Abstract
Spine tumors comprise a small percentage of reasons for back pain and other symptoms originating in the spine. The majority of the tumors involving the spinal column are metastases of visceral organ cancers which are mostly seen in older patients. Primary musculoskeletal system sarcomas involving the spinal column are rare. Benign tumors and tumor-like lesions of the musculoskeletal system are mostly seen in young patients and often cause instability and canal compromise. Optimal diagnosis and treatment of spine tumors require a multidisciplinary approach and thorough knowledge of both spine surgery and musculoskeletal tumor surgery. Either primary or metastatic tumors involving the spine are demanding problems in terms of diagnosis and treatment. Spinal instability and neurological compromise are the main and critical problems in patients with tumors of the spinal column. In the past, only a few treatment options aiming short-term control were available for treatment of primary and metastatic spine tumors. Spine surgeons adapted their approach for spine tumors according to orthopaedic oncologic principles in the last 20 years. Advances in imaging, surgical techniques and implant technology resulted in better diagnosis and surgical treatment options, especially for primary tumors. Also, modern chemotherapy drugs and regimens with new radiotherapy and radiosurgery options caused moderate to long-term local and systemic control for even primary sarcomas involving the spinal column.
Keywords: Spinal column, Sarcoma, Metastasis, Spinal neoplasms, Palliative surgery
Core tip: Primary tumors involving the spine are rare, while spinal column metastases are present in up to 70% of cancer patients. Both primary and metastatic tumors of the spine are often asymptomatic or have non-specific symptoms because in spine tumors, delayed diagnosis is not very unusual. Goal of treatment in spinal column metastases is to optimize the patient’s quality of life by providing effective pain relief and preserving or restoring neurological functions. Treatment strategy for primary tumors should be planned after both oncological and surgical staging. Because of that, biopsy is a very important step in primary tumors. Surgery in metastatic tumors are mostly palliative, aiming short-term control. Primary benign and malignant lesions mainly cause canal compromise and are treated surgically according to oncological staging and Weinstein-Boriani-Biagini classification.
INTRODUCTION
Spine tumors are examined under two subtitles called primary tumors which originate from the spine itself and its adjacent structures and secondary (metastatic) tumors of distant organs which spread hematogenously and lymphatically and are located in the spine and its surrounding tissues. As the spine is well vascularized and has close relationship with regional lymphatic and venous drainage systems (especially Batson’s venous plexus), it is generally susceptible to metastasis. Metastatic tumors are most common (97%) tumors of the spine[1]. It is known that the adenocarcinomas which mostly originate from the lung, breast, prostate, kidney, gastrointestinal tract and thyroid tend to metastasize especially to the spine[2]. It was found that the percentage of cancer patients who have had bone metastasis before death is between 50% and 70%, and especially in case of breast cancer this percentage rose up to 85%. Up to 10% of patients who have symptomatic spine metastases can be treated by surgery[3]. The most common (70%) sites for spine metastasis are thoracic and thoracolumbar spine, and lumbar spine and sacrum have more than 20% of metastatic lesions. Cervical spine is a less frequent metastasis site[1].
As primary tumors of the spine are rare and most of these lesions are asymptomatic, their real incidence is unknown. It is estimated that the incidence of hemangiomas and enostoses, which were accepted as the most common primary tumors of the spine, is between 11% and 14%. This ratio has been found to be dependent on lesions which have been detected incidentally in performing diagnostic procedures for another reasons. Proper diagnosis of these asymptomatic lesions which are seen in the spine very common and do not require treatment will prevent the performance of unnecessary diagnostic procedures[3]. Except some primary tumors (osteoblastoma, chordoma) which tend to effect especially the spine, tumors originate from the skeleton system itself are not seen in the spine frequently. Differential diagnosis of primary tumors of the spine from especially spinal infections is extremely important. Primary malignant tumors of the spine is the rarest tumor type in the spine. In all bone and soft tissue sarcomas, only 10% of them are related with the spine[4].
Clinical features
Patients with spine tumors have medical histories and physical examination findings which are not directly associated with current disease. However, these findings need to be perfectly understood and evaluated in order to give some clues about the disease to the physician. In patients with spine tumors, the most common and leading symptom is pain[4]. As in almost all skeletal system tumors, the patients with spine tumors believe that their pain is relevant to a real or suspected traumatic event in the recent past. This condition sometimes indicates a pathological fracture which occurs by collapsing of the vertebral body due to a current destruction as a result of a minor trauma. The pain that slowly starts, gradually increases, is usually persistent at night and eventually disturbs the patient even at rest is considered the most typical sign for spine tumors. An acute pain that starts without any trauma in a patient without any previous symptom should also be considered a pathological fracture. Pain in spinal tumors can occur as a result of many reasons. Generally a tumor that grows inside the vertebral body with expansion causes bone remodalization and thinning of the cortex at first, then causes pathologic fracture and invasion of paravertebral structures. At the beginning of disease the main source of pain is stretched periosteum as a result of cortical expansion. After the development of the fracture, pain due to neural compression, neurological deficits and instability comes foreground[3]. Waist and back pain is commonly seen in the population. However, most patients with spine tumors have local tenderness which is a sign that was not observed in other non-traumatic spine problems. Benign tumors in children can sometimes appear as secondary scoliosis or torticollis due to pain. In the case of pathological fracture, kyphotic posture may be seen.
In patients with spine tumors, radicular signs are also frequent. Radicular signs could also be as a result of invasion or compression of the nerve root by the tumor itself, and sometimes pathologic fractures could make root irritation. In patients who developed a neurological deficit, it is important to evaluate the processes associated with the development of this deficit[4]. There is a major difference in terms of prognosis and behavior of the tumor between a patient with a sudden onset of paraparesia and paraplegia who previously had pain and a patient who developed a neurological deficit in months.
Another important point about evaluating patients with spine tumors is the patient’s age. Metastatic tumors, which are the most common tumors of the spine, and hematological malignancies are usually seen after 50 years of age.
In cases under the age of 18, usually benign primary bone tumors such as hemangiomas, eosinophilic granuloma, osteoid osteoma, osteoblastoma, aneurysmal bone cyst and giant cell tumor should be considered in the foreground[3,5]. The most common malignant tumors in young patients are osteosarcoma and Ewing’s sarcoma[3].
When evaluating patients with spine tumors, the current and potential cancer and carcinogen contact history must be investigated. There are reports about cases who had spine metastases years after successful cancer treatment[3]. It must be kept in mind that benign musculoskeletal lesions in any part of the body may cause spine metastasis after malignant or sarcomatous transformation[6]. Especially history of mammographic examination in female patients over 40 years of age, and the smoking history in male patients must be questioned[4].
Diagnostic procedures
Plain radiographs must always take the first line in imaging for spinal diseases. In patients with suspected spine tumors, other parts of the spine and pelvis must be screened in addition to the plain radiographs of the suspected region. Plain radiographs can help to identify nearly 80% of the benign tumors that have a more specific appearance and some of malignant tumors and metastatic lesions[1]. Plain radiographic findings are present in 40% of patients with spine metastasis. At least 50% loss of the trabecular bone is required for a destructive spine lesion to be visualised on plain radiographs[4]. In many hematological malignancies, plain radiographic findings may not be seen until the advanced stages of disease. Plain radiographic characteristics of metastatic lesions can be osteoblastic, osteolytic or mixed. Spine metastases of prostate and breast carcinomas are generally osteoblastic or mixed-type lesions, but lung and thyroid carcinomas as well as renal cell carcinoma are usually in the form of lytic metastatic lesions[3].
Radiopaque lesions which extend outside of the rectangle that draws the boundaries of the vertebral body generally indicate primary malignant lesions of the spine like osteosarcoma or chondrosarcoma. Radiographic sign known as “winking owl sign” can be defined as a faint shadow obscuring the visibility of one pedicle on anteroposterior radiograph, indicates extending of the tumor mass from vertebral body to paraspinal area (Figure 1). Winking owl sign is generally accepted as the earliest direct radiographic sign of a metastatic lesion. Another plain radiographic finding for spine tumors is presence of one or more lytic lesions. Lytic lesions indicate bone destruction. However, destruction pattern gives information about nature of the tumor in the spine as well as in all bone tumors. Geographical destruction suggests that tumor is slowly progressive, moth-eaten lesions suggest that tumor grows faster, and permeative destruction suggests that tumor is very rapidly progressive[5]. Another plain radiographic finding is collapse of the vertebral body that can be called compression fracture. It is not easy to distinguish pathological compression fractures from benign osteoporotic ones. Bone scintigraphy is the most helpful diagnostic procedure in cases whose plain radiographs are negative or suspicious[4]. Bone scintigraphy is a diagnostic procedure performed by radioisotopes. Even though bone scintigraphy has a low specifity except in some tumors such as osteoid osteoma, it is a useful tool for diagnosis because of its high sensitivity and the ability to scan the entire body that is not found in other diagnostic tools. It is also useful in terms of recognizing the primary disease in metastatic tumors which have unknown primary origins and guiding biopsy.
Figure 1.
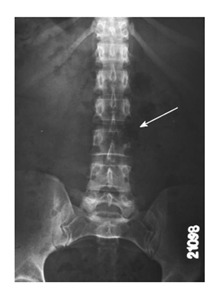
The (absent) pedicle sign known as the winking owl sign (arrow). A reliable sign of osteolytic spinal metastases on antero-posterior radiographs is loss of the normal pedicle contour.
Computed tomography (CT) is the most advantageous method in examination of mineralized tissues. Even complex anatomical structures like the spine could be evaluated by CT, which is superior to plain radiographs with regards to its ability of 3 plane examinaton. However, poor affinity and efficacy of CT in soft tissue lesions are disadvantages of this method .
Magnetic resonance imaging (MRI) is superior to all diagnostic procedures in spine tumors, especially in the evaluation of bone marrow and spinal canal, relationship of the tumor with neurovascular structures and tumor vascularity. In patients with spinal canal involvement, MRI is a useful technique for scanning the adjacent levels with wide, cross-sectional sagittal images. In 10% of spine metastases with spinal canal involvement, neurological compromise in adjacent or distant levels has been shown[3]. An important point about the MRI is its ability to differentiate osteoporotic compression fractures and pathological spinal fractures. Although there is no consensus so far, pathological fractures show low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences, but osteoporotic compressions show low signal intensity in both sequences. However, this finding is not valid for acute osteoporotic fractures. Osteoporotic compression fractures in the acute state (3-6 wk after the fracture) will be able to show low signal intensity on T1-weighted sequences and high signal intensity on T2-weighted sequences due to oedema and congestion within the trabecular bone. In such cases, the bone marrow signal pattern should be evaluated. Gadolinium contrast enhanced MRI can also distinguish intra and extra-dural tumors and also intra and extra-medullary tumors[7].
In spine tumors, especially those with unknown primary origin, biopsy is the latest and the most crucial step of the diagnostic process. Before planning the biopsy, all diagnostic tools should be used in a rational manner and precise localization of the lesion should be determined[8]. Biopsy in spine tumors can be performed as fine needle aspiration, tru-cut biopsy, incisional or excisional biopsy. Fine needle biopsy and tru-cut biopsy are percutaneously applied procedures. It should be kept in mind that biopsy tract is contaminated by tumor cells, and biopsies must be performed far from the neurovascular structures by small incisions which could then be removed with tumor mass in definitive surgical procedure.
Staging
In orthopedic oncologic surgery, where multidisciplinary approach is necessary, use of classifications that guide the treatment steps is inevitable[9]. The effective use of these classifications requires knowledge about the surgical margins in orthopedic oncologic surgery. Surgical treatment of bone tumors should be performed by targeting one of the four surgical margins[10]. In intralesional surgery, the tumor is removed in small pieces by destroying the anatomical structure and integrity of tumor. This type of surgery is usually performed in benign tumors, because it is not possible to obtain a clean surgical margin by this method. In marginal resection, the tumor is removed en bloc but, even in a small area of its surface, it is covered by the capsule or pseudo-capsule. In wide resection, the tumor is removed en bloc entirely enwrapped by a continious layer of normal tissue. Finally, in the radical resection, the tumor is removed en bloc with the entire anatomical compartments of origin bounded by its natural barriers such as the disc, fascia, cortex and end plate[11].
Enneking classification has been used for the classification of benign and malignant tumors of the musculoskeletal system for over 25 years[12]. In Enneking classification, benign tumors are indicated with arabic numbers (1, 2, 3) according to the nature of tumor and its histopathological grade. Benign tumors are classified as inactive (latent), active and aggressive. Malignant tumors are indicated with Roman numbers (I, II, III) according to histopathological grade, localization and the relationship of tumor with natural barriers and whether the tumor metastasizes or not[12]. However, Enneking classification in treatment of spine tumors has been found to be insufficient for surgical planning over time. Because of that, in 1997, Boriani et al[13] have published a study about the new terminology and surgical staging for primary tumors of the spine. The authors stated a new classification system known as Weinstein-Boriani-Biagini classification, which is still actively in use today. In this classification, the spine is radially divided into 12 equal radial segments (clock-face) in axial plane and examined in 5 layers from superficial to deep plane (Figure 2).
Figure 2.
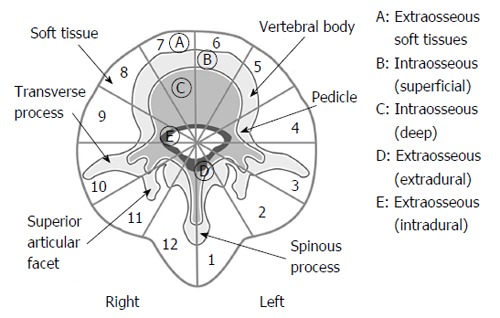
The Weinstein-Boriani-Biagini surgical staging system[13]. In this classification, the spine is radially divided into 12 equal radial segments (clock-face) in axial plane and examined in 5 layers from superficial to deep plane. Adapted with permission from Spine 1997; 22(9):1036-1044.
In 2005, Tokuhashi et al[14] have published a study about preoperative prognostic classification for patients with spine metastases. The classification system was based on general condition of the patient, extraspinal bone metastases, number of metastatic foci in the spine, major visceral metastases, primary cancer focus (origin) and the patient’s neurological status. The authors have stated that the patients with a Tokuhashi score between 12 and 15 points have a life expectancy more than 1 year and this patient group should be treated by tumor excision. The patients with a Tokuhashi score between 9 and 11 points have a life expectancy more than 6 mo, and patients in this group with single level spine metastasis but without major internal organ (visceral) metastases should be treated by tumor excision, while the rest should be treated by palliative surgery. The patients with a Tokuhashi score less than 8 points have a life expectancy less than 6 mo, and these patients should be treated by palliative surgery or conservative treatment[14].
Tomita et al[15] have published a classification regarding surgical strategy in spinal metastases in 2001. According to this classification, patient evaluation was based on 3 prognostic factors: Histopathologic grade of primary tumor, visceral metastasis to vital organs (the lungs, liver, kidneys and brain) and bone metastases including spine metastases (Table 1). Spine metastases were also evaluated in 7 types (Figure 3). As long-term regional control could be provided, the patients whose score is 2-3 points from Tomita classification are suggested to be treated by total en bloc spondylectomy which means marginal or wide resection; as medium-term regional control could be provided, the patients whose score is 4-5 points are suggested to be treated by marginal resection or intralesional treatment (total en bloc spondylectomy or curettage); as they are appropriate for short-term palliation, the patients whose score is 6-7 points are suggested to be treated by palliative surgery like spinal canal decompression and stabilization; the patients whose score is 8 and above points are suggested to be treated by conservative support treatment instead of surgical treatment with the idea that they are at the terminal stage.
Table 1.
Surgical strategy for spinal metastases
| Point |
Scoring system |
Prognostic score | Treatment goal | Surgical strategy | ||
|
Prognostic factors |
||||||
| Primary tumor | Visceral metastases | Bone metastases1 | ||||
| 1 | Slow growth (breast, thyroid, etc.) | Solitary or isolated | 2 | Long-term local control | Wide or marginal excision | |
| 3 | ||||||
| 2 | Moderate growth (kidney, uterus, etc.) | Treatable | Multiple | 4 | Middle-term local control | Marginal or intralesonal excision |
| 5 | ||||||
| 4 | Rapid growth (lung, stomach, etc.) | Untreatable | 6 | Short-term palliation | Palliative surgery | |
| 7 | ||||||
| 8 | Terminal care | Supportive care | ||||
| 9 | ||||||
| 10 | ||||||
No visceral metastases = 0 points;
Bone metastases: Including spinal metastases.
Figure 3.
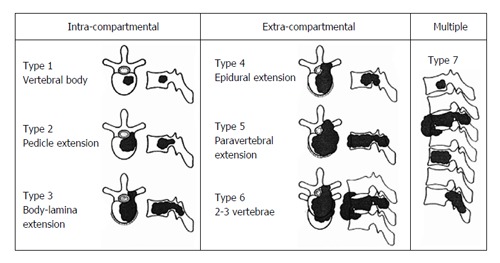
Schematic diagram of surgical classification of spinal tumors according to Tomita et al[15]. Adapted with permission from Spine 2001; 26(3): 298-306.
Surgical treatment
In spine tumors, main goal of surgical treatment is local control for local disease and at least one year survival for spine metastases. Surgery is the best treatment option for the pain and neurological symptoms caused by spinal instability. Spinal instability, vertebral collapse with or without any neurological deficit, radiotherapy resistant tumors, intolerable pain unresponsive to conventional therapy and neurological deficit before, during or after the radiotherapy are the indications for surgery[7]. General principals for spine tumor surgery are decompression of tumor compression to the spinal cord, establishing a tumor-free solid spine and performing the surgery with minimal morbidity.
Surgical modalities in metastatic spine tumors are palliative interventions including posterior decompression of spinal canal with posterior instrumentation, restoring the bone loss of the vertebral body with cement augmentation techniques (vertebroplasty/kyphoplasty), and total spondylectomy with anterior and posterior stabilization[15]. In primary tumors of the spine, total/partial laminectomy, total/partial vertebral body resection, piece-meal resection and curettage, in addition to the surgical procedures described above, can be used.
Total spondylectomy or vertebrectomy, which is the wide resection procedure for spine tumors, can be performed in 1 or 2 stage operation. In 2 stage total spondylectomy, initially posterior instrumentation is applied and total laminectomy via bilateral pedicle section is done. After that, the patient is turned in supine position and vertebral body removal is performed via an anterior approach. In total spondylectomy, segmentary nerve roots and vessels are ligated and sectioned as well as caudal and cranial discs. After vertebral body removal, anterior defect must be reconstructed[13]. One stage total en bloc spondylectomy was introduced by Tomita et al[16]. In this procedure, removal of vertebral body is performed through a posterior approach after blunt dissection of vertebral body from surrounding structures and large vessels that lie at the anterior of the spinal column[16] (Table 2).
Table 2.
Tokuhashi classification[14]
| Characteristic | Score |
| General condition (performance status) | |
| Poor (10%-40%) | 0 |
| Moderate (50%-70%) | 1 |
| Good (80%-100%) | 2 |
| No. of extraspinal bone metastasis foci | |
| ≥ 3 | 0 |
| 1-2 | 1 |
| 0 | 2 |
| No. of extraspinal metastasis foci in the vertebral body | |
| ≥ 3 | 0 |
| 1-2 | 1 |
| 0 | 2 |
| Metastases to the major internal organs | |
| Unremovable | 0 |
| Removable | 1 |
| No metastases | 2 |
| Primary site of the cancer | |
| Lung, osteosarcoma, stomach, bladder, esophagus, pancreas | 0 |
| Liver, gallbladder, unidentified | 1 |
| Others | 2 |
| Kidney, uterus | 3 |
| Rectum | 4 |
| Thyroid, breast, prostate, carcinoid tumor | 5 |
| Palsy | |
| Complete (Frankel A, B) | 0 |
| Incomplete (Frankel C, D) | 1 |
| None (Frankel E) | 2 |
Criteria of predicted prognosis: Total score (TS) 0-8: < 6 mo; TS 9-11: ≥ 6 mo; TS 12-15: ≥ 1 year.
Radical resection is not easy in spine tumors. According to surgical oncologic principles, total removal of involved level vertebral body as well as that level of dural sac, spinal cord and spinal nerves have to be sectioned[13]. Even though this procedure is extremely morbid, it is rarely used as a salvage procedure[7].
Primary benign spinal tumors
Osteoid osteoma, osteoblastoma, osteochondroma, giant cell tumor of the bone, aneurysmal bone cyst, eosinophilic granuloma and neurofibroma are the most common primary benign spine tumors. Primary benign tumors of the spine are more common than primary malignant ones. Benign aggresive tumors, such as giant cell tumor of the bone, osteoblastoma and aneurysmal bone cyst, tend to relapse. Because of that, surgical treatment of these tumors must include local adjuvant agents with marginal resection[17].
Osteochondromas as they are mostly seen in the other benign tumors of the spine, originate from posterior elements and become symptomatic by spinal canal compromise or nerve root compression. One should not forget that, if the cartilage cap of the osteochondroma is not completely removed, the tumor can recur[18].
Osteoid osteoma is a frequent primary benign spinal tumor. Osteoid osteoma is commonly seen in adolescents and young adults with painful secondary scoliosis and pain that worsens at night and is relieved by non-steroidal anti inflammatory agents, especially acetyl-salicylate. Treatment of osteoid osteoma is based on removal or ablation of entire nidus. Symptoms dramatically disappear after treatment[19].
The most common site for osteoblastoma in the entire skeleton is the spinal column. Although histopatologically similar to osteoid osteoma, osteoblastoma has different clinical and radiological characteristics. Osteoblastomas mostly originate from posterior elements, as other benign tumors. In contrast to osteoid osteoma, osteoblastoma can grow into the spinal canal and may cause dural sac compression. Treatment of osteoblastomas consists of intralesional excision or marginal resection according to histopatological grade. Postoperative radiotherapy may be feasible in terms of local control in some cases[20].
Hemangioma is the most frequent benign tumor involving the spinal column. Typically hemangiomas involve vertebral body and they are usually asymptomatic lesions. According to autopsy findings, hemangiomas are seen in 10% of the general population[3]. Even though hemangiomas are asymptomatic, they may cause pathological fractures. Also, hemangiomas can cause symptoms in the 3rd trimester of pregnancy.
Aneurysmal bone cysts (ABC) are commonly seen in the posterior elements of the spinal column in patients under the age of 20. ABC have a tendency to involve more than one segment. ABC are continuously growing and expanding active or aggresive (stage 2-3) lesions. Treatment of ABC consists of embolization or wide resection after embolization. ABC have an overall recurrence rate of 25%[21].
Giant cell bone tumor (GCT) is commonly seen in the sacrum more than other parts of the spinal column. It is difficult to obtain clean surgical margins in the surgical treatment of GCTs because of their localization within the vertebral body. Surgical margins should aim wide resection, because piece-meal removal is associated with a recurrence rate of 50% in surgical treatment of GCT. Postoperative radiotherapy for local control is controversial because of high risk of sarcomatous transformation. This transformation is generally to secondary osteosarcoma. Even though GCT is a benign tumor, it is capable of lung metastasis.
Eosinophilic granuloma is a benign tumor which is more often seen in children and adolescents. Eosinophilic granuloma generally causes uniform, rapid flattening of the vertebral body. Radiological apperance of this type of vertebral body involvement is called vertebra plana[3]. Always it heals spontaneously. Classical treatment is observation and bracing in some cases to prevent development of kyphosis.
Primary musculoskeletal system sarcomas in spine
Three types of major primary musculoskeletal system sarcomas that are mostly seen in the spinal column are osteosarcoma, Ewing’s sarcoma and chondrosarcoma. These tumors can be seen in any part of the entire spinal column. Osteosarcoma and Ewing’ sarcoma are more often seen in children and adolescents but chondrosarcomas are more often seen in adults and older individuals[22].
Ewing’s sarcoma is more frequent in patients between the age of 5 and 20. Because of the inflammatory characteristics of Ewing’s sarcoma, this tumor may be misdiagnosed as infection and diagnosis can be delayed[1]. Swelling, local tenderness, fever and increase in sedimentation rate are the significant characteristics of patients with Ewing’s sarcoma. Spinal column involvement is seen in only 5% of patients with Ewing’s sarcoma. In patients with axial skeleton involvement, Ewing’s sarcoma is most commonly seen in the pelvis. In contrast to long bones in which periosteal reaction and permeative destruction are predominant, lytic lesions associated with soft tissue masses in the vertebral body is the main radiological finding of spine involvement of Ewing’s sarcoma. Preservation of contiguous discs help to distinguish Ewing’s sarcoma from spondylodiscitis. In pediatric spinal infections, disease generally starts from disc space and extends to vertebral end plates, but in Ewing’s sarcoma involvement starts from the core trabecular bone of the vertebral body, and endplate involvement may be seen in late phase of the tumor invasion[7]. Because of high cellularity of the tumor tissue, Ewing’s sarcomas located in extremities and the spine respond well to chemotherapy. Wide resection with clear surgical margins is possible after neo-adjuvant chemotherapy in Ewing’s sarcoma. Postoperative radiotherapy should be given in cases with contaminated surgical margins[5].
Even though osteosarcoma is the most commonly seen primary malignant tumor of bone, spinal involvement is rare. Approximately 2% of all osteosarcomas originate from the spine. Classic osteosarcoma is most commonly seen in the second decade of life. Occasionally, osteosarcoma may have its second peak incidence in the 6th decade of life as secondary osteosarcomas which arise from sarcomatous transformation of presarcomatous lesions such as Paget’s disease of bone and fibrous dysplasia[3]. Paget’s osteosarcomas more commonly occur in the spine and pelvis. Treatment of osteosarcomas involving the spinal column is similar to that for extermity osteosarcomas. Treatment of osteosarcoma has evolved in the last 40 years. Before 1970s, 5-year-survival rate was 10% for osteosarcomas, with 70%-80% of the patients surviving with no evidence of disease today. Current treatment of osteosarcoma consists of 2 episodes of neoadjuvant chemotherapy followed by wide or radical resection and at least 4 more chemotherapy episodes. Tumor response to chemotherapy is important for prognosis[3,5].
Chondrosarcomas are more common than the other primary sarcomas in the spine. As a result of highly avascular characteristics of cartilage tissue, chondrosarcoma is unresponsive to chemotherapy and radiotherapy so that main determinative for prognosis is surgical treatment with wide or radical surgical margins[3]. In surgical treatment main target should address radical resection.
Chordoma does not originate from the musculoskeletal system, and this tumor arises from remnants of the notochordal cells. Even though it is not a primary skeletal tumor, chordoma involves the spinal column and affects mainly the sacrum and lower lumbar vertebrae with its destructive behaviour. Chordoma is one of the most common tumors of the sacrum. While 60% of chordomas arise in the sacrum, 25% are seen at the skull base and the remaining 15% seen in the rest of the axial skeleton[23]. Chordomas are generally seen in the midline and caudal half of the sacrum (S3 and more caudal levels). Constipation, coccygodynia, hemorrhoids and urinary incontinance are the most common symptoms. Half of the sacral chordomas are palpable in digital rectal examination. As an original soft tissue tumor, chordoma is best evaluated using MRI. Surgical margins have great importance in prognosis of chordoma, therefore especially in the treatment of sacral chordoma wide resection should be aimed.
CONCLUSION
Spinal column represents the major portion of the axial skeleton that supports vital organs. Metastatic tumors are the most frequent tumors that involve the spine. In terms of frequency, benign bone tumors follow the metastatic tumors and primary bone sarcomas are the least frequent tumors that involve the spine. Sometimes it is difficult to distinguish primary tumors from metastatic tumors in the spine. Metastatic tumors with unknown origin are also common in the spine. Knowledge about the primary lesion has critical importance in treatment protocol for metastatic tumors of the spine. Therefore, in primary unknown metastases, biopsy is an important step which affects the treatment modalities. It should be kept in mind that metastatic lesions that involve the spinal are a part of systemic malignancies. Surgical staging is important for determining treatment protocol. Treatment of metastatic tumors should aim pain relief with preservation of mechanical and neurological functions of the spine. In primary tumors treatment strategy should address removal of local disease while preserving the mechanical and neurological functions of the spine. As in all oncological surgery procedures, all diagnostic and interventional procedures in primary or metastatic tumors of the spine, as well as the general management of the patient should be performed in a multidisciplinary approach.
Footnotes
Conflict-of-interest statement: The authors declare that they have no conflict of interest related to the work.
Open-Access: This article is an open-access article which was selected by an in-house editor and fully peer-reviewed by external reviewers. It is distributed in accordance with the Creative Commons Attribution Non Commercial (CC BY-NC 4.0) license, which permits others to distribute, remix, adapt, build upon this work non-commercially, and license their derivative works on different terms, provided the original work is properly cited and the use is non-commercial. See: http://creativecommons.org/licenses/by-nc/4.0/
Peer-review started: May 18, 2015
First decision: August 19, 2015
Article in press: November 17, 2015
P- Reviewer: Anand A, Robertson GA S- Editor: Qiu S L- Editor: Wang TQ E- Editor: Liu SQ
References
- 1.Lewandrowski KU, Anderson ME, McLain RF. Tumors of the Spine. In: Herkowitz HN, Garfin SR, Eismont FJ, Bell GR, Balderston RA, et al., editors. Philadelphia: Elsevier Saunders; 2011. pp. 1480–1512. [Google Scholar]
- 2.Choi D, Crockard A, Bunger C, Harms J, Kawahara N, Mazel C, Melcher R, Tomita K. Review of metastatic spine tumour classification and indications for surgery: the consensus statement of the Global Spine Tumour Study Group. Eur Spine J. 2010;19:215–222. doi: 10.1007/s00586-009-1252-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Aboulaflia AJ, Levine AM. Musculoskeletal and Metastatic Tumors. In: Fardon DF, Garfin SR, et al., editors. OKU: Spine 2, Rosemont. American Academy of Orthopaedic Surgeons; 2002. pp. 411–431. [Google Scholar]
- 4.Deol GS, Haydol R, Phillips FM. Tumors of the Spine. In: Vaccaro AR. OKU 8, Rosemont. American Academy of Orthopaedic Surgeons; 2005. pp. 587–599. [Google Scholar]
- 5.Boos N, Fuchs B. Primary Tumors of the Spine. In: Boos N, Aebi M, et al., editors. Spinal disorders: Fundamentals of Diagnosis and Treatment. Berlin: Springer-Verlag; 2008. pp. 951–976. [Google Scholar]
- 6.Boriani S, Bandiera S, Casadei R, Boriani L, Donthineni R, Gasbarrini A, Pignotti E, Biagini R, Schwab JH. Giant cell tumor of the mobile spine: a review of 49 cases. Spine (Phila Pa 1976) 2012;37:E37–E45. doi: 10.1097/BRS.0b013e3182233ccd. [DOI] [PubMed] [Google Scholar]
- 7.Davies AM, Cassar-Pullicino VN. Principles of Detection and Diagnosis. In: Davies AM, Sundaram M, James SLJ, et al., editors. Imaging of Bone tumors and Tumor-like Lesions. Berlin: Springer-Verlag; 2009. pp. 111–135. [Google Scholar]
- 8.Aebi M. Spinal Metastasis in the Elderly. In: Aebi M, Gunzburg R, Szpalski M, et al., editors. Aging Spine. Berlin: Springer-Verlag; 2005. pp. 120–131. [Google Scholar]
- 9.Copuroglu C, Yalniz E. Spinal oncologic reconstruction. World Spinal Column J. 2010;1:176–183. [Google Scholar]
- 10.Campanacci M. Bone and Soft Tissue Tumors. 2nd ed. Padova: Piccin Nuova Libraria; 1999. pp. 46–52. [Google Scholar]
- 11.Campanacci M. Bone and Soft Tissue Tumors. 2nd ed. Padova: Piccin Nuova Libraria; 1999. pp. 54–56. [Google Scholar]
- 12.Enneking WF, Spanier SS, Goodman MA. A system for the surgical staging of musculoskeletal sarcoma. Clin Orthop Relat Res. 1980;(153):106–120. [PubMed] [Google Scholar]
- 13.Boriani S, Weinstein JN, Biagini R. Primary bone tumors of the spine. Terminology and surgical staging. Spine (Phila Pa 1976) 1997;22:1036–1044. doi: 10.1097/00007632-199705010-00020. [DOI] [PubMed] [Google Scholar]
- 14.Tokuhashi Y, Matsuzaki H, Oda H, Oshima M, Ryu J. A revised scoring system for preoperative evaluation of metastatic spine tumor prognosis. Spine (Phila Pa 1976) 2005;30:2186–2191. doi: 10.1097/01.brs.0000180401.06919.a5. [DOI] [PubMed] [Google Scholar]
- 15.Tomita K, Kawahara N, Kobayashi T, Yoshida A, Murakami H, Akamaru T. Surgical strategy for spinal metastases. Spine (Phila Pa 1976) 2001;26:298–306. doi: 10.1097/00007632-200102010-00016. [DOI] [PubMed] [Google Scholar]
- 16.Tomita K, Kawahara N, Baba H, Tsuchiya H, Fujita T, Toribatake Y. Total en bloc spondylectomy. A new surgical technique for primary malignant vertebral tumors. Spine (Phila Pa 1976) 1997;22:324–333. doi: 10.1097/00007632-199702010-00018. [DOI] [PubMed] [Google Scholar]
- 17.Campanacci M. Bone and Soft Tissue Tumors. 2nd ed. Padova: Piccin Nuova Libraria; 1999. pp. 58–63. [Google Scholar]
- 18.Mavrogenis AF, Papagelopoulos PJ, Soucacos PN. Skeletal osteochondromas revisited. Orthopedics. 2008:31. [PubMed] [Google Scholar]
- 19.Crist BD, Lenke LG, Lewis S. Osteoid osteoma of the lumbar spine. A case report highlighting a novel reconstruction technique. J Bone Joint Surg Am. 2005;87:414–418. doi: 10.2106/JBJS.C.01499. [DOI] [PubMed] [Google Scholar]
- 20.Atesok KI, Alman BA, Schemitsch EH, Peyser A, Mankin H. Osteoid osteoma and osteoblastoma. J Am Acad Orthop Surg. 2011;19:678–689. doi: 10.5435/00124635-201111000-00004. [DOI] [PubMed] [Google Scholar]
- 21.Ofluoglu O, Boriani S, Gasbarrini A, De Iure F, Donthineni R. Diagnosis and planning in the management of musculoskeletal tumors: surgical perspective. Semin Intervent Radiol. 2010;27:185–190. doi: 10.1055/s-0030-1253516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Yalniz E, Ozcan M, Copuroglu C, Memisoglu S, Yalçin O. Osteosarcoma of the lumbar vertebra: case report and a review of the literature: rare localization with long survival. Arch Orthop Trauma Surg. 2009;129:1701–1705. doi: 10.1007/s00402-009-0896-7. [DOI] [PubMed] [Google Scholar]
- 23.Boriani S, Bandiera S, Biagini R, Bacchini P, Boriani L, Cappuccio M, Chevalley F, Gasbarrini A, Picci P, Weinstein JN. Chordoma of the mobile spine: fifty years of experience. Spine (Phila Pa 1976) 2006;31:493–503. doi: 10.1097/01.brs.0000200038.30869.27. [DOI] [PubMed] [Google Scholar]


