Abstract
Mammalian cells are rapidly becoming the system of choice for the production of recombinant glycoproteins for structural biology applications. Their use has enabled the structural investigation of a whole new set of targets including large, multi-domain and highly glycosylated eukaryotic cell surface receptors and their supra-molecular assemblies. We summarize the technical advances that have been made in mammalian expression technology and highlight some of the structural insights that have been obtained using these methods. Looking forward, it is clear that mammalian cell expression will provide exciting and unique opportunities for an integrative approach to the structural study of proteins, especially of human origin and medically relevant, by bridging the gap between the purified state and the cellular context.
Introduction
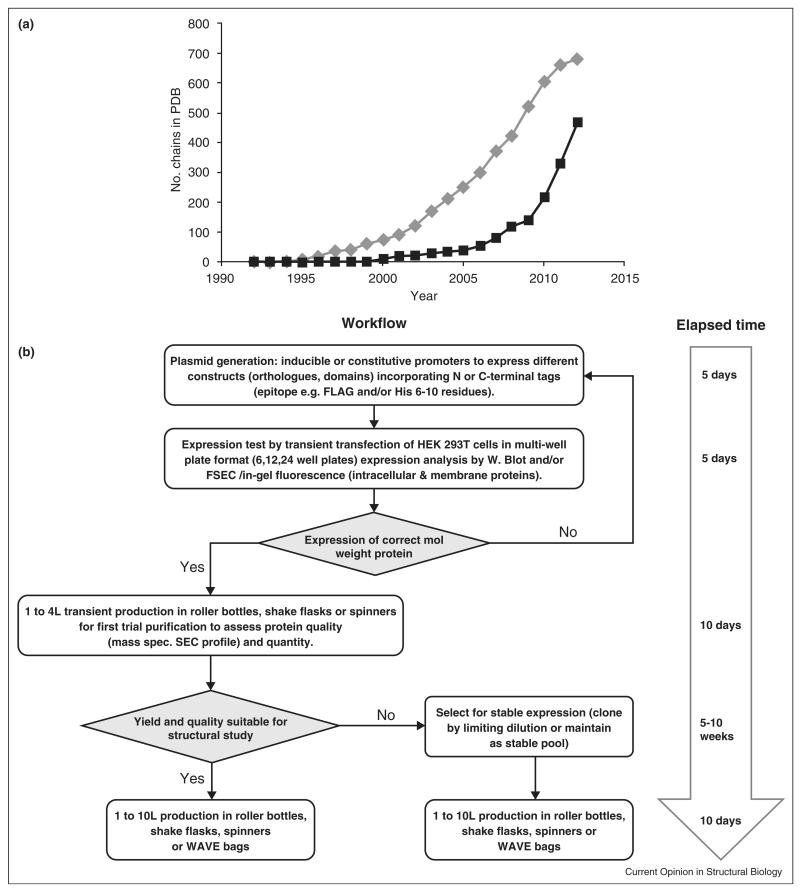
Mammalian cells in culture have long been used in the manufacture of biopharmaceuticals, in particular monoclonal antibodies engineered for human therapy, for example, Herceptin® and Avastin® [1]. Processes for protein production in mammalian cells for both pre-clinical and clinical studies are well-established, using both transient and stable cell line expression systems. However, due to perceived difficulties including the need for specialized culture facilities and higher costs compared to microbial alternatives, only relatively recently have mammalian expression systems become routinely used in laboratory-scale production of recombinant proteins for structural biology applications. Although only ~3% of the unique structures in the Protein Data Bank currently come from mammalian cell expression [2], their number has increased by 40% in the last two years (Figure 1a). Considering the recent technological progress, this trend is likely to continue.
Figure 1.
Mammalian expression technology applied to structural biology. (a) Plot of the cumulative total number of chains deposited in the PDB whose expression system was identified as either HEK-293 (Human Embryonic Kidney) or CHO (Chinese hamster ovary) cells by year of deposition. Expression data were parsed from the set of PDB files available from ftp://ftp.wwpdb.org/pub/pdb/data/structures/divided/pdb as of November 2012. Chains were counted rather than PDB entries as expression information is recorded by chain in the PDB. Note: entries marked ‘obsolete’ could not be included, which might have excluded a small number of early 1990s structures. (b) Workflow for the production of recombinant proteins in HEK-293 cells for structural biology applications.
Mammalian expression is particularly beneficial for the production of human and other vertebrate proteins, especially large, multi-domain, cell surface and/or secreted constructs, which require a complex folding machinery and post-translational modifications. For example, over 50% of the proteins encoded by the human genome undergo glycosylation of either serine/threonine (O-linked) or asparagine (N-linked) residues [3]. Such modifications are often essential during the folding process, but typically hamper crystallographic work [4]. Therefore, an important early development was the derivation of mutant Chinese Hamster Ovary (CHO) cell lines deficient in glycan processing which produce samples with defined glycoforms and minimal micro-heterogeneity [5]. One of these CHO glycosylation mutants (CHO LecR 3.2.8.1), in combination with a highly efficient selection system based on glutamine synthetase [6,7], was used to produce the extracellular region of rat CD2 [8] and opened the way for crystallographic analyses of glycoproteins in the early 1990s (Figure 1a). Other developments, such as selenomethionine (SeMet) labeling of proteins expressed in CHO cells for crystallographic phasing by multiple anomalous dispersion methods were soon reported [9,10]. Introduction of large-scale transient expression of Human Embryonic Kidney 293 cells (HEK-293) [11–13] in early/mid 2000s has been associated with an exponential increase in the number of structures of mammalian-expressed proteins (Figure 1a). In this article we review the current status of the use of mammalian cells for sample preparation in structural biology. Examples of how the application of these methods has contributed to obtaining new structural information, in particular novel principles of cell surface receptor organization and signaling, will be discussed.
Enabling technology
A contemporary workflow for mammalian cell production of glycoproteins is shown in Figure 1b. HEK-293 cells have become the host of choice due to their ease of culture in either attached or suspension formats, high transfection efficiency and capacity to express recombinant proteins in large amounts. Large-scale transient transfection of HEK-293 cells has become economically feasible since the discovery that the inexpensive linear cationic polymer, polyethylenimine, is an excellent DNA condensing agent [11,14]. Yields of purified proteins range between 1 and 80 mg/L of culture [13,15,16•] (unpublished), though for a highly optimized process production of 1 g/L of a recombinant antibody has been reported [17]. In our experience yields of secreted proteins from HEK-293 cells exceed those for the same product expressed in baculovirus infected insect cells [13]. Cells are typically grown attached, in either roller bottles [13] or cell factories [18], or in suspension, in either Erlenmeyer or spinner flasks [19•], but can be scaled up to multi-litre volumes in bioreactors such as the Wavebag® system [12]. Automation of the culture processes for both attached and suspension-adapted HEK-293 cells has been successfully implemented in medium/large-sized academic laboratories, thereby reducing manual handling and greatly increasing batch reproducibility and cost-effectiveness [16•,20].
Three main variants of HEK-293 cells are currently in use for large-scale protein production: first, 293T which expresses the SV40 large-T antigen [12,13,21]; second, 293E which expresses the Epstein-Barr virus nuclear antigen 1 [11,21] and third, 293S-GnTI−, deficient in N-acetylglucosaminyl transferase I activity and therefore lacking complex N-glycans [22]. For sustained production of a particular protein, or to increase yields compared to transient expression, stable cell lines may be constructed by co-selection or further streamlined by site-specific integration of the target gene into the host cell genome, at transcriptionally active sites, removing the need to clone out high producers [23•]. For some targets, notably integral membrane proteins, it is important to control the timing of gene expression, typically by using an inducible system [24•]; the most widely used one exploits the prokaryotic tetracycline repressor/operator to control expression [25].
Management of N-linked glycosylation
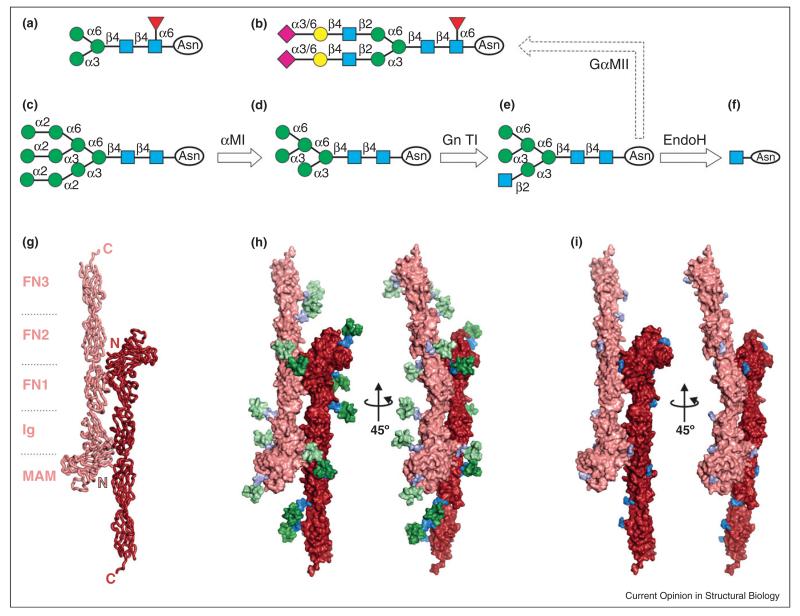
The structure of mammalian N-linked glycan chains, unlike insect equivalents, is highly variable (Figure 2a and b) and management of this heterogeneity is usually essential for structural biology studies [4]. Treatment of HEK-293T cells with small molecule inhibitors such as kifunensine, which targets α-mannosidase I [26]; swainsonine, which blocks α-mannosidase II activity [27]; or the use of cells deficient in N-acetylglucosaminyl tranferase I [22] results in relatively simple and chemically uniform glycans (Figure 2c–e), cleavable using endoglycosidase (endo) H or endo F1 to leave only one GlcNAc residue attached to the N-glycosylation site (Figure 2f) [4]. Ultimately, the strategy chosen for a particular recombinant protein depends on the glycan structure that needs to be preserved. The large number of glycosylation sites typically present on cell surface proteins brings a considerable extra volume and flexibility (Figure 2g–i), therefore enzymatic treatment is often a pre-requisite for crystallization [28]. Recent examples where de-glycosylation was beneficial include the EphA2-ephrin [29••], netrinG-NGL [30••] and semaphorin-plexin/neuropilin signaling complexes [31••,32••], the Wnt inhibitory factor WIF-1 [33•], heteromeric kainate receptor amino-terminal domains [34••] and the HIV GP120 [35•]. Complete removal of N-linked glycans, achievable via peptide-N-glycosidase F (PNGAse F) treatment, should be avoided as it introduces an Asn to Asp mutation and the Asn-linked GlcNAc may establish contacts with protein surface residues and play an important structural role. It is also important to note that, in some circumstances, removal of glycans is not required, or indeed not desirable, for crystallization, as exemplified by the structures of FcER1α [36•], human IgG1-Fc [37•], cytokine receptor–ligand complexes [38••,39••] and neurexin 1α [40•].
Figure 2.
The structure and management of canonical N-linked glycans. (a) Paucimannose glycans typically produced by insect cell lines (e.g. Sf9), difficult to remove enzymatically [4]. (b) Mammalian cell glycans, in contrast, have a larger size and chemical heterogeneity. (c)–(e) Glycosylation inhibitors or mutant cell lines are used to trap specific structures in the biosynthetic pathway. These include Man9GlcNAc2, upon kifunensine treatment (c), Man5GlcNAc2, in GnTI− cells (d) and hybrid mannose, following treatment with swainsonine (e). (f) All these intermendiates (c)–(e) can be trimmed down to a single GlcNAc by Endo H/F1 treatment. (g) Crystal structure of the human RPTPμ trans-adhesive interaction [28]. (h) Each monomer carries 12 N-glycosylation sites (around one per 100 residues, a typical amount for cell surface receptors). Man9GlcNAc2 glycans were modeled at nine sites per monomer, to illustrate their considerable volume and coverage of the protein surface. (i) Successful crystallization could only be achieved upon EndoH glycan trimming [28]. Colour/shape coding of glycan units: GlcNAc, blue square; Man, green circle; Gal, yellow circle; NeuNAc, pink diamond; Fuc, red triangle.
Furthermore, while N-linked glycans may pose a problem for crystallization, their ability to mask relatively extended protein surface patches has been used to restrict protein conformation (for example in the integrin head-piece [41]) or to probe protein–protein interactions. Engineering of ‘glycan wedges’ at structure-guided locations is increasingly employed to functionally validate and manipulate the architecture of oligomeric proteins (for example the interaction interface between GABAB receptor subunits [42]), receptor–ligand assemblies (e.g. EphA2-ephrin [29••], netrinG-NGL [30••]) or predict interaction sites in protein complexes (e.g. WIF1-Wnt3a) [33•].
Specific amino acid labeling in mammalian cells
Biosynthetic labeling of glycoproteins expressed in mammalian cells with SeMet for crystallographic phasing has been successfully adapted to the HEK-293 transient expression format [13,29••,43–47]. Furthermore, increased availability of isotope-labeled media has also opened-up mammalian expression to NMR studies (reviewed in [48•]). Relatively high levels of incorporation (approximately 50% sequence coverage) have been achieved using a medium containing an 15N amino acid mix (GKLQSTVW) [49]. More recently, over 90% isotope enrichment has been reported [50]. Such developments, combined with the rapid refinement of mammalian in-cell NMR approaches (reviewed in [51]), hold great promise for the structural characterization of suitable mammalian proteins within their cellular milieu. In this context, an atomic resolution NMR description of the complete post-translational maturation process of human superoxide dismutase 1 (SOD1), endogeneously expressed in HEK-293T cells, has been achieved [52••].
Recent efforts aimed at ‘expanding’ the genetic code led to successful incorporation of non-natural amino acids at specific sites in mammalian cell proteins [53,54]. This involves co-transfection with vectors encoding paired bacterial aminoacyl-tRNA synthetases, engineered to recognize specific non-natural amino acids, and an amber-supressor tRNA that recognizes UAG codons introduced into the target sequence. The technology has already opened up a multitude of exciting applications, including novel possibilities for experimental phasing or isotope-labeling [53,54], probing protein–protein interactions in situ using residues with light-induced cross-linking activity [53,55] and site-specific protein labeling with fluorophores for live cell imaging experiments [56•–58•].
Novel structural insights into cell signaling mechanisms
The last three years have witnessed a sharp increase in structural studies that have made use of mammalian cell expression technology. In this short review we cannot do justice to all these investigations, therefore we will highlight examples in which a combination of structural, or structural and cellular, techniques has been employed to yield new concepts in cellular signaling.
Receptor–ligand complexes: specificity determinants and viral interference
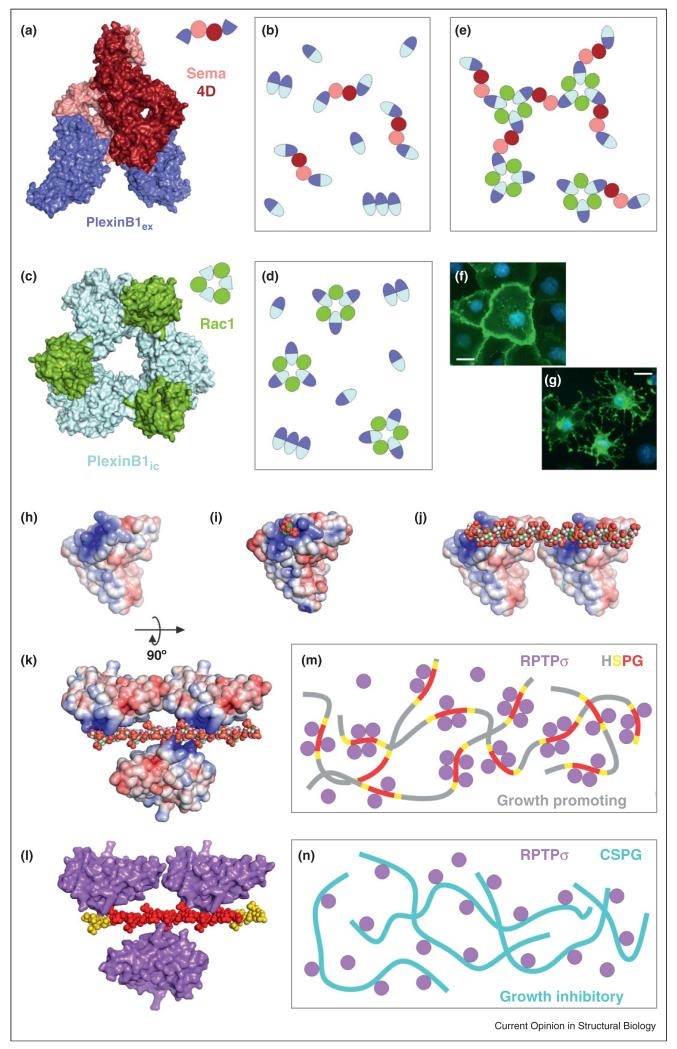
A combined approach involving mammalian cell expression and live cell fluorescence microscopy has recently shed light on two key aspects of brain wiring, one of the most puzzling and fascinating processes in developmental biology. Firstly, the comprehensive crystallographic investigation of all possible netrinG-NGL trans-synaptic molecular pairs [30••] explained how migrating axons connect to specific subdomains on the dendrites of target neurons. This is a remarkable example of molecular patterning on an individual cell surface, very likely of broad significance in biology. Secondly, three groups have independently described crystal structures of semaphorin-plexin complexes [31••,59••,60••], providing unprecedented insights into this prototypical neuronal guidance system. On the extracellular side, binding of dimeric Sema ligands triggers Plexin receptor dimerization (Figure 3a and b). This is facilitated by a co-receptor, neuropilin-1, in the case of Sema3-PlexinA pairs [32••]. However, a mature signaling assembly appears to be much larger because the intracellular region of Plexin-B1, in complex with a Rho GTPase (Rac1), was found to form a non-crystallographic 3:3 arrangement (Figure 3c and d) [61•]. This points towards an intriguing honeycomb-like cluster of receptor–ligand complexes that may facilitate the transduction of bi-directional (inside-out and outside-in) signals (Figure 3e). The functional impact of the interactions that stabilize this assembly has been validated in cell collapse assays (Figure 3f and g) using structure-guided mutant constructs [61•].
Figure 3.
Emerging concepts in cell surface signaling: examples of combined structure/function approaches that have revealed supramolecular receptor organization. Such assemblies are driven by either protein–protein interactions (e.g. the semaphoring/plexin system) or by non-protein ligands (e.g. type IIa RPTPs interactions with HSPGs and CSPGs). (a) Crystal structure of a Sema4D-PlexinB1 ectodomain complex [31••]. (b) Binding of dimeric Sema4D ligands (pink/red) to the PlexinB1 extracellular region (dark blue) triggers receptor dimerization. Note that non-ligand-dependent PlexinB1 ectodomain oligomerization has also been reported, but its extent or architecture are still unclear [31••]. (c) Crystal structure of the PlexinB1 intracellular region in complex with Rac1 [61•]. (d) This interaction leads to the formation of a 3:3 receptor–ligand assembly. (e) Schematic representation of the putative impact of simultaneous Sema4D and Rac1 binding to PlexinB1. The combined 2-fold and 3-fold interactions may lead to a hexagonal ‘honeycomb’ arrangement (shown in part here) that facilitates bi-directional signaling. (f)–(g) COS7 cell collapse assays were used to validate crystallographic interfaces using structure-guided mutagenesis. Scalebar: 40 μm. (h) Electrostatic potential representation (±5kT/e) of the glycosaminoglycan (GAG)-binding region of human RPTPσ [45••]. (i) A basic residue cluster, conserved in all family members, interacts with sulphated sugars (shown here is human LAR in complex with sucrose-octasulphate). (j)–(k) The polymeric nature of heparan sulphate (HS) triggers receptor clustering. (l)–(m) Importantly, the distribution of sulphate groups along the HS chains is not even: 12–14 sulphate-rich units (red), flanked by intermediate sulphation regions (yellow) are separated by long low-sulphation portions. This imposes an uneven distribution of receptors on the cell surface (~four receptors per cluster), which is essential to promote neuronal motility [45••]. (n) In contrast, the distribution of sulphate groups on chondroitin sulphate molecules prevents formation of RPTPσ clusters and inhibits cell motility [45••].
Remarkable progress has also been made in the study of signaling pathways that drive the development of the mammalian immune system, in particular those centered around the colony stimulating factor-1 receptor (CSF-1R or FMS), a class III receptor protein tyrosine kinase. A combination of structural methods, as well as mammalian expression techniques, has revealed surprisingly conserved principles of organization in the architecture of CSF-1R extracellular complexes with their structurally and functionally distinct cytokine ligands, CSF-1 and IL-34 [62••,63••].
Two of the pathways discussed above are also targeted during viral infections, and recent structural data provided important new insights into the molecular mechanisms of viral mimicry. BARF1, a secreted Epstein-Barr virus-encoded decoy receptor, acts as a molecular ‘trap’ for CSF-1 ligands that are stabilized in an inactive conformation upon attachment to its toroid-shaped hexamer [39••]. By contrast, Vaccinia (smallpox) virus and Alcelaphine herperviruses secrete semaphorin homologues (A39R and AHVsema) that bind to and activate the PlexinC1 receptor. Importantly, while the overall arrangement of a dimeric A39R in complex to PlexinC1 is highly similar to that observed in the Sema7A-PlexinC1 complex, the A39R has evolved a smaller yet more efficient receptor interface, leading to a 20-fold tighter interaction compared to the endogenous Sema7A ligand [59••].
Higher order organization of cell surface receptors
The rapid expansion of structural information has recently set the foundations for a fundamentally novel concept of receptor organization at the cell surface. Increasingly, instead of appearing ‘isolated’ or simply dimerized upon ligand binding, it is now clear that many such proteins have the potential to form supra-molecular clusters or arrays, driven or stabilized by cis (same cell) and trans (across the inter-cellular space) interactions. The initial observation of ‘molecular zippers’ in the crystal packing of C-cadherin [64], a homophilic cell adhesion molecule, gained further support from cryo-electron tomographic analysis of desmosomal cadherins in human skin samples [65]. Importantly, the C-cadherin arrangement has been confirmed by two additional crystal structures (for E-cadherin and N-cadherin ectodomains), and also visualized outside the constraints of crystalline packing by cryo-electron microscopy of cadherin-coated liposomes [66••]. The physiological relevance of these assemblies was demonstrated by mutational analysis of protein–protein interfaces in cellular assays [66••]. Furthermore, the wealth of experimental data that emerged from the extensive, multi-disciplinary, analysis of cadherin interactions has now been rationalized within a robust theoretical framework [67••].
While the concept of an array-like arrangement might have been expected at molecularly homogeneous cell contacts, a similar scenario is currently emerging from the study of heterophilic inter-cellular interactions. For example, a complex between neurexin-1β and its adhesive partner, neuroligin-1, has also revealed crystallographic packing consistent with a trans 2D-array [68•]. Importantly, this architecture was supported by correlative fluorescence-electron microscopy observations at points of contacts between cells expressing recombinant neurexin-1β and neuroligin-1, respectively, on their surface [68•]. The relevance of this molecular architecture, even over a short range, within the physiological environment of a neuronal synaptic cleft remains to be demonstrated. If genuine, it will likely have a key impact on synapse formation, function and remodelling.
The importance of supra-molecular interactions is better understood in the case of receptor enzymes. For example, the multi-domain, conformationally flexible, ectodomain of erythropoietin-producing hepatoma A2 (EphA2) kinase tends to cluster in linear arrays. Certain relative arrangements within such arrays are signaling-compatible and appear to be stabilized by interactions with trans ligands, the ephrins [29••,69••]. This architecture facilitates signal amplification and its discovery solved a long-standing puzzle in the field: while Eph signal triggering is strictly dependent on direct contact with a small area of pre-clustered ephrins, the regions of receptor activation vastly exceed these points of contact [70].
Receptor clustering may also be modulated by non-protein ligands, as recently demonstrated by crystallographic and functional analysis of type IIa receptor protein tyrosine phosphatases, such as RPTPσ (Figure 3h–n) [45••]. The linear glycosaminoglycan chains of extracellular or cell surface attached proteoglycans, a ubiquitous presence around eukaryotic cells, play fundamental roles in signaling regulation. In the case of RPTPσ, the ratio between heparan and chondroitin sulphate proteoglycans it encounters determines whether receptors cluster or not (Figure 3m and n), which in turn represents a key control of cellular motility as demonstrated in the case of neuronal extension [45••]. This mechanism is likely to be widespread in cell signaling systems, including the semaphorin-plexin discussed above, to which it adds an additional level of complexity [71].
Novel insights into antibody complexes
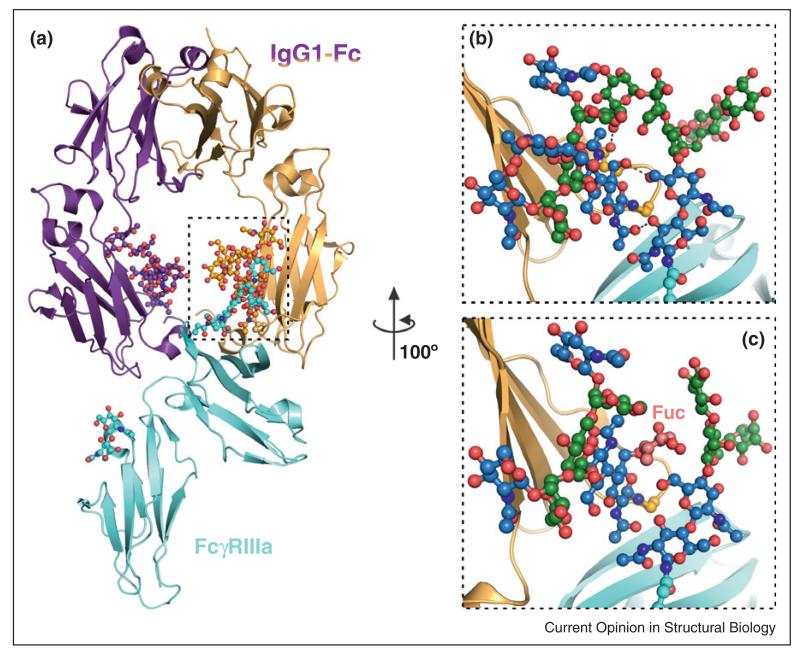
The interaction between IgG and Fc receptors is modulated by the structure of the IgG N-linked glycans. Antibodies lacking core fucosylation show a significant increase in FcRγIIIa binding, which results in improved receptor-mediated effector activity [72••]. This is important for the function of therapeutic antibodies, whose efficacy has been improved by glyco-engineering. Structural studies of the complex between glycosylated IgG-Fc and FcRγIIIa revealed a unique type of carbohydrate interface, involving the N-glycans of both proteins, which is only formed in the absence of core fucosylation (Figure 4) [72••]. The use of mammalian cells to produce defined glycoforms of both proteins was essential for this discovery.
Figure 4.
The impact of N-linked glycans in modulating protein complexes. (a) Crystal structure of the glycosylated complex between the IgG1-Fc region and its FcγRIIIa receptor, essential for antibody-mediated cellular cytotoxicity [72••]. (b) Antibodies lacking core fucosylation show a large increase in affinity for FcγRIIIa, due to additional glycan–glycan and glycan–protein interactions (putative hydrogen bonds are shown as dashed lines). This leads to an improved receptor-mediated effector function and forms the basis for a next generation of therapeutics, glycoengineered antibodies. (c) In the complex structure containing fucosylated IgG1-Fc, such contacts are very limited, explaining the decreased affinity for the FcγRIIIa receptor. Fuc: fucose attached to the Asn297-linked GlcNAc in IgG1-Fc. Colour coding of carbon atoms in the glycan units (sphere and stick representation) in panels (b) and (c) is as described in Figure 2.
More generally, mammalian cells are increasingly being used for the production of antibodies and Fab fragments for structural studies. Novel antigen-binding specificities identified by phage-display technology have been converted into recombinant IgGs from which Fab fragments have been derived by papain cleavage for structural analysis of antibody–antigen complexes [73,74]. Alternatively, recombinant Fab fragments have been produced directly in mammalian cells for co-crystallization studies [75,76,77••]. Mapping the epitopes recognized by broadly neutralizing antibodies using X-ray crystallography became a powerful guide for the development of ‘universal’ vaccines against viruses including HIV-1, influenza and hepatitis C [73,77••–80••]. Similarly, the crystallographic characterization of a human EphrinA2 receptor construct in complex with an agonistic antibody has implications for the development of novel anti-cancer therapies [81•].
Conclusion and future directions
Expression of proteins in mammalian cells for structural biology applications is set to increase over the next few years. Technical developments have lowered the barriers of cost and time, and increased the throughput of protein production. Strategies for incorporation of selenomethionine and amino acid isotopes, extending the genetic code with non-natural amino acids and controlling glycosylation heterogeneity have all been reliably established. The combined structural and cellular investigation of the same protein constructs, afforded by the use of mammalian cells, is likely to expand and reveal novel mechanistic insights into molecular pathways relevant to human health. The power of such an interdisciplinary approach will likely allow a direct observation of complex processes, such as signal transduction across cellular membranes, in genuine molecular detail: current efforts directed at understanding epidermal growth factor receptor signaling represent a spectacular example [82••–84••]. Further technological developments in super-resolution microscopy [85,86••], correlative fluorescence-electron microscopy [87,88••] and X-ray microscopy [89•,90•] will offer more exciting opportunities for a modern, integrative approach to the structural study of recombinant proteins across the resolution scale, bridging the gap between the purified state and the quasi-physiological, cellular context. The successful achievement of such goals will vitally depend on a broad and seamless accessibility to cutting-edge technologies. Therefore, efforts aimed and coordinating and integrating infrastructure, exemplified by the European INSTRUCT (http://www.structuralbiology.eu/), BioStruct-X (http://www.biostruct-x.eu/) or Bio-NMR (http://www.bionmr.net/) projects, are likely to expand and play a crucial role.
Acknowledgements
A.R.A. was supported by a UK Medical Research Council Career Development Award Fellowship. The OPPF-UK is funded by the MRC and BBSRC research councils. We are grateful to Jon Diprose for the analysis of PDB data and to Max Crispin for help in modeling the glycosylated RPTPμ ectodomain structure.
References and recommended reading
Papers of particular interest, published within the period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Reichert JM. Marketed therapeutic antibodies compendium. MAbs. 2012;4:413–415. doi: 10.4161/mabs.19931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Nettleship JE, Assenberg R, Diprose JM, Rahman-Huq N, Owens RJ. Recent advances in the production of proteins in insect and mammalian cells for structural biology. J Struct Biol. 2010;172:55–65. doi: 10.1016/j.jsb.2010.02.006. [DOI] [PubMed] [Google Scholar]
- 3.Apweiler R, Hermjakob H, Sharon N. On the frequency of protein glycosylation, as deduced from analysis of the SWISS-PROT database. Biochim Biophys Acta. 1999;1473:4–8. doi: 10.1016/s0304-4165(99)00165-8. [DOI] [PubMed] [Google Scholar]
- 4.Chang VT, Crispin M, Aricescu AR, Harvey DJ, Nettleship JE, Fennelly JA, Yu C, Boles KS, Evans EJ, Stuart DI, et al. Glycoprotein structural genomics: solving the glycosylation problem. Structure. 2007;15:267–273. doi: 10.1016/j.str.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Stanley P. Chinese hamster ovary cell mutants with multiple glycosylation defects for production of glycoproteins with minimal carbohydrate heterogeneity. Mol Cell Biol. 1989;9:377–383. doi: 10.1128/mcb.9.2.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cockett MI, Bebbington CR, Yarranton GT. High level expression of tissue inhibitor of metalloproteinases in Chinese hamster ovary cells using glutamine synthetase gene amplification. Biotechnology (NY) 1990;8:662–667. doi: 10.1038/nbt0790-662. [DOI] [PubMed] [Google Scholar]
- 7.Bebbington CRH, Hentschel CCG, editors. DNA Cloning. IRL Press; Oxford: 1987. [Google Scholar]
- 8.Jones EY, Davis SJ, Williams AF, Harlos K, Stuart DI. Crystal structure at 2.8 A resolution of a soluble form of the cell adhesion molecule CD2. Nature. 1992;360:232–239. doi: 10.1038/360232a0. [DOI] [PubMed] [Google Scholar]
- 9.Lustbader JW, Wu H, Birken S, Pollak S, Gawinowicz Kolks MA, Pound AM, Austen D, Hendrickson WA, Canfield RE. The expression, characterization, and crystallization of wild-type and selenomethionyl human chorionic gonadotropin. Endocrinology. 1995;136:640–650. doi: 10.1210/endo.136.2.7835298. [DOI] [PubMed] [Google Scholar]
- 10.Wu H, Lustbader JW, Liu Y, Canfield RE, Hendrickson WA. Structure of human chorionic gonadotropin at 2.6 A resolution from MAD analysis of the selenomethionyl protein. Structure. 1994;2:545–558. doi: 10.1016/s0969-2126(00)00054-x. [DOI] [PubMed] [Google Scholar]
- 11.Durocher Y, Perret S, Kamen A. High-level and high-throughput recombinant protein production by transient transfection of suspension-growing human 293-EBNA1 cells. Nucleic Acids Res. 2002;30:E9. doi: 10.1093/nar/30.2.e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geisse S, Henke M. Large-scale transient transfection of mammalian cells: a newly emerging attractive option for recombinant protein production. J Struct Funct Genomics. 2005;6:165–170. doi: 10.1007/s10969-005-2826-4. [DOI] [PubMed] [Google Scholar]
- 13.Aricescu AR, Lu W, Jones EY. A time- and cost-efficient system for high-level protein production in mammalian cells. Acta Crystallogr D Biol Crystallogr. 2006;62(Pt 10):1243–1250. doi: 10.1107/S0907444906029799. [DOI] [PubMed] [Google Scholar]
- 14.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baldi L, Hacker DL, Adam M, Wurm FM. Recombinant protein production by large-scale transient gene expression in mammalian cells: state of the art and future perspectives. Biotechnol Lett. 2007;29:677–684. doi: 10.1007/s10529-006-9297-y. [DOI] [PubMed] [Google Scholar]
- 16 •.Zhao Y, Bishop B, Clay JE, Lu W, Jones M, Daenke S, Siebold C, Stuart DI, Jones EY, Aricescu AR. Automation of large scale transient protein expression in mammalian cells. J Struct Biol. 2011;175:209–215. doi: 10.1016/j.jsb.2011.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]; This manuscript reports the successful implementation of automation for large-scale cell maintenance, transient transfection and protein production in mammalian cells within a medium/large size academic laboratory. A detailed description of protocols, optimized for the commonly used HEK293 cell lines is provided.
- 17.Backliwal G, Hildinger M, Chenuet S, Wulhfard S, De Jesus M, Wurm FM. Rational vector design and multi-pathway modulation of HEK 293E cells yield recombinant antibody titers exceeding 1 g/l by transient transfection under serum-free conditions. Nucleic Acids Res. 2008;36:e96. doi: 10.1093/nar/gkn423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lee JE, Fusco ML, Saphire EO. An efficient platform for screening expression and crystallization of glycoproteins produced in human cells. Nat Protoc. 2009;4:592–604. doi: 10.1038/nprot.2009.29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19 •.Chaudhary S, Pak JE, Gruswitz F, Sharma V, Stroud RM. Overexpressing human membrane proteins in stably transfected and clonal human embryonic kidney 293S cells. Nat Protoc. 2012;7:453–466. doi: 10.1038/nprot.2011.453. [DOI] [PMC free article] [PubMed] [Google Scholar]; Article provides detailed protcol for the production of membrane proteins in mammalian cells using the inducible expression system in the HEK 293 S GnTI− cell line first developed by Reeves et al. [22].
- 20.Gonzalez R, Jennings LL, Knuth M, Orth AP, Klock HE, Ou W, Feuerhelm J, Hull MV, Koesema E, Wang Y, et al. Screening the mammalian extracellular proteome for regulators of embryonic human stem cell pluripotency. Proc Natl Acad Sci U S A. 2010;107:3552–3557. doi: 10.1073/pnas.0914019107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Van Craenenbroeck K, Vanhoenacker P, Haegeman G. Episomal vectors for gene expression in mammalian cells. Eur J Biochem. 2000;267:5665–5678. doi: 10.1046/j.1432-1327.2000.01645.x. [DOI] [PubMed] [Google Scholar]
- 22.Reeves PJ, Callewaert N, Contreras R, Khorana HG. Structure and function in rhodopsin: high-level expression of rhodopsin with restricted and homogeneous N-glycosylation by a tetracycline-inducible N-acetylglucosaminyltransferase I-negative HEK293S stable mammalian cell line. Proc Natl Acad Sci U S A. 2002;99:13419–13424. doi: 10.1073/pnas.212519299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23 •.Wilke S, Groebe L, Maffenbeier V, Jager V, Gossen M, Josewski J, Duda A, Polle L, Owens RJ, Wirth D, et al. Streamlining homogeneous glycoprotein production for biophysical and structural applications by targeted cell line development. PLoS ONE. 2011;6:e27829. doi: 10.1371/journal.pone.0027829. [DOI] [PMC free article] [PubMed] [Google Scholar]; Several strategies are currently pursued to speed up generation of genetically stable and highly productive mammalian cell lines. This paper describes novel CHO Lec3.2.8.1-derived master cell lines that can be efficiently converted to produce a protein of interest with limited glycosylation, via Flp recombinase-mediated cassette exchange.
- 24 •.Andréll J, Tate CG. Overexpression of membrane proteins in mammalian cells for structural studies. Mol Membr Biol. 2012;30:52–63. doi: 10.3109/09687688.2012.703703. [DOI] [PMC free article] [PubMed] [Google Scholar]; A review of the current state of the field with a useful compilation of the results for the epxression of integral membrane proteins in both transient and stable mammlain expression systems.
- 25.Reeves PJ, Kim JM, Khorana HG. Structure and function in rhodopsin: a tetracycline-inducible system in stable mammalian cell lines for high-level expression of opsin mutants. Proc Natl Acad Sci U S A. 2002;99:13413–13418. doi: 10.1073/pnas.212519199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Elbein AD, Tropea JE, Mitchell M, Kaushal GP. Kifunensine, a potent inhibitor of the glycoprotein processing mannosidase I. J Biol Chem. 1990;265:15599–15605. [PubMed] [Google Scholar]
- 27.Elbein AD, Solf R, Dorling PR, Vosbeck K. Swainsonine: an inhibitor of glycoprotein processing. Proc Natl Acad Sci U S A. 1981;78:7393–7397. doi: 10.1073/pnas.78.12.7393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Aricescu AR, Siebold C, Choudhuri K, Chang VT, Lu W, Davis SJ, van der Merwe PA, Jones EY. Structure of a tyrosine phosphatase adhesive interaction reveals a spacer-clamp mechanism. Science. 2007;317:1217–1220. doi: 10.1126/science.1144646. [DOI] [PubMed] [Google Scholar]
- 29 ••.Seiradake E, Harlos K, Sutton G, Aricescu AR, Jones EY. An extracellular steric seeding mechanism for Eph-ephrin signaling platform assembly. Nat Struct Mol Biol. 2010;17:398–402. doi: 10.1038/nsmb.1782. [DOI] [PMC free article] [PubMed] [Google Scholar]; Together with Ref. [69••], this manuscript provides crucial structural insights into the supra-molecular architecture of Eph receptor — ephrin ligand complexes and explains the long-standing puzzle regarding the ability of ephrins to rapidly trigger receptor activation well beyond the regions of direct receptor–ligand contact.
- 30 ••.Seiradake E, Coles CH, Perestenko PV, Harlos K, McIlhinney RA, Aricescu AR, Jones EY. Structural basis for cell surface patterning through NetrinG–NGL interactions. EMBO J. 2011;30:4479–4488. doi: 10.1038/emboj.2011.346. [DOI] [PMC free article] [PubMed] [Google Scholar]; A comprehensive biophysical and structural analysis of the netrin G and netrin G ligands (NGL) family members, as well as their complexes, which has unraveled the basis for NGL sorting into seggregated cell surface subdomains. Such mechanisms appear to be broadly employed to determine the correct neuronal connectivity and patterning within the central nervous system.
- 31 ••.Janssen BJ, Robinson RA, Perez-Branguli F, Bell CH, Mitchell KJ, Siebold C, Jones EY. Structural basis of semaphorin-plexin signalling. Nature. 2010;467:1118–1122. doi: 10.1038/nature09468. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study along with Refs. [59••,60••] constitute the culmination of a genuinely outstanding structural tour-de-force to describe structures of representative Plexin A, B and C family members and their complexes with Semaphorin ligands. These interactions are of crucial importance during the development and homeostasis of the nervous, immune and cardiovascular systems.
- 32 ••.Janssen BJ, Malinauskas T, Weir GA, Cader MZ, Siebold C, Jones EY. Neuropilins lock secreted semaphorins onto plexins in a ternary signaling complex. Nat Struct Mol Biol. 2012;19:1293–1299. doi: 10.1038/nsmb.2416. [DOI] [PMC free article] [PubMed] [Google Scholar]; Secreted semaphorin 3 family members require a co-receptor, Neuropilin-1, to signal through Plexin A receptors. This paper describes the ternary complex architecture, highlighting the unexpected role of neuropilin to act as a molecular ‘staple’, thus adding a further level of control over the ‘classical’ ligand-induced dimerization strategy commonly encoutered in cell-surface receptors.
- 33 •.Malinauskas T, Aricescu AR, Lu W, Siebold C, Jones EY. Modular mechanism of Wnt signaling inhibition by Wnt inhibitory factor 1. Nat Struct Mol Biol. 2011;18:886–893. doi: 10.1038/nsmb.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper reveals the crystal structure of Wnt inhibitory factor 1 (WIF1), an important modulator of multiple Wnt morphogen family members. Extensive ‘glycan–wedge’ mutagenesis, coupled with biophysical and cellular assays, led to the mapping of a complex, multi-domain, Wnt interaction interface.
- 34 ••.Kumar J, Schuck P, Mayer ML. Structure and assembly mechanism for heteromeric kainate receptors. Neuron. 2011;71:319–331. doi: 10.1016/j.neuron.2011.05.038. [DOI] [PMC free article] [PubMed] [Google Scholar]; A study of outstanding importance to the understanding of heteromeric ionotropic glutamate receptors’ assembly. Furthermore, this work illustrates an ingenious approach to prevent the formation of high order oligomers/aggregates and to influence the crystallographic packing mode through the use of structure-guided placement of ‘glycan wedges’.
- 35 •.Kwon YD, Finzi A, Wu X, Dogo-Isonagie C, Lee LK, Moore LR, Schmidt SD, Stuckey J, Yang Y, Zhou T, et al. Unliganded HIV-1 gp120 core structures assume the CD4-bound conformation with regulation by quaternary interactions and variable loops. Proc Natl Acad Sci U S A. 2012;109:5663–5668. doi: 10.1073/pnas.1112391109. [DOI] [PMC free article] [PubMed] [Google Scholar]; A series of unliganded HIV-1 gp120 core crystal structures, from three viral clades (B, C and E), provides important insights into the gp120 conformational mobility, with implications for understanding the mechanism of immune evasion and designing inhibitors of conformational translations for prophylactic and therapeutic purposes.
- 36 •.Holdom MD, Davies AM, Nettleship JE, Bagby SC, Dhaliwal B, Girardi E, Hunt J, Gould HJ, Beavil AJ, McDonnell JM, et al. Conformational changes in IgE contribute to its uniquely slow dissociation rate from receptor FcvarepsilonRI. Nat Struct Mol Biol. 2011;18:571–576. doi: 10.1038/nsmb.2044. [DOI] [PMC free article] [PubMed] [Google Scholar]; High affinity binding of the IgE immunoglobulin to its FcεRI receptor represents a key step in allergic reactions. This paper describes crystal structures of the IgE-Fc and the IgE-Fc-FcεRIα complex, which reveal unexpected conformational changes in both proteins upon binding and may inform the development of small molecule allosteric modulators to reduce the affinity of this complex.
- 37 •.Bowden TA, Baruah K, Coles CH, Harvey DJ, Yu X, Song BD, Stuart DI, Aricescu AR, Scanlan CN, Jones EY, et al. Chemical and structural analysis of an antibody folding intermediate trapped during glycan biosynthesis. J Am Chem Soc. 2012;134:17554–17563. doi: 10.1021/ja306068g. [DOI] [PMC free article] [PubMed] [Google Scholar]; The use of glycosidase inhibitors and mammalian cell-lines with modified glycan processing enzymes allows production of proteins with defined glycan structures. This approach has been exploited here to evaluate the impact of glycan maturation on the structural stability of the human IgG Fc region.
- 38 ••.Verstraete K, Vandriessche G, Januar M, Elegheert J, Shkumatov AV, Desfosses A, Van Craenenbroeck K, Svergun DI, Gutsche I, Vergauwen B, et al. Structural insights into the extracellular assembly of the hematopoietic Flt3 signaling complex. Blood. 2011;118:60–68. doi: 10.1182/blood-2011-01-329532. [DOI] [PubMed] [Google Scholar]; The trio of Refs. [38••,62••,63••] further our understanding of the organization and activation principles of class III receptor tyrosine kinases (RTKIII) and their cytokine ligands. The architecture of Flt3:FL complex is unexpectedly different compared to other family members (such as CSF-1R:CSF-1), in both ligand binding-mode and the lack of preformed dimers at the cell surface. On the other hand, despite the marked differences in sequences, IL-34 and CSF-1 establish suprrisingly similar extracellular assemblies with their common receptor, CSF-1R. The successful structural characterization of such large, multi-domain, protein complexes, is one of the best illustrations of the remarkable benefits emerging from a creative combination of mammalian expression strategies and multiple biophysical techniques.
- 39 ••.Elegheert J, Bracke N, Pouliot P, Gutsche I, Shkumatov AV, Tarbouriech N, Verstraete K, Bekaert A, Burmeister WP, Svergun DI, et al. Allosteric competitive inactivation of hematopoietic CSF-1 signaling by the viral decoy receptor BARF1. Nat Struct Mol Biol. 2012;19:938–947. doi: 10.1038/nsmb.2367. [DOI] [PubMed] [Google Scholar]; An outstanding combination of structural, biophysical and cellular techniques that lead to the discovery of a novel strategy employed by the Epstein-Barr virus to evade detection and elimination by the human immune system. The secreted lytic cycle protein BARF-1 forms a hexameric toroid that traps and allosterically inactivates hematopoietic human colony-stimulating factor 1 molecules.
- 40 •.Miller MT, Mileni M, Comoletti D, Stevens RC, Harel M, Taylor P. The crystal structure of the alpha-neurexin-1 extracellular region reveals a hinge point for mediating synaptic adhesion and function. Structure. 2011;19:767–778. doi: 10.1016/j.str.2011.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]; A concerted structural effort led to three publications in 2010–2011 focused on the electron microscopic and crystallographic characterization of a key synaptic organizer, neurexin 1α. The paper highlighted here relied on mammalian cell expression to produce a large (7-domain) neurexin 1α ectodomain, solve the crystal structure, and characterize its interaction with a post-synaptic partner, neuroligin-1.
- 41.Luo BH, Springer TA, Takagi J. Stabilizing the open conformation of the integrin headpiece with a glycan wedge increases affinity for ligand. Proc Natl Acad Sci U S A. 2003;100:2403–2408. doi: 10.1073/pnas.0438060100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rondard P, Huang S, Monnier C, Tu H, Blanchard B, Oueslati N, Malhaire F, Li Y, Trinquet E, Labesse G, et al. Functioning of the dimeric GABA(B) receptor extracellular domain revealed by glycan wedge scanning. EMBO J. 2008;27:1321–1332. doi: 10.1038/emboj.2008.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Aricescu AR, Hon WC, Siebold C, Lu W, van der Merwe PA, Jones EY. Molecular analysis of receptor protein tyrosine phosphatase mu-mediated cell adhesion. EMBO J. 2006;25:701–712. doi: 10.1038/sj.emboj.7600974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Barton WA, Tzvetkova-Robev D, Erdjument-Bromage H, Tempst P, Nikolov DB. Highly efficient selenomethionine labeling of recombinant proteins produced in mammalian cells. Protein Sci. 2006;15:2008–2013. doi: 10.1110/ps.062244206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45 ••.Coles CH, Shen Y, Tenney AP, Siebold C, Sutton GC, Lu W, Gallagher JT, Jones EY, Flanagan JG, Aricescu AR. Proteoglycan-specific molecular switch for RPTPsigma clustering and neuronal extension. Science. 2011;332:484–488. doi: 10.1126/science.1200840. [DOI] [PMC free article] [PubMed] [Google Scholar]; Virtually all mammalian cells are surrounded by membrane bound or extracellular matrix proteoglycans, which are major modulators of cellular signalling. Yet, very little is still known about their mechanisms of action and specificity of interaction with cell surface receptors. This paper explains the antagonistic effect exerted by heparan versus chondroitin sulphate proteoglycans on the RPTPsigma receptor clustering and, as a result, neuronal extension and repair.
- 46.Clayton A, Siebold C, Gilbert RJ, Sutton GC, Harlos K, McIlhinney RA, Jones EY, Aricescu AR. Crystal structure of the GluR2 amino-terminal domain provides insights into the architecture and assembly of ionotropic glutamate receptors. J Mol Biol. 2009;392:1125–1132. doi: 10.1016/j.jmb.2009.07.082. [DOI] [PubMed] [Google Scholar]
- 47.Bishop B, Aricescu AR, Harlos K, O’Callaghan CA, Jones EY, Siebold C. Structural insights into hedgehog ligand sequestration by the human hedgehog-interacting protein HHIP. Nat Struct Mol Biol. 2009;16:698–703. doi: 10.1038/nsmb.1607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48 •.Dutta A, Saxena K, Schwalbe H, Klein-Seetharaman J. Isotope labeling in mammalian cells. Methods Mol Biol. 2012;831:55–69. doi: 10.1007/978-1-61779-480-3_4. [DOI] [PMC free article] [PubMed] [Google Scholar]; Article provides detailed protocols for producing 15N and 13N amino acid-labelled proteins in mammalian cells for NMR studies.
- 49.Werner K, Richter C, Klein-Seetharaman J, Schwalbe H. Isotope labeling of mammalian GPCRs in HEK293 cells and characterization of the C-terminus of bovine rhodopsin by high resolution liquid NMR spectroscopy. J Biomol NMR. 2008;40:49–53. doi: 10.1007/s10858-007-9205-3. [DOI] [PubMed] [Google Scholar]
- 50.Egorova-Zachernyuk TA, Bosman GJ, Degrip WJ. Uniform stable-isotope labeling in mammalian cells: formulation of a cost-effective culture medium. Appl Microbiol Biotechnol. 2011;89:397–406. doi: 10.1007/s00253-010-2896-5. [DOI] [PubMed] [Google Scholar]
- 51.Tochio H. Watching protein structure at work in living cells using NMR spectroscopy. Curr Opin Chem Biol. 2012;16:609–613. doi: 10.1016/j.cbpa.2012.10.022. [DOI] [PubMed] [Google Scholar]
- 52 ••.Banci LBL, Bertini I, Luchinat E, Secci E, Zhao Y, Aricescu AR. Atomic resolution monitoring of protein maturation in live human cells. Nat Chem Biol. 2013 doi: 10.1038/nchembio.1202. http://dx.doi.org/10.1038/nchembio.1202, in press. [DOI] [PMC free article] [PubMed] [Google Scholar]; Mammalian expression was employed to reach a key milestone in the quest for structural characterization of protein molecules withing their cellular environment. In-cell NMR was employed to follow for the first time, in live cells and in atomic detail, the complete maturation process of a protein, human SOD1, recombinantly expressed at close to physiological levels.
- 53.Hino N, Hayashi A, Sakamoto K, Yokoyama S. Site-specific incorporation of non-natural amino acids into proteins in mammalian cells with an expanded genetic code. Nat Protoc. 2006;1:2957–2962. doi: 10.1038/nprot.2006.424. [DOI] [PubMed] [Google Scholar]
- 54.Jones DH, Cellitti SE, Hao X, Zhang Q, Jahnz M, Summerer D, Schultz PG, Uno T, Geierstanger BH. Site-specific labeling of proteins with NMR-active unnatural amino acids. J Biomol NMR. 2010;46:89–100. doi: 10.1007/s10858-009-9365-4. [DOI] [PubMed] [Google Scholar]
- 55.Hino N, Okazaki Y, Kobayashi T, Hayashi A, Sakamoto K, Yokoyama S. Protein photo-cross-linking in mammalian cells by site-specific incorporation of a photoreactive amino acid. Nat Methods. 2005;2:201–206. doi: 10.1038/nmeth739. [DOI] [PubMed] [Google Scholar]
- 56 •.Borrmann A, Milles S, Plass T, Dommerholt J, Verkade JM, Wiessler M, Schultz C, van Hest JC, van Delft FL, Lemke EA. Genetic encoding of a bicyclo[6.1.0]nonyne-charged amino acid enables fast cellular protein imaging by metal-free ligation. Chembiochem. 2012;13:2094–2099. doi: 10.1002/cbic.201200407. [DOI] [PubMed] [Google Scholar]; See comment to Ref. [58•].
- 57 •.Lang K, Davis L, Torres-Kolbus J, Chou C, Deiters A, Chin JW. Genetically encoded norbornene directs site-specific cellularprotein labelling via a rapid bioorthogonal reaction. Nat Chem. 2012;4:298–304. doi: 10.1038/nchem.1250. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [58].
- 58 •.Lang K, Davis L, Wallace S, Mahesh M, Cox DJ, Blackman ML, Fox JM, Chin JW. Genetic encoding of bicyclononynes and trans-cyclooctenes for site-specific protein labeling in vitro and in live mammalian cells via rapid fluorogenic Diels–Alder reactions. J Am Chem Soc. 2012;134:10317–10320. doi: 10.1021/ja302832g. [DOI] [PMC free article] [PubMed] [Google Scholar]; Refs. [56•–58•] illustrate the spectacular and rapidly evolving field of specific fluorogenic protein labeling in live mammalian cells using genetically endoded unnatural amino acids.
- 59 ••.Liu H, Juo ZS, Shim AH, Focia PJ, Chen X, Garcia KC, He X. Structural basis of semaphorin-plexin recognition and viral mimicry from Sema7A and A39R complexes with PlexinC1. Cell. 2010;142:749–761. doi: 10.1016/j.cell.2010.07.040. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [31••]. Furthermore, this paper explains a strategy employed by the Vaccinia (smallpox) virus to enhance its survival in the host, which involves the secretion of semaphorin mimetics to bind and activate Plexin C1 signalling, and thus achieve immunomodulation.
- 60 ••.Nogi T, Yasui N, Mihara E, Matsunaga Y, Noda M, Yamashita N, Toyofuku T, Uchiyama S, Goshima Y, Kumanogoh A, et al. Structural basis for semaphorin signalling through the plexin receptor. Nature. 2010;467:1123–1127. doi: 10.1038/nature09473. [DOI] [PubMed] [Google Scholar]; See comment to Ref. [31••].
- 61 •.Bell CH, Aricescu AR, Jones EY, Siebold C. A dual binding mode for RhoGTPases in plexin signalling. PLoS Biol. 2011;9:e1001134. doi: 10.1371/journal.pbio.1001134. [DOI] [PMC free article] [PubMed] [Google Scholar]; Plexin receptors can simultaneously bind an extracellular semaphorin ligand and an intracellular Rho GTPase. The combination of crystallographic and cellular assays used here led to the discovery of a trimeric arrangement of cytoplasmic Plexin-B1-Rac1 complexes. Coupled with the ligand-induced Plexin ectodomain dimerization, this arrangement suggest the formation of intriguing supramolecular honeycomb structures during plexin signalling.
- 62 ••.Elegheert J, Desfosses A, Shkumatov AV, Wu X, Bracke N, Verstraete K, Van Craenenbroeck K, Brooks BR, Svergun DI, Vergauwen B, et al. Extracellular complexes of the hematopoietic human and mouse CSF-1 receptor are driven by common assembly principles. Structure. 2011;19:1762–1772. doi: 10.1016/j.str.2011.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [38].
- 63 ••.Felix J, Elegheert J, Gutsche I, Shkumatov AV, Wen Y, Bracke N, Pannecoucke E, Vandenberghe I, Devreese B, Svergun DI, et al. Human IL-34 and CSF-1 establish structurally similar extracellular assemblies with their common hematopoietic receptor. Structure. 2013;21:528–539. doi: 10.1016/j.str.2013.01.018. [DOI] [PubMed] [Google Scholar]; See comment to Ref. [38].
- 64.Boggon TJ, Murray J, Chappuis-Flament S, Wong E, Gumbiner BM, Shapiro L. C-cadherin ectodomain structure and implications for cell adhesion mechanisms. Science. 2002;296:1308–1313. doi: 10.1126/science.1071559. [DOI] [PubMed] [Google Scholar]
- 65.Al-Amoudi A, Diez DC, Betts MJ, Frangakis AS. The molecular architecture of cadherins in native epidermal desmosomes. Nature. 2007;450:832–837. doi: 10.1038/nature05994. [DOI] [PubMed] [Google Scholar]
- 66 ••.Harrison OJ, Jin X, Hong S, Bahna F, Ahlsen G, Brasch J, Wu Y, Vendome J, Felsovalyi K, Hampton CM, et al. The extracellular architecture of adherens junctions revealed by crystal structures of type I cadherins. Structure. 2011;19:244–256. doi: 10.1016/j.str.2010.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]; The 2D arrays (‘molecular layers’) previously observed in the C-cadherin crystallographic packing [64] have been extended here to E-cadherin and N-cadherin. Importantly, these have been shown to form on artificial membranes and be physiologically relevant at cellular adhesive junctions, thus representing a paradigm of higher order cell surface protein organization.
- 67 ••.Wu Y, Vendome J, Shapiro L, Ben-Shaul A, Honig B. Transforming binding affinities from three dimensions to two with application to cadherin clustering. Nature. 2011;475:510–513. doi: 10.1038/nature10183. [DOI] [PMC free article] [PubMed] [Google Scholar]; This seminal paper has established the theoretical framework for understanding the molecular principles of homophilic cell adhesion.
- 68 •.Tanaka H, Miyazaki N, Matoba K, Nogi T, Iwasaki K, Takagi J. Higher-order architecture of cell adhesion mediated by polymorphic synaptic adhesion molecules neurexin and neuroligin. Cell Rep. 2012;2:101–110. doi: 10.1016/j.celrep.2012.06.009. [DOI] [PubMed] [Google Scholar]; The combination of cis/trans interactions between heterophilic cell adhesion molecules may also lead to the formation of 2D array-type ‘molecular layers’. The extent and physiological relevance of such arrangements, described here for the neurexin1β-neuroligin 1 pairs, remain to be characterized.
- 69 ••.Himanen JP, Yermekbayeva L, Janes PW, Walker JR, Xu K, Atapattu L, Rajashankar KR, Mensinga A, Lackmann M, Nikolov DB, et al. Architecture of Eph receptor clusters. Proc Natl Acad Sci U S A. 2010;107:10860–10865. doi: 10.1073/pnas.1004148107. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [29••].
- 70.Wimmer-Kleikamp SH, Janes PW, Squire A, Bastiaens PI, Lackmann M. Recruitment of Eph receptors into signaling clusters does not require ephrin contact. J Cell Biol. 2004;164:661–666. doi: 10.1083/jcb.200312001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kantor DB, Chivatakarn O, Peer KL, Oster SF, Inatani M, Hansen MJ, Flanagan JG, Yamaguchi Y, Sretavan DW, Giger RJ, et al. Semaphorin 5A is a bifunctional axon guidance cue regulated by heparan and chondroitin sulfate proteoglycans. Neuron. 2004;44:961–975. doi: 10.1016/j.neuron.2004.12.002. [DOI] [PubMed] [Google Scholar]
- 72 ••.Ferrara C, Grau S, Jager C, Sondermann P, Brunker P, Waldhauer I, Hennig M, Ruf A, Rufer AC, Stihle M, et al. Unique carbohydrate–carbohydrate interactions are required for high affinity binding between FcgammaRIII and antibodies lacking core fucose. Proc Natl Acad Sci U S A. 2011;108:12669–12674. doi: 10.1073/pnas.1108455108. [DOI] [PMC free article] [PubMed] [Google Scholar]; Example of how structural analysis by X-ray crystallography of proteins produced in mammalian cells has provided insight into the role of glycosylation in receptor-mediated antibody-dependent cytotoxocity.
- 73.Ekiert DC, Kashyap AK, Steel J, Rubrum A, Bhabha G, Khayat R, Lee JH, Dillon MA, O’Neil RE, Faynboym AM, et al. Cross-neutralization of influenza A viruses mediated by a single antibody loop. Nature. 2012;489:526–532. doi: 10.1038/nature11414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lee JE, Fusco ML, Hessell AJ, Oswald WB, Burton DR, Saphire EO. Structure of the Ebola virus glycoprotein bound to an antibody from a human survivor. Nature. 2008;454:177–182. doi: 10.1038/nature07082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Oganesyan V, Damschroder MM, Phipps S, Wilson SD, Cook KE, Wu H, Dall’Acqua WF. Crystallization and preliminary X-ray diffraction analysis of the complex of a human anti-ephrin type-A receptor 2 antibody fragment and its cognate antigen. Acta Crystallogr Sect F Struct Biol Cryst Commun. 2010;66(Pt 6):730–733. doi: 10.1107/S1744309110015861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Nettleship JE, Ren J, Rahman N, Berrow NS, Hatherley D, Barclay AN, Owens RJ. A pipeline for the production of antibody fragments for structural studies using transient expression in HEK 293T cells. Protein Expr Purif. 2008;62:83–89. doi: 10.1016/j.pep.2008.06.017. [DOI] [PubMed] [Google Scholar]
- 77 ••.Kong L, Giang E, Robbins JB, Stanfield RL, Burton DR, Wilson IA, Law M. Structural basis of hepatitis C virus neutralization by broadly neutralizing antibody HCV1. Proc Natl Acad Sci U S A. 2012;109:9499–9504. doi: 10.1073/pnas.1202924109. [DOI] [PMC free article] [PubMed] [Google Scholar]; Refs. [77••–80•••] illustrate the spectacular progress recently made in the quest to developing ‘universal vaccines’ against the major viral pathogens. Once thought to be an intractable problem, due to extreme sequence diversity/variability of viral proteins, the discovery and crystallographic characterization of viral vulnerability epitopes represents a major breakthrough.
- 78 ••.Kong L, Giang E, Nieusma T, Robbins JB, Deller MC, Stanfield RL, Wilson IA, Law M. Structure of hepatitis C virus envelope glycoprotein E2 antigenic site 412 to 423 in complex with antibody AP33. J Virol. 2012;86:13085–13088. doi: 10.1128/JVI.01939-12. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [77••].
- 79 ••.McLellan JS, Pancera M, Carrico C, Gorman J, Julien JP, Khayat R, Louder R, Pejchal R, Sastry M, Dai K, et al. Structure of HIV-1 gp120 V1/V2 domain with broadly neutralizing antibody PG9. Nature. 2011;480:336–343. doi: 10.1038/nature10696. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [77••].
- 80 ••.Ekiert DC, Friesen RH, Bhabha G, Kwaks T, Jongeneelen M, Yu W, Ophorst C, Cox F, Korse HJ, Brandenburg B, et al. A highly conserved neutralizing epitope on group 2 influenza A viruses. Science. 2011;333:843–850. doi: 10.1126/science.1204839. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [77••].
- 81 •.Peng L, Oganesyan V, Damschroder MM, Wu H, Dall’Acqua WF. Structural and functional characterization of an agonistic anti-human EphA2 monoclonal antibody. J Mol Biol. 2011;413:390–405. doi: 10.1016/j.jmb.2011.08.018. [DOI] [PubMed] [Google Scholar]; The complex between an Eph receptor ligand-binding domain and a Fab fragment with agonistic properties provides an important structural framework for the development of anti-tumour reagents.
- 82 ••.Arkhipov A, Shan Y, Das R, Endres NF, Eastwood MP, Wemmer DE, Kuriyan J, Shaw DE. Architecture and membrane interactions of the EGF receptor. Cell. 2013;152:557–569. doi: 10.1016/j.cell.2012.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]; A combination of molecular dynamics simulations, NMR and cellular assays led to the discovery of novel, key steps in epidermal growth factor receptor signal transduction. Ligand binding removes extracellular steric constraints that allow association of transmembrane helices and the formation of assymmetric kinase dimers. Taken together, Refs. [82••,83••] and the large body of previous knowledge have generated the most detailed understanding to date of transmembrane signalling in type I membrane protein receptors.
- 83 ••.Endres NF, Das R, Smith AW, Arkhipov A, Kovacs E, Huang Y, Pelton JG, Shan Y, Shaw DE, Wemmer DE, et al. Conformational coupling across the plasma membrane in activation of the EGF receptor. Cell. 2013;152:543–556. doi: 10.1016/j.cell.2012.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [82••].
- 84 ••.Mi LZ, Lu C, Li Z, Nishida N, Walz T, Springer TA. Simultaneous visualization of the extracellular and cytoplasmic domains of the epidermal growth factor receptor. Nat Struct Mol Biol. 2011;18:984–989. doi: 10.1038/nsmb.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]; A single-particle negative-staining electron microscopy study of the (nearly) full length EGF receptor, the first structural study ever that allowed visualization of the overall conformational changes driven by ectodomain–ligand interactions in a single-pass membrane protein.
- 85.Kanchanawong P, Waterman CM. Advances in light-based imaging of three-dimensional cellular ultrastructure. Curr Opin Cell Biol. 2012;24:125–133. doi: 10.1016/j.ceb.2011.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86 ••.Kanchanawong P, Shtengel G, Pasapera AM, Ramko EB, Davidson MW, Hess HF, Waterman CM. Nanoscale architecture of integrin-based cell adhesions. Nature. 2010;468:580–584. doi: 10.1038/nature09621. [DOI] [PMC free article] [PubMed] [Google Scholar]; An outstanding three-dimensional super-resolution fluorescence microscopy study that describes the multi-layered molecular organization of focal adhesions in cultured mammalian cells. Such an unprecedented view of a very complex molecular network of plasma-membrane integrins and cytoplasmic proteins will inspire the application of this technology to characterize other supra-molecular assemblies.
- 87.Lidke DS, Lidke KA. Advances in high-resolution imaging-techniques for three-dimensional imaging of cellular structures. J Cell Sci. 2012;125(Pt 11):2571–2580. doi: 10.1242/jcs.090027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88 ••.Kukulski W, Schorb M, Welsch S, Picco A, Kaksonen M, Briggs JA. Correlated fluorescence and 3D electron microscopy with high sensitivity and spatial precision. J Cell Biol. 2011;192:111–119. doi: 10.1083/jcb.201009037. [DOI] [PMC free article] [PubMed] [Google Scholar]; An excellent illustration of the combined power of electron tomography and fluorescence microscopy to characterize dynamic cellular structures with high sensitivity and precision.
- 89 •.Hagen C, Guttmann P, Klupp B, Werner S, Rehbein S, Mettenleiter TC, Schneider G, Grunewald K. Correlative VIS-fluorescence and soft X-ray cryo-microscopy/tomography of adherent cells. J Struct Biol. 2012;177:193–201. doi: 10.1016/j.jsb.2011.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]; X-ray microscopy is a revolutionary technique that, for the first time ever, has opened up the possibility to directly visualize the detailed subcellular architecture (at better than 50 nm resolution) of unfixed, uncut and unstained/unlabelled cells. Reports [89•,90•] establish key methodological aspects and provide a comprehensive analysis of the mammalian cell landscape using X-ray microscopy.
- 90 •.Muller WG, Heymann JB, Nagashima K, Guttmann P, Werner S, Rehbein S, Schneider G, McNally JG. Towards an atlas of mammalian cell ultrastructure by cryo soft X-ray tomography. J Struct Biol. 2012;177:179–192. doi: 10.1016/j.jsb.2011.11.025. [DOI] [PMC free article] [PubMed] [Google Scholar]; See comment to Ref. [89•].