Abstract
Background
Previous studies have demonstrated that the rising mortality due to mesothelioma and asbestosis can be predicted from historic asbestos usage. Mortality due to idiopathic pulmonary fibrosis (IPF) is also rising, without any apparent explanation.
Aims
To compare mortality due to these conditions and examine the relationship between mortality and national asbestos imports.
Methods
Mortality data for IPF and asbestosis in England and Wales were available from the Office for National Statistics. Data for mesothelioma deaths in England and Wales and historic UK asbestos import data were available from the Health & Safety Executive. The numbers of annual deaths due to each condition were plotted separately by gender, against UK asbestos imports 48 years earlier. Linear regression models were constructed.
Results
For mesothelioma and IPF, there was a significant linear relationship between the number of male and female deaths each year and historic UK asbestos imports. For asbestosis mortality, a similar relationship was found for male but not female deaths. The annual numbers of deaths due to asbestosis in both sexes were lower than for IPF and mesothelioma.
Conclusions
The strength of the association between IPF mortality and historic asbestos imports was similar to that seen in an established asbestos-related disease, i.e. mesothelioma. This finding could in part be explained by diagnostic difficulties in separating asbestosis from IPF and highlights the need for a more accurate method of assessing lifetime occupational asbestos exposure.
Key words: Asbestos, asbestosis; idiopathic pulmonary fibrosis, mesothelioma.
Introduction
World Health Organization (WHO) mortality data have established that historic asbestos consumption is a significant predictor of mortality due to long-latency asbestos-related disease [1]. Knowledge of previous asbestos usage has also been utilized to develop models capable of predicting future mortality from asbestosis and mesothelioma in a number of different countries [2–6].
Asbestosis is a form of chronic interstitial lung disease that occurs in a proportion of individuals following prolonged and usually heavy occupational exposure to asbestos [7]. It most commonly occurs several decades after exposure and is more common in men and in older age groups [8]. The main differential diagnosis for asbestosis is idiopathic pulmonary fibrosis (IPF), where a similar pattern of fibrosis [usual interstitial pneumonitis (UIP)] occurs with no identifiable cause [9].
Differentiating asbestosis from IPF is important, as the former may be eligible for benefits/compensation, whereas the latter may be considered for anti-fibrotic drug treatments [10]. Diagnosis may be challenging, however, as patient recall of previous exposures to asbestos is variable [11], radiological features may be identical [12] and few patients undergo surgical lung biopsy [13].
Mortality due to IPF has risen steadily in the UK over recent decades, with the condition now accounting for approximately ~5000 deaths each year [14]. IPF shares several of the demographic risk factors of asbestosis mortality, yet epidemiological studies of IPF have not identified links with previous asbestos exposure [15]. Certain occupations have however been linked with an increased risk of IPF in Britain, particularly workers exposed to metal and wood dusts [16,17].
Although the reason for the rising IPF mortality in the UK remains unexplained [14], it has previously been noted to be following a similar pattern to an established asbestos-related disease, i.e. mesothelioma [18]. Given this observation and the difficulties of differentiating idiopathic from asbestos-induced fibrosis, this study aimed to compare IPF, asbestosis and mesothelioma mortality and examine their relation to historic national asbestos imports.
Methods
This analysis utilized mortality data as published by the Office for National Statistics (ONS) [19], and by the Health & Safety Executive (HSE) [20]. Annual numbers of male and female deaths (England and Wales), where IPF (1962–2012) and asbestosis (1967–2012) were listed as the underlying cause, were requested from the ONS. ICD codes for IPF were used as per the method described by Navaratnam et al. [14]. Asbestosis ICD codes used were ICD8 515.2 asbestosis, ICD9 501 asbestosis and ICD10 J61 pneumoconiosis due to asbestos and other mineral fibres.
Annual mortality figures for mesothelioma for Britain were available from 1968 to 2012 from the HSE. These figures are derived from ONS data but include all deaths where mesothelioma was mentioned on the death certificate. Figures for England and Wales were calculated by subtracting Scottish mesothelioma deaths. Total asbestos imports for each year between 1914 and 1965 were calculated by adding annual import data for chrysotile, amosite and croccidolite [21,22]. Where individual annual import data were not available, figures were calculated by linear interpolation. A latent period of 48 years was selected based on a previously developed US asbestosis model [2].
Annual numbers of male and female deaths in England and Wales due to IPF, asbestosis and mesothelioma were plotted for each year, against data for historic UK asbestos imports expressed as hundreds of tonnes. In addition, total annual deaths from mesothelioma and IPF for men and women were plotted separately, and Pearson correlation coefficients calculated. Finally, the total number of deaths per year due to mesothelioma, asbestosis and IPF for men and women was plotted against historic asbestos imports, and regression models constructed.
Results
Given the 48-year latent interval selected, the time period 1962–2013 in Figures 1 and 2 corresponds to historic asbestos imports between 1914 and 1965. Total asbestos imports to the UK rose steadily over this time, apart from a fall in the period between 1941 and 1947, during and shortly after the Second World War. Over the period included in the analysis, male mortality due to IPF, mesothelioma and asbestosis rose steadily (Figure 1). The total numbers of male deaths due to IPF and mesothelioma for each year were of a similar magnitude, with a much lower number of deaths attributed to asbestosis.
Figure 1.
Annual male mortality due to IPF, mesothelioma and asbestosis in England and Wales. Historic annual UK asbestos imports (as hundreds of tonnes 48 years earlier) are shown for comparison (black line).
Figure 2.
Annual female mortality due to IPF, mesothelioma and asbestosis in England and Wales. Historical annual UK asbestos imports (as hundreds of tonnes 48 years earlier) are shown for comparison (black line).
For female mortality (shown in Figure 2), the number of deaths due to IPF and mesothelioma also increased over this period, but there was no increase in deaths due to asbestosis. In contrast to male mortality, the annual number of female deaths due to IPF was consistently higher than deaths due to mesothelioma. The ratio of male deaths directly attributed to asbestosis versus IPF remained relatively constant over the study period, at approximately 1:16 in 1967 and 1:13 in 2012. For female mortality, however, this ratio rose by a factor of ten, from approximately 1:38 in 1967 to 1:381 in 2012.
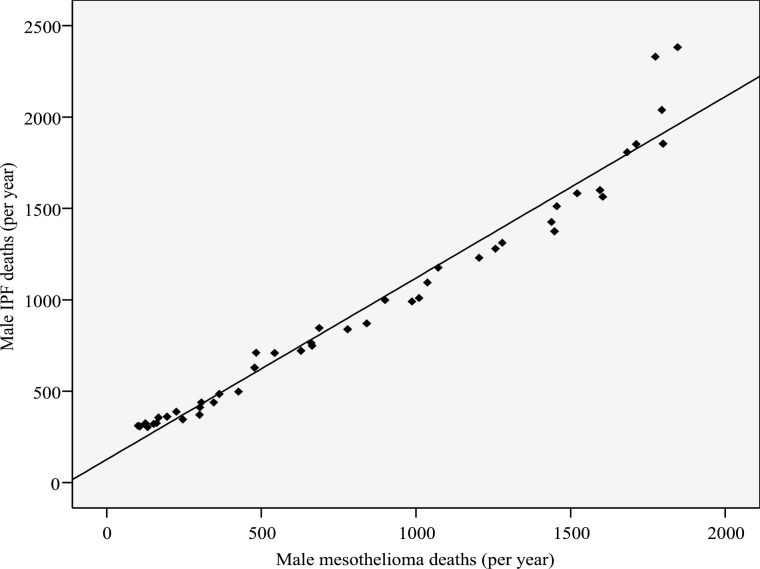
Annual mortality due to mesothelioma and IPF showed a significant linear relationship for both men and women, with Pearson correlation coefficients of 0.98 (P < 0.001) and 0.97 (P < 0.001), respectively (Figures 3 and 4).
Figure 3.
Number of annual male deaths (England and Wales) due to IPF and mesothelioma between 1968 and 2012.
Figure 4.
Number of annual female deaths (England and Wales) due to IPF and mesothelioma between 1968 and 2011.
The results of the regression analyses are seen in Table 1. A positive linear relation was found, both for males and females, between annual deaths due to mesothelioma and annual asbestos imports 48 years earlier. A similar relationship for male asbestosis mortality was seen with a slightly lower R 2 value. In contrast, there was no significant relationship between imports and female asbestosis mortality, with very low numbers of deaths each year. Linear relationships for male and female mortality due to IPF were also seen in relation to historic asbestos imports, with R 2 values similar to those seen for mesothelioma. Individual plots with regression lines are shown in the online Supplement (available at Occupational Medicine Online).
Table 1.
Regression analyses for annual number of deaths due to mesothelioma (meso), asbestosis and IPF in males (m) and females (f) versus historical asbestos imports 48 years earlier
| n | B 0 (95% CI) | SE | P value | B 1 (95% CI) | Adjusted R 2 | P value | |
|---|---|---|---|---|---|---|---|
| Meso (m) | 45 | −100 (−203 to 3) | 51 | 0.06 | 0.011 (0.010 to 0.012) | 0.91 | < 0.001 |
| Meso (f) | 44 | −15 (−38 to 7) | 11 | 0.17 | 0.002 (0.002 to 0.002) | 0.87 | < 0.001 |
| Asbestosis (m) | 46 | −10 (−23 to 4) | 7 | 0.16 | 0.001 (0.001 to 0.001) | 0.76 | < 0.001 |
| Asbestosis (f) | 45 | 5 (4 to 6) | 1 | <0.001 | 0.000 (0.000 to 0.000) | 0.00 | NS |
| IPF (m) | 51 | 19 (−68 to 106) | 43 | 0.66 | 0.011 (0.010 to 0.012) | 0.92 | < 0.001 |
| IPF (f) | 51 | 20 (−29 to 69) | 25 | 0.42 | 0.007 (0.006 to 0.008) | 0.93 | < 0.001 |
B 0, intercept of regression line; B 1, slope of regression line; n, number of years included in the analysis; regression model: number of deaths = B 0 + B 1 × national asbestos imports 48 years earlier (in hundreds of tonnes); SE, standard error.
Discussion
This study has confirmed the expected relationship between the rising mortality due to asbestos-related diseases in England and Wales and historic national asbestos imports [1]. A significant linear relation was seen for mesothelioma mortality in both genders and asbestosis deaths in males. In contrast, there was no significant rise in female asbestosis mortality over the period included in the study and no significant relation to asbestos imports. In addition, this study has demonstrated that the rising male and female mortality attributed to an idiopathic disease (IPF) has risen in parallel to that of mesothelioma, with the number of deaths each year also significantly related to previous UK asbestos imports.
A relatively simple analytical approach was utilized in this study, comparing the number of certified deaths due to three different conditions and investigating their relationship with historic UK asbestos imports. The analyses were conducted separately for male and female mortality, due to known differences in asbestos-related mortality [11]. Total asbestos imports for the UK were used, as data for England and Wales were not available. This estimate of asbestos usage was crude, as it was calculated by adding the total imports of chrysotile, crocidolite and amosite for each year, rather than imports per capita. It could therefore not make any allowance for whether the asbestos imported each year was used or stored, or allow for exposures to existing asbestos products. Another potential limitation was that a 48-year latent period was utilized based on an asbestosis model developed in the US [2], rather than selecting the latent period that would best fit the study data.
The findings in this study are in keeping with the results from an international study using WHO mortality data for 2000–2004 and mean per head asbestos consumption between 1960 and 1969 [1]. Data were used from 33 countries (including the UK), accounting for 63% of the total worldwide asbestos consumption in the time period selected. Mortality rates due to mesothelioma were consistently higher than mortality rates due to asbestosis, in keeping with the findings in our study. Lin et al. [1], using regression techniques, demonstrated that historic asbestos consumption was a significant predictor of mesothelioma mortality in both sexes and asbestosis mortality in males. Stronger relationships were found for male asbestosis (adjusted R 2 = 0.79) and male mesothelioma (adjusted R 2 = 0.74), than were seen for female mesothelioma (adjusted R 2 = 0.58). These findings were slightly different to the results of our analysis, where the relationships between historic asbestos imports and mesothelioma mortality were stronger and similar for both sexes, with R 2 values higher than for male asbestosis. These differences are likely to relate to the different study designs, as Lin et al. [1] utilized pooled international mortality data, a shorter latent interval, and per capita asbestos consumption. The low number of annual female deaths due to asbestosis seen in our study was also in keeping with US data, where 96% of asbestosis mortality between 1968 and 2004 was in men [2].
Although there have been a number of studies confirming the relationship between long-latency asbestos-related disease mortality and historic asbestos usage [1–6], our study is (to our knowledge) the first to investigate a possible relationship for IPF. The strength of the relationship seen between IPF and asbestos imports was seen for both sexes and was similar to that seen for mesothelioma mortality. Although this relationship cannot establish causation, and may be entirely coincidental, it raises the question as to whether a proportion of IPF mortality is in fact due to unrecognized asbestos exposure.
The hypothesis that IPF mortality and asbestos exposure may be linked is at odds with the findings of a number of case-control studies, designed to explore occupational risk factors for this disease [15–17]. Although no significant link to asbestos exposure was found in these studies, indirect evidence to support the importance of asbestos exposure in the workers at increased risk of IPF can be found from their markedly increased risk of mesothelioma around the time of these studies [11]. The increased mesothelioma risk in sheet metal workers has been attributed to bystander (i.e. indirect) exposure to asbestos (not metal dust) [7], whereas for carpenters/joiners it has been attributed to the use of asbestos insulation board used in the construction industry (not wood dust exposure) [11].
In Britain, significant occupational asbestos exposure was common amongst the working population up to the early 1980s. Control data from a mesothelioma case-control study published in 2009 found that in the general population born in the 1940s, two-thirds of men and over a fifth of women had worked in a high- or medium-risk exposure job, as assessed objectively by a job-exposure matrix (JEM) [11]. This study highlighted that whilst many individuals with this disease did not give a clear history of asbestos exposure, their mesothelioma risk could be determined by their likely exposures as assessed by the JEM. If patients of this age group developing pulmonary fibrosis are similar to the general population, many will have worked with asbestos, irrespective of whether they are able to provide a suitable history of exposure. This raises the question as to how we clinically assess how much asbestos exposure is ‘enough’ for diffuse pulmonary fibrosis to be classified as asbestosis rather than idio pathic [23, 24].
Clinicians are often reliant on patients with pulmonary fibrosis having an accurate knowledge and recall of asbestos exposure many decades prior to the onset of disease. In addition, the clinician must be satisfied that the exposure has been of sufficient dose to be capable of inducing fibrosis, which may be somewhat subject ive, without a detailed knowledge of historic levels of asbestos exposure in different British industries. Where no definite history of asbestos exposure can be provided, or where the exposure assessment concludes that the exposure has not been prolonged and/or heavy enough to cause asbestosis, it is likely that the patient will be diagnosed with idiopathic disease (IPF).
A 25 fibre/ml-years threshold of asbestos exposure is often stated as a pre-requisite for the development of asbestosis, yet this is not universally accepted. For example, individual cases of asbestosis have been reported with lower lifetime exposures [25], and a threshold of 5 fibre/ml-years is used in the Dutch JEM for assessing asbestosis benefits [26]. In addition, analyses of asbestosis mortality data from American and Chinese cohorts have not supported a ‘no effect’ threshold and have demonstrated that a ‘power’ model best fits the data [27,28]. The concept that all individual workers would respond in the same way to the same dose of asbestos is biologically implausible, given that many with high levels of exposure never develop fibrosis. An absolute threshold requirement makes no consideration of individual susceptibility to fibrosis, for which there is an emerging evidence base [29,30].
In summary, the findings of this study are in keeping with the hypothesis that in England and Wales, a proportion of UIP pattern pulmonary fibrosis currently diagnosed as idiopathic may in fact be due to the difficulties of accurately assessing historic asbestos exposure. There is therefore a clear need to develop an asbestos exposure JEM, using British industry data, to facilitate accurate diagnosis and future case-control studies.
Key points
This study has confirmed the expected relationship between historic UK asbestos imports and the rising mortality due to mesothelioma (both sexes) and asbestosis (in men but not women).
In addition, it has identified a similarly strong linear relationship (for both sexes) between asbestos imports and mortality due to idiopathic pulmonary fibrosis (a disease with no known cause).
These data raise the possibility that a significant proportion of asbestosis may be being misclassified as idiopathic pulmonary fibrosis and highlight the need for evidence-based guidance in the differential diagnosis of these conditions.
Funding
Health and Safety Laboratory.
Conflicts of interest
None declared.
Supplementary Material
Acknowledgements
The authors would like to acknowledge the staff at the ONS and Andy Darnton of the Epidemiology Unit of HSE for their assistance in providing the data utilized in this study.
References
- 1. Lin RT, Takahashi K, Karjalainen A, et al. Ecological association between asbestos-related diseases and historical asbestos consumption: an international analysis. Lancet 2007;369:844–849. [DOI] [PubMed] [Google Scholar]
- 2. Antao VC, Pinheiro GA, Wassell JT. Asbestosis mortality in the USA: facts and predictions. Occup Environ Med 2009;66:335–338. [DOI] [PubMed] [Google Scholar]
- 3. Banaei A, Auvert B, Goldberg M, et al. Future trends in mortality of French men from mesothelioma. Occup Environ Med 2000;57:488–494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Price B, Ware A. Time trend of mesothelioma incidence in the United States and projection of future cases: an update based on SEER data for 1973 through 2005. Crit Rev Toxicol 2009;39:576–588. [DOI] [PubMed] [Google Scholar]
- 5. Tan E, Warren N, Darnton AJ, et al. Projection of mesothelioma mortality in Britain using Bayesian methods. Br J Cancer 2010;103:430–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Takahashi K, Huuskonen MS, Tossavainen A, et al. Ecological relationship between mesothelioma incidence/mortality and asbestos consumption in ten western countries and Japan. J Occup Health 1999;41:8–11. [Google Scholar]
- 7. American Thoracic Society. Diagnosis and initial management of non malignant diseases related to asbestos. Am J Respir Crit Care Med 2004;170:691–715. [DOI] [PubMed] [Google Scholar]
- 8. Harding AH, Darnton AJ. Asbestosis and mesothelioma among British asbestos workers (1971–2005). Am J Ind Med 2010;53:1070–1080. [DOI] [PubMed] [Google Scholar]
- 9. Raghu G, Collard HR, Egan JJ, et al. ; on behalf of the ATS/ERS/JRS/ALAT Committee on Idiopathic Pulmonary Fibrosis. An Official ATS/ERS/JRS/ALAT Statement: Idiopathic Pulmonary Fibrosis: evidence-based Guidelines for Diagnosis and Management. Am J Respir Crit Care Med 2011;183:788–824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Noble PW, Albera C, Bradford WZ, et al. ; CAPACITY Study Group. Pirfenidone in patients with idiopathic pulmonary fibrosis (CAPACITY): two randomised trials. Lancet 2011;377:1760–1769. [DOI] [PubMed] [Google Scholar]
- 11. Rake C, Gilham C, Hatch J, et al. Occupational, domestic and environmental mesothelioma risks in the British population: a case-control study. Br J Cancer 2009;100:1175–1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Copley SJ, Wells AU, Sivakumaran P, et al. Asbestosis and idiopathic pulmonary fibrosis: comparison of thin-section CT features. Radiology 2003;229:731–736. [DOI] [PubMed] [Google Scholar]
- 13. Harris JM, Johnston ID, Rudd R, et al. Cryptogenic fibrosing alveolitis and lung cancer: the BTS study. Thorax 2010;65:70–76. [DOI] [PubMed] [Google Scholar]
- 14. Navaratnam V, Fleming KM, West J, et al. The rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax 2011;66:462–467. [DOI] [PubMed] [Google Scholar]
- 15. Ley B, Collard HR. Epidemiology of idiopathic pulmonary fibrosis. Clinical Epidemiology 2013;5:483–492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Hubbard R, Lewis S, Richards K, et al. Occupational exposure to metal or wood dust and aetiology of cryptogenic fibrosing alveolitis. Lancet 1996;347:284–289. [DOI] [PubMed] [Google Scholar]
- 17. Hubbard R, Cooper M, Antoniak M, et al. Risk of cryptogenic fibrosing alveolitis in metal workers. Lancet 2000;355:466–467. [DOI] [PubMed] [Google Scholar]
- 18. Barber CM, Fishwick D. Importance of past occupational exposures in the rising incidence of idiopathic pulmonary fibrosis in the U.K. Thorax 2012;67:264. [DOI] [PubMed] [Google Scholar]
- 19. Office for National Statistics. Mortality Statistics. http://www.ons.gov.uk/. (1 January 2014, date last accessed).
- 20. HSE. Mesothelioma Statistics http://www.hse.gov.uk/statistics/causdis/mesothelioma/index.htm (1 June 2014, date last accessed).
- 21. Shuker L, Harrison P, Poole S. Fibrous materials in the environment. A review of asbestos and man-made mineral fibres. Institute for Environment and Health 1997:11–29. [Google Scholar]
- 22.Asbestos Information Centre. Asbestos Imports http://www.aic.org.uk/asbestos-imports/ (1 June 2014, date last accessed).
- 23. Van Cleemput J, De Raeve H, Verschakelen JA, et al. Surface of localized pleural plaques quantitated by computed tomography scanning: no relation with cumulative asbestos exposure and no effect on lung function. Am J Respir Crit Care Med 2001;163:705–710. [DOI] [PubMed] [Google Scholar]
- 24. Monsó E, Tura JM, Pujadas J, et al. Lung dust content in idiopathic pulmonary fibrosis: a study with scanning electron microscopy and energy dispersive x ray analysis. Br J Ind Med 1991;48:327–331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Dement JM, Harris RL, Symons M J, et al. Exposures and mortality among chrysotile asbestos workers. Part II Mortality. Am J Ind Med 1983;4:310–322. [DOI] [PubMed] [Google Scholar]
- 26. Burdorf A, Swuste P. An expert system for the evaluation of historical asbestos exposure as diagnostic criterion in asbestos-related diseases. Ann Occup Hyg 1999;43:57–66. [PubMed] [Google Scholar]
- 27. Hein MJ, Stayner LT, Lehman E, et al. Follow-up study of chrysotile textile workers: cohort mortality and exposure-response. Occup Environ Med 2007;64:616–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Deng Q, Wang X, Wang M, Lan Y. Exposure-response relationship between chrysotile exposure and mortality from lung cancer and asbestosis. Occup Environ Med 2012;69:81–86. [DOI] [PubMed] [Google Scholar]
- 29. Nemery B, Bast A, Behr J, et al. Interstitial lung disease induced by exogenous agents: factors governing susceptibility. Eur Respir J Suppl 2001;32:30s–42s. [PubMed] [Google Scholar]
- 30. Putman RK, Rosas IO, Hunninghake GM. Genetics and early detection in idiopathic pulmonary fibrosis. Am J Respir Crit Care Med 2014;189:770–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.