surgery has become the standard primary surgical procedure for treatment of capitellar OCD. This minimally invasive approach shows good results, low risk of operative morbidity, and early recuperation postoperatively. Open surgery is indicated for more advanced cases or for those that failed previous operative treatment.
Footnotes
Conflict-of-interest statement: The authors have no conflict of interest.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: June 1, 2015
First decision: August 4, 2015
Article in press: December 2, 2015
P- Reviewer: Schmitz MR S- Editor: Ji FF L- Editor: A E- Editor: Liu SQ
References
- 1.Bruns J. [Osteochondrosis dissecans] Orthopade. 1997;26:573–584. doi: 10.1007/PL00003414. [DOI] [PubMed] [Google Scholar]
- 2.Kida Y, Morihara T, Kotoura Y, Hojo T, Tachiiri H, Sukenari T, Iwata Y, Furukawa R, Oda R, Arai Y, et al. Prevalence and Clinical Characteristics of Osteochondritis Dissecans of the Humeral Capitellum Among Adolescent Baseball Players. Am J Sports Med. 2014;42:1963–1971. doi: 10.1177/0363546514536843. [DOI] [PubMed] [Google Scholar]
- 3.Baratz M, Yi SJ. Osteochondritis dissecans of the elbow. In: Eygendaal D, editor. The elbow. The treatment of basic elbow pathology. Nieuwegein: Arko Sports Media; 2009. pp. 139–148. [Google Scholar]
- 4.Kenniston JA, Beredjiklian PK, Bozentka DJ. Osteochondritis dissecans of the capitellum in fraternal twins: case report. J Hand Surg Am. 2008;33:1380–1383. doi: 10.1016/j.jhsa.2008.05.008. [DOI] [PubMed] [Google Scholar]
- 5.Douglas G, Rang M. The role of trauma in the pathogenesis of the osteochondroses. Clin Orthop Relat Res. 1981;(158):28–32. [PubMed] [Google Scholar]
- 6.Takahara M, Ogino T, Takagi M, Tsuchida H, Orui H, Nambu T. Natural progression of osteochondritis dissecans of the humeral capitellum: initial observations. Radiology. 2000;216:207–212. doi: 10.1148/radiology.216.1.r00jl29207. [DOI] [PubMed] [Google Scholar]
- 7.Heijink A, Gomoll AH, Madry H, Drobnič M, Filardo G, Espregueira-Mendes J, Van Dijk CN. Biomechanical considerations in the pathogenesis of osteoarthritis of the knee. Knee Surg Sports Traumatol Arthrosc. 2012;20:423–435. doi: 10.1007/s00167-011-1818-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.van Dijk CN, Reilingh ML, Zengerink M, van Bergen CJ. The natural history of osteochondral lesions in the ankle. Instr Course Lect. 2010;59:375–386. [PubMed] [Google Scholar]
- 9.van Bergen CJ, Kox LS, Maas M, Sierevelt IN, Kerkhoffs GM, van Dijk CN. Arthroscopic treatment of osteochondral defects of the talus: outcomes at eight to twenty years of follow-up. J Bone Joint Surg Am. 2013;95:519–525. doi: 10.2106/JBJS.L.00675. [DOI] [PubMed] [Google Scholar]
- 10.Bauer M, Jonsson K, Josefsson PO, Lindén B. Osteochondritis dissecans of the elbow. A long-term follow-up study. Clin Orthop Relat Res. 1992;(284):156–160. [PubMed] [Google Scholar]
- 11.Takahara M, Ogino T, Sasaki I, Kato H, Minami A, Kaneda K. Long term outcome of osteochondritis dissecans of the humeral capitellum. Clin Orthop Relat Res. 1999;(363):108–115. [PubMed] [Google Scholar]
- 12.Takahara M, Shundo M, Kondo M, Suzuki K, Nambu T, Ogino T. Early detection of osteochondritis dissecans of the capitellum in young baseball players. Report of three cases. J Bone Joint Surg Am. 1998;80:892–897. doi: 10.2106/00004623-199806000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kijowski R, De Smet AA. Radiography of the elbow for evaluation of patients with osteochondritis dissecans of the capitellum. Skeletal Radiol. 2005;34:266–271. doi: 10.1007/s00256-005-0899-6. [DOI] [PubMed] [Google Scholar]
- 14.Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. Surgical technique. J Bone Joint Surg Am. 2008;90 Suppl 2 Pt 1:47–62. doi: 10.2106/JBJS.G.01135. [DOI] [PubMed] [Google Scholar]
- 15.Harada M, Takahara M, Sasaki J, Mura N, Ito T, Ogino T. Using sonography for the early detection of elbow injuries among young baseball players. AJR Am J Roentgenol. 2006;187:1436–1441. doi: 10.2214/AJR.05.1086. [DOI] [PubMed] [Google Scholar]
- 16.Takahara M, Ogino T, Tsuchida H, Takagi M, Kashiwa H, Nambu T. Sonographic assessment of osteochondritis dissecans of the humeral capitellum. AJR Am J Roentgenol. 2000;174:411–415. doi: 10.2214/ajr.174.2.1740411. [DOI] [PubMed] [Google Scholar]
- 17.Takenaga T, Goto H, Nozaki M, Yoshida M, Nishiyama T, Otsuka T. Ultrasound imaging of the humeral capitellum: a cadaveric study. J Orthop Sci. 2014;19:907–912. doi: 10.1007/s00776-014-0637-9. [DOI] [PubMed] [Google Scholar]
- 18.Dewan AK, Chhabra AB, Khanna AJ, Anderson MW, Brunton LM. MRI of the elbow: techniques and spectrum of disease: AAOS exhibit selection. J Bone Joint Surg Am. 2013;95:e99 1–13. doi: 10.2106/JBJS.L.01621. [DOI] [PubMed] [Google Scholar]
- 19.Theodoropoulos JS, Dwyer T, Wolin PM. Correlation of preoperative MRI and MRA with arthroscopically proven articular cartilage lesions of the elbow. Clin J Sport Med. 2012;22:403–407. doi: 10.1097/JSM.0b013e318266c735. [DOI] [PubMed] [Google Scholar]
- 20.Minami M, Nakashita K, Ishii S, Usui M, Muramatsu I. Twenty-five cases of osteochondritis dissecans of the elbow. Rinsho Seikei Geka. 1979;14:805–810. [Google Scholar]
- 21.Itsubo T, Murakami N, Uemura K, Nakamura K, Hayashi M, Uchiyama S, Kato H. Magnetic Resonance Imaging Staging to Evaluate the Stability of Capitellar Osteochondritis Dissecans Lesions. Am J Sports Med. 2014;42:1972–1977. doi: 10.1177/0363546514532604. [DOI] [PubMed] [Google Scholar]
- 22.Brittberg M, Winalski CS. Evaluation of cartilage injuries and repair. J Bone Joint Surg Am. 2003;85-A Suppl 2:58–69. doi: 10.2106/00004623-200300002-00008. [DOI] [PubMed] [Google Scholar]
- 23.Takahara M, Mura N, Sasaki J, Harada M, Ogino T. Classification, treatment, and outcome of osteochondritis dissecans of the humeral capitellum. J Bone Joint Surg Am. 2007;89:1205–1214. doi: 10.2106/JBJS.F.00622. [DOI] [PubMed] [Google Scholar]
- 24.Mihara K, Tsutsui H, Nishinaka N, Yamaguchi K. Nonoperative treatment for osteochondritis dissecans of the capitellum. Am J Sports Med. 2009;37:298–304. doi: 10.1177/0363546508324970. [DOI] [PubMed] [Google Scholar]
- 25.Satake H, Takahara M, Harada M, Maruyama M. Preoperative imaging criteria for unstable osteochondritis dissecans of the capitellum. Clin Orthop Relat Res. 2013;471:1137–1143. doi: 10.1007/s11999-012-2462-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kijowski R, De Smet AA. MRI findings of osteochondritis dissecans of the capitellum with surgical correlation. AJR Am J Roentgenol. 2005;185:1453–1459. doi: 10.2214/AJR.04.1570. [DOI] [PubMed] [Google Scholar]
- 27.Bradley JP, Petrie RS. Osteochondritis dissecans of the humeral capitellum. Diagnosis and treatment. Clin Sports Med. 2001;20:565–590. doi: 10.1016/s0278-5919(05)70270-2. [DOI] [PubMed] [Google Scholar]
- 28.Takahara M, Ogino T, Fukushima S, Tsuchida H, Kaneda K. Nonoperative treatment of osteochondritis dissecans of the humeral capitellum. Am J Sports Med. 1999;27:728–732. doi: 10.1177/03635465990270060701. [DOI] [PubMed] [Google Scholar]
- 29.de Graaff F, Krijnen MR, Poolman RW, Willems WJ. Arthroscopic surgery in athletes with osteochondritis dissecans of the elbow. Arthroscopy. 2011;27:986–993. doi: 10.1016/j.arthro.2011.01.002. [DOI] [PubMed] [Google Scholar]
- 30.Iwasaki N, Kato H, Ishikawa J, Saitoh S, Minami A. Autologous osteochondral mosaicplasty for capitellar osteochondritis dissecans in teenaged patients. Am J Sports Med. 2006;34:1233–1239. doi: 10.1177/0363546506286343. [DOI] [PubMed] [Google Scholar]
- 31.Kuwahata Y, Inoue G. Osteochondritis dissecans of the elbow managed by Herbert screw fixation. Orthopedics. 1998;21:449–451. doi: 10.3928/0147-7447-19980401-10. [DOI] [PubMed] [Google Scholar]
- 32.Takeda H, Watarai K, Matsushita T, Saito T, Terashima Y. A surgical treatment for unstable osteochondritis dissecans lesions of the humeral capitellum in adolescent baseball players. Am J Sports Med. 2002;30:713–717. doi: 10.1177/03635465020300051501. [DOI] [PubMed] [Google Scholar]
- 33.Vogt S, Siebenlist S, Hensler D, Weigelt L, Ansah P, Woertler K, Imhoff AB. Osteochondral transplantation in the elbow leads to good clinical and radiologic long-term results: an 8- to 14-year follow-up examination. Am J Sports Med. 2011;39:2619–2625. doi: 10.1177/0363546511420127. [DOI] [PubMed] [Google Scholar]
- 34.van den Ende KI, McIntosh AL, Adams JE, Steinmann SP. Osteochondritis dissecans of the capitellum: a review of the literature and a distal ulnar portal. Arthroscopy. 2011;27:122–128. doi: 10.1016/j.arthro.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 35.Baumgarten TE, Andrews JR, Satterwhite YE. The arthroscopic classification and treatment of osteochondritis dissecans of the capitellum. Am J Sports Med. 1998;26:520–523. doi: 10.1177/03635465980260040801. [DOI] [PubMed] [Google Scholar]
- 36.Byrd JW, Jones KS. Arthroscopic surgery for isolated capitellar osteochondritis dissecans in adolescent baseball players: minimum three-year follow-up. Am J Sports Med. 2002;30:474–478. doi: 10.1177/03635465020300040401. [DOI] [PubMed] [Google Scholar]
- 37.O’Driscoll SW. The healing and regeneration of articular cartilage. J Bone Joint Surg Am. 1998;80:1795–1812. [PubMed] [Google Scholar]
- 38.Wulf CA, Stone RM, Giveans MR, Lervick GN. Magnetic resonance imaging after arthroscopic microfracture of capitellar osteochondritis dissecans. Am J Sports Med. 2012;40:2549–2556. doi: 10.1177/0363546512458765. [DOI] [PubMed] [Google Scholar]
- 39.Schoch B, Wolf BR. Osteochondritis dissecans of the capitellum: minimum 1-year follow-up after arthroscopic debridement. Arthroscopy. 2010;26:1469–1473. doi: 10.1016/j.arthro.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 40.Bojanić I, Ivković A, Borić I. Arthroscopy and microfracture technique in the treatment of osteochondritis dissecans of the humeral capitellum: report of three adolescent gymnasts. Knee Surg Sports Traumatol Arthrosc. 2006;14:491–496. doi: 10.1007/s00167-005-0693-y. [DOI] [PubMed] [Google Scholar]
- 41.Jones KJ, Wiesel BB, Sankar WN, Ganley TJ. Arthroscopic management of osteochondritis dissecans of the capitellum: mid-term results in adolescent athletes. J Pediatr Orthop. 2010;30:8–13. doi: 10.1097/BPO.0b013e3181c3be83. [DOI] [PubMed] [Google Scholar]
- 42.Miyake J, Masatomi T. Arthroscopic debridement of the humeral capitellum for osteochondritis dissecans: radiographic and clinical outcomes. J Hand Surg Am. 2011;36:1333–1338. doi: 10.1016/j.jhsa.2011.05.024. [DOI] [PubMed] [Google Scholar]
- 43.Rahusen FT, Brinkman JM, Eygendaal D. Results of arthroscopic debridement for osteochondritis dissecans of the elbow. Br J Sports Med. 2006;40:966–969. doi: 10.1136/bjsm.2006.030056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Elfeddali R, Schreuder MH, Eygendaal D. Arthroscopic elbow surgery, is it safe? J Shoulder Elbow Surg. 2013;22:647–652. doi: 10.1016/j.jse.2013.01.032. [DOI] [PubMed] [Google Scholar]
- 45.Nelson GN, Wu T, Galatz LM, Yamaguchi K, Keener JD. Elbow arthroscopy: early complications and associated risk factors. J Shoulder Elbow Surg. 2014;23:273–278. doi: 10.1016/j.jse.2013.09.026. [DOI] [PubMed] [Google Scholar]
- 46.Hennrikus WP, Miller PE, Micheli LJ, Waters PM, Bae DS. Internal Fixation of Unstable In Situ Osteochondritis Dissecans Lesions of the Capitellum. J Pediatr Orthop. 2014;35:467–473. doi: 10.1097/BPO.0000000000000308. [DOI] [PubMed] [Google Scholar]
- 47.Nobuta S, Ogawa K, Sato K, Nakagawa T, Hatori M, Itoi E. Clinical outcome of fragment fixation for osteochondritis dissecans of the elbow. Ups J Med Sci. 2008;113:201–208. doi: 10.3109/2000-1967-232. [DOI] [PubMed] [Google Scholar]
- 48.Kosaka M, Nakase J, Takahashi R, Toratani T, Ohashi Y, Kitaoka K, Tsuchiya H. Outcomes and failure factors in surgical treatment for osteochondritis dissecans of the capitellum. J Pediatr Orthop. 2013;33:719–724. doi: 10.1097/BPO.0b013e3182924662. [DOI] [PubMed] [Google Scholar]
- 49.Hangody L, Füles P. Autologous osteochondral mosaicplasty for the treatment of full-thickness defects of weight-bearing joints: ten years of experimental and clinical experience. J Bone Joint Surg Am. 2003;85-A Suppl 2:25–32. doi: 10.2106/00004623-200300002-00004. [DOI] [PubMed] [Google Scholar]
- 50.Maruyama M, Takahara M, Harada M, Satake H, Takagi M. Outcomes of an open autologous osteochondral plug graft for capitellar osteochondritis dissecans: time to return to sports. Am J Sports Med. 2014;42:2122–2127. doi: 10.1177/0363546514538759. [DOI] [PubMed] [Google Scholar]
- 51.Ovesen J, Olsen BS, Johannsen HV. The clinical outcomes of mosaicplasty in the treatment of osteochondritis dissecans of the distal humeral capitellum of young athletes. J Shoulder Elbow Surg. 2011;20:813–818. doi: 10.1016/j.jse.2010.09.001. [DOI] [PubMed] [Google Scholar]
- 52.Lyons ML, Werner BC, Gluck JS, Freilich AM, Dacus AR, Diduch DR, Chhabra AB. Osteochondral autograft plug transfer for treatment of osteochondritis dissecans of the capitellum in adolescent athletes. J Shoulder Elbow Surg. 2015;24:1098–1105. doi: 10.1016/j.jse.2015.03.014. [DOI] [PubMed] [Google Scholar]
- 53.Nishimura A, Morita A, Fukuda A, Kato K, Sudo A. Functional recovery of the donor knee after autologous osteochondral transplantation for capitellar osteochondritis dissecans. Am J Sports Med. 2011;39:838–842. doi: 10.1177/0363546510388386. [DOI] [PubMed] [Google Scholar]
- 54.Kiyoshige Y, Takagi M, Yuasa K, Hamasaki M. Closed-Wedge osteotomy for osteochondritis dissecans of the capitellum. A 7- to 12-year follow-up. Am J Sports Med. 2000;28:534–537. doi: 10.1177/03635465000280041401. [DOI] [PubMed] [Google Scholar]
- 55.Nishinaka N, Tsutsui H, Yamaguchi K, Uehara T, Nagai S, Atsumi T. Costal osteochondral autograft for reconstruction of advanced-stage osteochondritis dissecans of the capitellum. J Shoulder Elbow Surg. 2014;23:1888–1897. doi: 10.1016/j.jse.2014.06.047. [DOI] [PubMed] [Google Scholar]
- 56.Shimada K, Tanaka H, Matsumoto T, Miyake J, Higuchi H, Gamo K, Fuji T. Cylindrical costal osteochondral autograft for reconstruction of large defects of the capitellum due to osteochondritis dissecans. J Bone Joint Surg Am. 2012;94:992–1002. doi: 10.2106/JBJS.J.00228. [DOI] [PMC free article] [PubMed] [Google Scholar]


