Abstract
Synovial cells from patients with rheumatoid arthritis (RA) when grown in vitro in media supplemented with 20% fetal calf serum failed to show any difference in growth rate, life span, uptake of tritiated thymidine or cellular and nuclear characteristics when compared with synovial cells from patients with osteoarthritis or other joint diseases grown similarly in 20% serum enriched medium. There was also no evidence that lymphocytes and/or sera from RA patients were more cytotoxic to autologous synovial cells than sera and/or lymphocytes from OA patients. It is unlikely that antisynovial antibodies or lymphocytes from RA patients act as triggers for synovial damage.
Full text
PDF

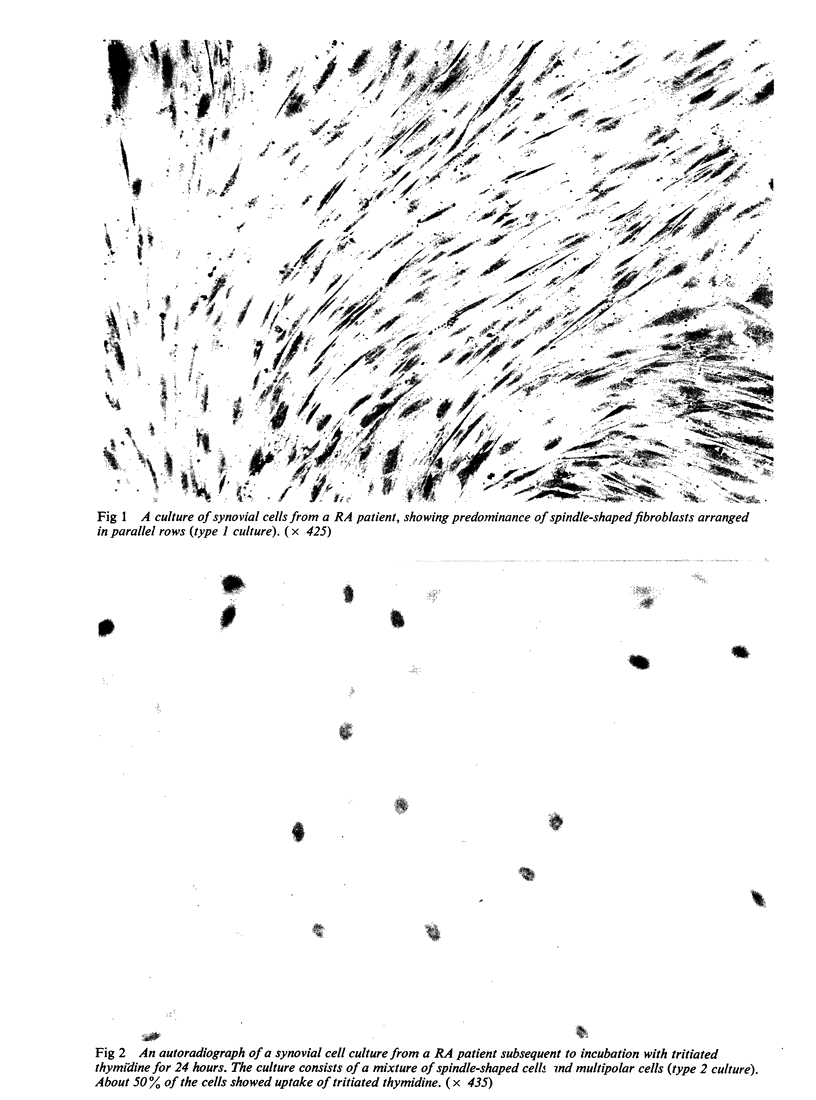


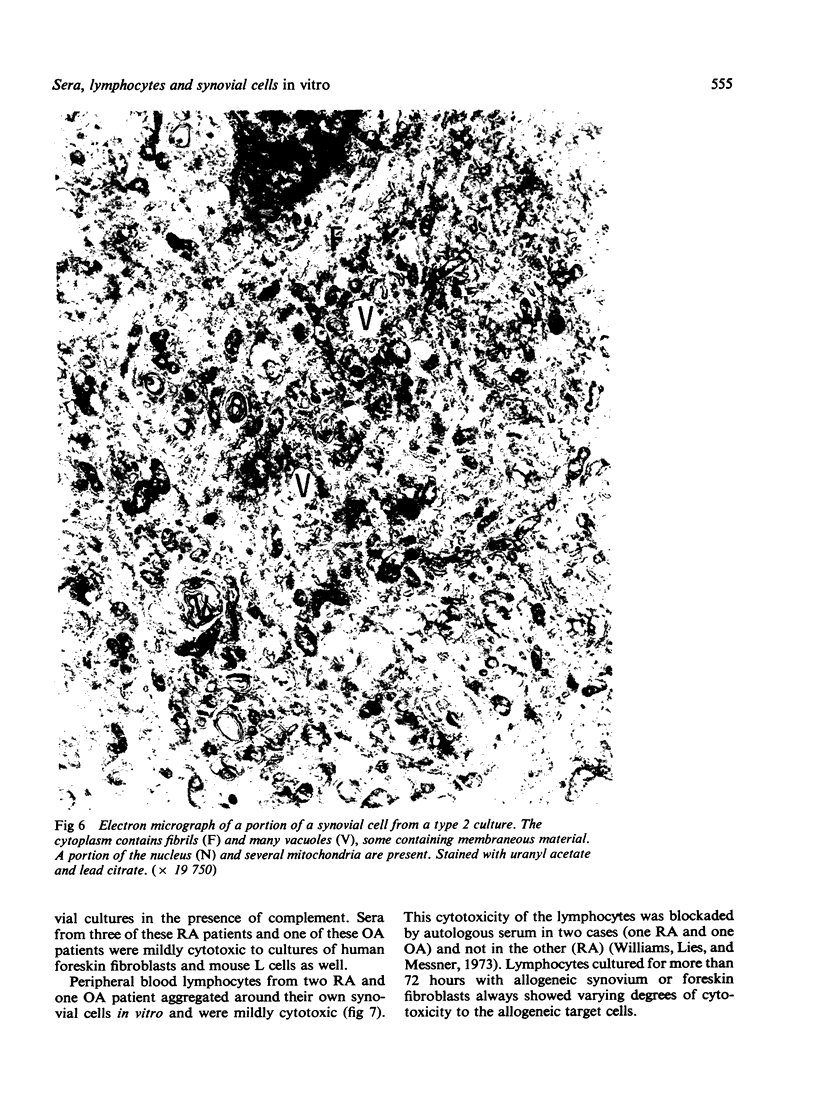



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Astorga G. P., Williams R. C., Jr Altered reactivity in mixed lymphocyte culture of lymphocytes from patients with rheumatoid arthritis. Arthritis Rheum. 1969 Dec;12(6):547–554. doi: 10.1002/art.1780120602. [DOI] [PubMed] [Google Scholar]
- BARLAND P., NOVIKOFF A. B., HAMERMAN D. Electron microscopy of the human synovial membrane. J Cell Biol. 1962 Aug;14:207–220. doi: 10.1083/jcb.14.2.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BARTFELD H. RHEUMATOID ARTHRITIC AND NON-RHEUMATOID SYNOVIUM IN CELL CULTURE. MORPHOLOGICAL OBSERVATIONS, ACRIDINE ORANGE, AND FLUORESCENT FRACTION II STUDIES. Ann Rheum Dis. 1965 Jan;24:31–39. doi: 10.1136/ard.24.1.31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bacon P. A., Cracchiolo A., Bluestone R., Goldberg L. S. Cell-mediated immunity to synovial antigens in rheumatoid arthritis. Lancet. 1973 Sep 29;2(7831):699–702. doi: 10.1016/s0140-6736(73)92537-3. [DOI] [PubMed] [Google Scholar]
- Brinkley B. R., Murphy P., Richardson L. C. Procedure for embedding in situ selected cells cultured in vitro. J Cell Biol. 1967 Oct;35(1):279–283. doi: 10.1083/jcb.35.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- CASTOR C. W., PRINCE R. K., DORSTEWITZ E. L. "Epithelial transformation" of human synovial connective tissue cells: cytologic and biochemical consequences. Proc Soc Exp Biol Med. 1961 Dec;108:574–578. doi: 10.3181/00379727-108-27001. [DOI] [PubMed] [Google Scholar]
- Castor C. W., Dorstewitz E. L. Abnormalities of connective tissue cells cultured from patients with rheumatoid arthritis. I. Relative unresponsiveness of rheumatoid synovial cells to hydrocortisone. J Lab Clin Med. 1966 Aug;68(2):300–313. [PubMed] [Google Scholar]
- Crocker J. F., Ghose T., Rozee K., Woodbury J., Stevenson B. Arthritis, deformities, and runting in C5-deficient mice injected with human rheumatoid arthritis synovium. J Clin Pathol. 1974 Feb;27(2):122–124. doi: 10.1136/jcp.27.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas W. H., Elser J. E. A method for in situ embedding of cultured cells grown on plastic surfaces. In Vitro. 1972 Jul-Aug;8(1):26–29. doi: 10.1007/BF02617940. [DOI] [PubMed] [Google Scholar]
- Fraser J. R., Clarris B. J. On the reactions of human synovial cells exposed to homologous leucocytes in vitro. Clin Exp Immunol. 1970 Feb;6(2):211–225. [PMC free article] [PubMed] [Google Scholar]
- Ghose T., Cerini M. Persistence of thyroid microsomal and thyroglobulin antigens in primary cultures of human thyroid epithelium. Clin Exp Immunol. 1969 Nov;5(5):515–524. [PMC free article] [PubMed] [Google Scholar]
- Ghose T., Landrigan P., Killeen R., Dill J. Immunopathological studies in patients with farmer's lung. Clin Allergy. 1974 Jun;4(2):119–129. doi: 10.1111/j.1365-2222.1974.tb01369.x. [DOI] [PubMed] [Google Scholar]
- Ghose T., Nairn R. C., Cerini M. Persistence of gastrointestinal-specific antigens in primary culture of human colon epithelium. Exp Cell Res. 1968 Mar;49(3):513–519. doi: 10.1016/0014-4827(68)90201-2. [DOI] [PubMed] [Google Scholar]
- Ghose T., Norvell S. T., Guclu A., Cameron D., Bodurtha A., MacDonald A. S. Immunochemotherapy of cancer with chlorambucil-carrying antibody. Br Med J. 1972 Aug 26;3(5825):495–499. doi: 10.1136/bmj.3.5825.495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghose T., Woodbury J. F., Ahmad S., Stevenson B. Immunopathological changes in rheumatoid arthritis and other joint diseases. J Clin Pathol. 1975 Feb;28(2):109–117. doi: 10.1136/jcp.28.2.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths M. M., Williams R. C., Jr In vitro peripheral blood and synovial fluid lymphocyte interactions. Arthritis Rheum. 1974 Mar-Apr;17(2):111–120. doi: 10.1002/art.1780170203. [DOI] [PubMed] [Google Scholar]
- HEDBERG H., KAELLEN B. STUDIES ON MONONUCLEAR CELLS OBTAINED FROM SYNOVIAL FLUID OF PATIENTS WITH DIFFERENT TYPES OF ARTHRITIS. CYTOTOXIC EFFECT ON TISSUE-CULTURED HUMAN FIBROBLASTS. Acta Pathol Microbiol Scand. 1964;62:177–188. doi: 10.1111/apm.1964.62.2.177. [DOI] [PubMed] [Google Scholar]
- Lundgren G., Zukoski C. F., Möller G. Differential effects of human granulocytes and lymphocytes on human fibroblasts in vitro. Clin Exp Immunol. 1968 Oct;3(8):817–836. [PMC free article] [PubMed] [Google Scholar]
- MacLennan I. C., Loewi G. The effect of peripheral lymphocytes from patients with inflammatory joint disease on human target cells in vitro. Clin Exp Immunol. 1968 Jun;3(5):385–391. [PMC free article] [PubMed] [Google Scholar]
- Maclennan I. C., Loewi G. The cytotoxic activity of mononuclear cells from joint fluid. Clin Exp Immunol. 1970 May;6(5):713–720. [PMC free article] [PubMed] [Google Scholar]
- McGiven A. R., Ghose T. Antinuclear factor in NZB-NZW mice: incidence and in vitro effects. Clin Exp Immunol. 1968 Sep;3(7):657–663. [PMC free article] [PubMed] [Google Scholar]
- Palmer D. G. Dispersed cell cultures of rheumatoid synovial membrane. Acta Rheumatol Scand. 1970;16(4):261–270. [PubMed] [Google Scholar]
- ROSS J. D. Cytotoxins and cytotoxic antibodies. Ann N Y Acad Sci. 1957 Dec 16;69(4):795–800. doi: 10.1111/j.1749-6632.1957.tb49716.x. [DOI] [PubMed] [Google Scholar]
- Rothenberger W., Thiele H. G. In vitro-Studie zur Pathogenese der primär-chronischen Polyarthritis mittels des Migrations-Inhibitionstests. Klin Wochenschr. 1970 Nov 1;48(21):1308–1311. doi: 10.1007/BF01485523. [DOI] [PubMed] [Google Scholar]
- Smith C., Hamerman D. Significance of persistent differences between normal and rheumatoid synovial membrane cells in culture. Arthritis Rheum. 1969 Dec;12(6):639–645. doi: 10.1002/art.1780120612. [DOI] [PubMed] [Google Scholar]
- Warren S. L., Marmor L., Liebes D. M., Rosenblatt H. M. An active agent from rheumatoid arthritis synovial tissue. Transmission by injection or ingestion in mice and rats. Arch Intern Med. 1972 Dec;130(6):899–903. [PubMed] [Google Scholar]
- Waxman J., Lockshin M. D., Schnapp J. J., Doneson I. N. Cellular immunity in rheumatic diseases. I. Rheumatoid arthritis. Arthritis Rheum. 1973 Jul-Aug;16(4):499–506. doi: 10.1002/art.1780160410. [DOI] [PubMed] [Google Scholar]
- Williams R. C., Jr, Lies R. B., Messner R. P. Inhibition of mixed leukocyte culture responses by serum and gamma-globulin fractions from certain patients with connective tissue disorders. Arthritis Rheum. 1973 Sep-Oct;16(5):597–605. doi: 10.1002/art.1780160504. [DOI] [PubMed] [Google Scholar]
- Wynne-Roberts C. R., Castor C. W. Ultrastructural comparison of rheumatoid and nonrheumatoid synovial fibroblasts grown in tissue culture. Arthritis Rheum. 1972 Jan-Feb;15(1):65–83. doi: 10.1002/art.1780150110. [DOI] [PubMed] [Google Scholar]
- Zvaifler N. J. The immunopathology of joint inflammation in rheumatoid arthritis. Adv Immunol. 1973;16(0):265–336. doi: 10.1016/s0065-2776(08)60299-0. [DOI] [PubMed] [Google Scholar]